Abstract
Phytoavailability of inorganic phosphorus (Pi) reduces within a few hours of application while continuous application of organic phosphorus (Po) has gained the attention of environmentalists. Therefore, continuous application of either Pi or Po would not be a desirable approach. In addition, the ameliorative effect of phosphorus (P) on plant growth under a saline environment has broadened the scope of this hazard. However, our knowledge about P release from amended soils and plant uptake under Cl or SO4 salt saturated soils is very limited. Therefore, the current study was designed with two objectives: 1) to evaluate the effect of Cl and SO4 salts on inorganic P release/uptake; 2) to evaluate wheat response to a new combined application of Po + Pi sources under a saline environment. In a greenhouse study, soil was salinized by adding NaCl and Na2SO4 salts and P was amended at the rate of 100 kg ha−1 in the form of composted livestock manure (Po), KH2PO4 (Pi) and Po (50 kg ha−1) + Pi (50 kg ha−1) (Pc) along with a control. Irrespective of salt types, application of P sources enhanced plant growth and P uptake compared to the control. Combined P sources (Pc) gave higher shoot and root dry matter than P source applied alone. Chloride salt suppressed shoot and root dry matter more severely than SO4 salt. Phosphorus uptake and recovery (%) increased in pots amended with Pc. Chloride salt was also more toxic for plant P uptake and recovery than SO4 salt. In the laboratory experiment, soils were amended with the same P sources. After first extraction with NaCl and Na2SO4, biologically available P (extracted with NaHCO3), Fe- and Al- bound P (extracted with NaOH) and stable Ca-bound P (extracted with HCl) fractions were measured. It was observed that SO4 salt released higher amounts of labile P fractions (salt and NaHCO3 extracted P) than Cl. This study clearly showed that Cl is more toxic for plant growth and P uptake, while SO4 has higher P desorption capacity. It is suggested that Pc would not only best meet wheat P requirements under saline conditions, but will also help to reduce the load of waste application on agricultural soils.
Introduction
Soil salinity and nutrient deficiencies are the main factors reducing plant productivity in arid and semi-arid areas (Ashraf & Waheed, Citation1993). It was estimated that about one-third of irrigated land has been affected by a salinity problem (Shannon, Citation1984). Increased salinity reduces water availability and nutrient uptake by plants (Pessarakli & Tucker, Citation1988). Ultimately, plant growth and yield potential are reduced (Shalhevet & Hsiao, Citation1986). The damage caused by salinity has been attributed mainly to excessive accumulation of Cl− and Na+ ions in plants, causing nutritional imbalances (Zekri & Parsons, Citation1992). Salt stress was found highly related to macro-nutrient deficiencies, e.g., high NaCl concentration has been shown to induce phosphorus and potassium deficiencies in tomato and cucumber (Adams, Citation1991; Sonneveld & Kreij, Citation1999). Usually, chloride and sulphate salts are dominant in salt-affected soils. However, most of the previous studies are confined to the effect of Cl salts only.
Addition of phosphorus (P) has been reported to increase the yields of certain cereals and pasture plants grown in saline soils at the same level of available P that would be sufficient to produce maximal yields on non-saline soils (Gibson, Citation1988; Manchanda et al., Citation1982). An antagonism between Cl and P absorption has been reported in tomato plants, and salt tolerance of plants was enhanced when the P levels were increased (Bernstein et al., Citation1974; Cerda & Bingham, Citation1978). However, our knowledge on P uptake by plants under SO4 salt is lacking. Similarly, the P release characteristics of soils and soil components have been studied extensively (Barrow, Citation1979; Chien & Clayton, Citation1980; Kuo & Lotse, Citation1974; Sharpley et al., Citation1981) and the factors which have been shown to influence desorption of P include duration of contact with added P, pH, temperature, ionic strength (Barrow, Citation1983), clay contents and soil organic C (Sharpley & Sisak, Citation1997). One of the principal experimental variables which affect the results of P sorption studies is the ionic composition, both species and concentrations, of the contacting solution. Schofield (Citation1955) suggested the use of 10−2 M CaCl2 on the basis that cation exchange is minimized by the use of calcium, and chloride at this concentration has no specific replacing power. KCl, NaCl and distilled water containing added P and associated cations have also been used as contacting media (Shapiro & Fried, Citation1959; Syers et al., Citation1970; Woodruff & Kamprath, Citation1965). In spite of the realization that ionic species, concentrations and their compositions affect P sorption and/or desorption, most of these studies are confined to Cl− (anion) in association with other cations.
The inorganic phosphorus applied in the form of fertilizer is immediately converted to unavailable forms by active aluminum and iron in neutral to acidic soils. Analogously, continuous manure application resulted in significant increase in soil P level (Dormaar & Chang, Citation1995; Vivekanandan & Fixen, Citation1990). As a result, surface soil accumulation of P occurred to such an extent that the loss of P in surface runoff became a priority management and environmental concern (Sharpley, Citation1995). Therefore, the use of manure or fertilizer alone would no longer be a desirable approach. The current study, therefore, was designed with two objectives: 1) to evaluate the Pi release from soils induced by Cl and SO4 salts and its uptake by the plant; 2) to evaluate wheat response to a new combined P source (Pc) application compared to Pi and Po under a saline environment.
Materials and methods
Soil analysis
Three soil types, sandune, masatsuchi and paddy were used in the study. According to the United Soil Classification System of Japan (Citation2002), the soils are Sandune Regosol, Terrestrial Regosol and Typic-Gray Paddy soil, respectively. The physico-chemical properties of the soils were determined as shown in . Soil texture was determined by the pipette method (Gee & Bauder, Citation1986). Soil pH and EC were measured in soil-water (1:5) suspensions. Total carbon and total nitrogen were determined by the dry combustion method using the SumiGraph NCH-21 analyzer (Model MT 700 Yonaco Company, Japan). Total P was determined by digesting soil samples in nitric-perchloric acid mixture and the P reading in the digest was measured with a spectrophotometer (Model U 2001, Hitachi Corp, Japan) at 710 nm using the sulpho-molybdo-phosphate blue color method. Exchangeable cations were leached from the soil with neutral ammonium acetate (Thomas, Citation1982) and the contents of Ca, Mg, Na and K were determined using an atomic absorption spectrophotometer (Model AA-670, Shimadzu Corp, Kyoto, Japan). Composted livestock (cow) manure was collected from the feedlot yard of Tottori University and its chemical composition was also determined ().
Table I. Selected characteristics of the soils and manure used for the study.
Soil salinization
Sandune soil was used in a pot experiment. Soil was passed through a 4 mm sieve and a sub-sample set aside as untreated control (non-saline). The remaining soil was salinized with NaCl (Cl) and Na2SO4 (SO4). Each salt was applied at rates of 75 and 150 mmolc l−1. The salinized soils were air dried and four kg of each sample were weighed into Wagner pots (size: height 30 cm, diameter 18 cm). Phosphorus was applied at the rate of 100 kg ha−1 in the form of organic phosphorus (Po), inorganic phosphorus (Pi) and combined Po (50 kg ha−1) + Pi (50 kg ha−1) (Pc). Composted livestock (cow) manure and potassium phosphate (KH2PO4) were used as sources of Po and Pi respectively. The control pot received no P or salinity treatments. Five levels of salts were factorially combined with four P sources to give a total of 20 treatments. Pots were arranged in a greenhouse in a randomized complete design in triplicate. Ten seeds of a Japanese spring wheat variety ‘Haruyutaka’ were sown in each pot and thinned to five plants after germination. Pots were irrigated and weighed. After six weeks the plants were harvested. Plant samples were rinsed with distilled water and separated into shoots and roots. Samples were oven dried at 65°C for 48 h for dry matter yield. Thereafter they were ground into powder and digested in nitric-perchloric acid mixture for P analysis. Post-harvest soil was also analysed for water-extractable P content. Phosphorus uptake was determined by multiplying P concentration by dry weight and P recovery was determined by using the following formula:
Phosphorus fractionation in soil
Five grams of masatsuchi and paddy soil samples were put into centrifuge tubes and amended with the same P sources used for the pot study. Soils were saturated with 0.5 M NaCl and Na2SO4 salts at a soil to solution ratio of 1:5 and shaken for 24 h at room temperature. The suspension was then centrifuged at 10×103 rpm for 10 min and filtered; then a second extraction of the same samples was performed with 0.5 M NaHCO3 (adjusted to pH 8.5) to remove labile Pi and Pi sorbed on the soil surface (Bowman & Cole, Citation1978). A third extraction was carried out with 0.1 M NaOH to extract Pi compounds held more strongly by chemisorption to iron and aluminum components of soil surfaces (Ryden et al., Citation1977; McLaughlin et al., Citation1977). Soil was sonicated with NaOH solution to extract the P fixed to inner layers of the particle and the readings of both NaOH extractions were combined. The final extraction was with 1 M HCl to remove mainly apatite-type minerals (Williams et al., Citation1971) but also could extract occluded P in more weathered soils (Williams et al., Citation1980). Each extraction was of 16 h duration and the soil-to-solution ratio was maintained at 1:5. The data were statistically analysed using StatView software (SAS, Citation1999). A probability level of <0.05 was considered significant and means were separated by Fisher's LSD test.
Results and discussion
Biomass production
Different P sources and salt types significantly (p<0.01) affected the wheat biomass production (). Both shoot and root dry matter differed for P sources in the order: Pc > Po > Pi > control (). Statistically, no difference was observed among the P sources for shoot-to- root ratio (). Irrespective of salt types, shoot-root ratio was high under both saline conditions compared to the non-saline treatment (). Shoot and root dry matter was significantly reduced under both saline conditions compared to the non-saline pots. Plants from highly salinized pots (150 mmol L−1) germinated but could not survive. Chloride salt retarded shoot growth more severely than SO4 salt. Shoot-to-root ratio was equally high under both salts compared to the non-saline treatment (). Shoot-to-root ratio was maximum in the pots amended with Po and treated with SO4 salinity.
Figure 1. Effect of P sources and salt types on shoot, root dry matter (g pot−1) and shoot-root dry matter ratio. Po = organic P source, Pi = inorganic P source and Pc = ½Po + ½Pi.
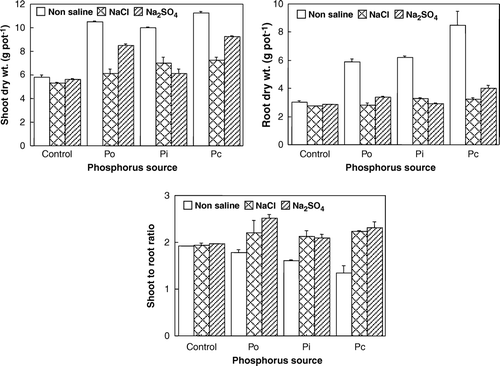
Table II. Summary of two-way analysis of variance for different parameters studied.
Tarafdar and Claassen (Citation2003) reported that with the increase in inorganic P concentration, plant dry weight and P concentration in shoot and root increased, which was further enhanced by the addition of the organic form of P. Similarly, Schlegel (Citation1992) found that composted manure plus fertilizer application resulted in greater grain sorghum yield than either source applied alone. Many researchers have reported the adverse effects of salinity on plant growth (Garg & Gupta, Citation1997; Mer et al., Citation2000; Ramoliya & Pandey, Citation2003). The mechanism of such effects is involved with reduced water absorption, reduced metabolic activities as a result of salt toxicity, and nutrient imbalances (Yeo, Citation1983). Champagnol (Citation1979) observed that P addition to saline soils increased crop growth and yield in 34 out of 37 crops tested. The adverse effect of SO4 salt on the plants was less visible than with Cl because sulphur is considered as an essential plant nutrient. Manchanda et al. (Citation1982) found that high SO4-treated plants yielded 3–6 times more dry matter of wheat and barley than Cl treatment. Similarly, Harsharn et al. (Citation2004) also observed an increase in shoot-root ratio with an increase in subsoil salinity for a wheat crop.
Phosphorus uptake and recovery
Effect of P sources
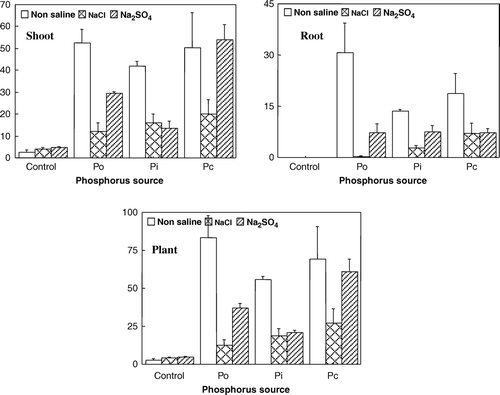
Wheat P uptake was significantly influenced by the P source applied (). Higher P uptake was recorded in shoot under Pc treatment, followed by Po, Pi and control, respectively (). Combined P source and Po gave 73% and 31% higher P uptake in the shoot than Pi treated pots, respectively. Irrespective of P sources (except non-P control pots), root P uptake was severely suppressed under both saline conditions than non-saline treatment (). Plant (shoot + root) P uptake was greater under Pc amended pots. Phosphorus recovery was calculated for each treatment and maximum P recovery (%) was observed from Pc amended pots.
Figure 2. Effect of different P sources and salt types on root, shoot and plant P uptake (mg DW−1). Po = organic P source, Pi = inorganic P source and Pc = ½Po + ½Pi.
The better response of the wheat crop under Pc treatment could possibly be due to less P sorption and continuous slow P release with time. The better performance of Pc compared to Po and Pi has also been reported by some researchers (Toor & Bahl, Citation1997; Reddy et al., Citation1999). Tarafdar and Claassen (Citation2003) reported a synergistic effect between Pi and Po (when applied together), and concluded that higher yields of plants were due to the higher P uptake under the effect of greater acid phosphatase activity. In addition, organic P sources not only improved physico-chemical conditions of the soil but also released inorganic P bound to the soil by inositol hexaphosphate (Anderson et al., Citation1974) and prevented P fixation in the soil (Kafkafi et al., Citation1988) due to the organic anions (Struthers & Sieling, Citation1950). Plant growth under Po and Pi treated pots was equally better, which might be due to the fact that P availability from Po is as available as from Pi (Gale et al., Citation2000; Meek et al., Citation1979). Elias-Azar et al. (Citation1980) also found that availability of P from fresh and composted dairy manure was similar to that of KH2PO4 fertilizer in alkaline sandy soils.
Effect of salt types
Phosphorus uptake in shoot and root was suppressed under both types of salts compared to normal soil (). Chloride salt was observed to be more toxic for shoot P uptake compared to SO4, and P uptake by roots was suppressed under both saline conditions compared to the non-saline treatment. In plant (root + shoot), Cl salt reduced P uptake more severely (about two-fold) than SO4 salt and control (four-fold). However, the salinity effect in non-P amended control did not differ. Phosphorus recovery was also low under both saline conditions compared to the non-saline treatment. However, among the salt types, P recovery was 125% higher in SO4 than Cl saturated pots. Manchanda et al. (Citation1982) also reported similar results and stated that P availability and its absorption were more adversely affected by excess Cl than SO4 salt. However, in the literature the interaction between salinity and phosphorus is reported to be very complex and there is no clear mechanistic explanation for decreased, increased or unchanged P uptake in response to salinization in different species, as reported by Grattan and Grieve (Citation1992). Few studies indicate that salinity either increased or had no effect on P uptake. However, most of the research studies showed a decrease in P concentration in the plant tissues under saline conditions (Güneş et al., Citation1999). Ravikovitch and Yoles (Citation1971) observed that increasing NaCl salinity suppressed P content and yield of clover, which was markedly improved with an increase in the P content of the soil. The suppressing effect of Cl salt on P uptake is attributed to a decrease in P solubility and availability owing to its complexity as insoluble Ca-P compounds (Bernstein et al., Citation1974).
Soluble phosphorus in post-harvest soil
Phosphorus sources and salt types significantly affected the results of residual P extracted with water from the soil after harvesting the plants (). The maximum amount of water soluble P was recorded in Po, followed by Pc and Pi treated pots. The amount of water soluble P in Pc was about one-third compared to P extracted from Po amended pots. Plant growth and P uptake from Pc amended pots were high, which might have reduced the water soluble P in soils after harvest. The lowest residual P-value in Pi might be due to P adsorption on the clay particles or immobilized as a result of other soil minerals. Laboski and Lamb (Citation2003) reported that P concentration in manure amended soils was greater than inorganic fertilizer at the same rate of P application.
It was observed that soils saturated with SO4 salt released higher amounts of water soluble P than Cl treatment. This might have been due to the anion exchange phenomena that occurred in salinized soils. Zahoor et al. (Citation2006) has also reported that release of labile P fractions is higher under SO4 salinity than Cl.
Effect of salt types on soil P fractions
Phosphorus release in salt extract was higher from both soils amended with Po and Pc (). However, masatsuchi soil released substantially higher amounts of P in the first salt extraction (salt-P) than paddy soil. NaHCO3 extracted P (NaHCO3-P) was also higher from Po and Pc but the P quantity was higher in paddy soil. The release of labile P fractions (salt-P and NaHCO3-P) was greater from soils saturated with SO4 than Cl salt. A substantial amount of P was released from all P sources with NaOH extraction (plus P extracted by sonication). No difference was observed in NaOH-P between the two salt types, but SO4-P was higher than non-saline soils. HCl extracted P (HCl-P) was higher from paddy soil amended with Po. Both salts released higher amounts of HCl-P than control.
Figure 3. Effect of different P sources and salt types on soil P fractions. Po = Organic P source, Pi = Inorganic P source and Pc = ½Po + ½Pi.
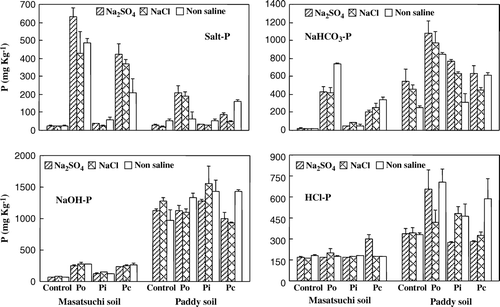
The greater amount of salt-P from masatsuchi soil might be due to the coarse texture of the soil. No previous study was found to compare the results of higher P release induced by SO4 than Cl salt. While most of the previous studies focused on anion sorption capacities, namely, oxalate (OX), SO4 and PO4 anions (Parfitt et al., Citation1977; Parfitt, Citation1980; Liu et al., Citation1999), the mechanism for their adsorption and the structures of their surface complexes are not fully known. Liu et al. (Citation1999) indicated that the order of addition of oxalate and SO4 had a strong influence on the adsorption of these anions on goethite at low pH values; the anion added first prevented the adsorption of the anion added secondly. When PO4 was added, SO4 desorption was strongly promoted as was, to a lesser extent, that of oxalate. Most authors are of the opinion that the mechanisms of SO4 and PO4 adsorption are similar, and that both ions compete for the same sorption sites (Kamprath et al., Citation1956; Couto et al., Citation1979; Pasricha & Fox, Citation1993). Although adsorbed SO4 does not compete strongly with PO4, there is probably some competition for sorption between these anions which may cause comparatively more P release by SO4 than Cl salt (Zahoor et al., Citation2006).
Among the P sources, most of the labile P fractions from Po and Pc were released in salt and NaHCO3 extractions. However, increase in P release from Pi and control was observed in NaOH and HCl extractions. Reduced Pi in the first extractions might be due to the fact that inorganic P easily fixes to the soil minerals and its availability starts to reduce just after application. Increased P availability from Po compared to Pi source might be due to the organic acid present in the Po source that may prevent P fixation and is able to replace P bound to soil minerals (Kafkafi et al., Citation1988) which in turn might have increased P release from the organic P amended treatment.
Achieved results have important implications not only from the viewpoint of P nutrition, but also from the research viewpoint, as most researchers focus on different cations only for evaluating P response to salinity (Adams, Citation1991; Shalhevet & Hsiao, Citation1986; Sonneveld & Kreij, Citation1999; Zekri & Parsons, Citation1992). However, our study made it clear that anions in association with cations also differed with regard to their effects on P release, and the SO4 ion has greater ionic strength to release P than Cl. It is therefore highly recommended that more than one anion species must be included when evaluating P response to a saline environment. Analogously, it was observed that salt effect was more prominent on labile soil P fractions (water, salt/H2O and NaHCO3-P) than other stable P fractions.
It is concluded that adverse effect of salt stress on plant growth can possibly be alleviated by combined application of organic and inorganic P fertilizers.
Acknowledgements
The author expresses thanks to A.E. Eneji, T. Endo and Shin Abe for their encouragement and help throughout the study. Thanks are also due to the Ministry of Education, Science, Sports and Culture-Japan for financial support during the research work.
References
- Adams , P. 1991 . Effect of increasing the salinity of the nutrient solution with major nutrients or sodium chloride on the yield, quality and composition of tomato grown in rockwool . Journal of Horticultural Sciences , 66 : 201 – 207 .
- Anderson , G. , Williams , E.G. and Moir , J.O. 1974 . A comparison of the sorption of inorganic orthophosphate and inositol hexaphosphate by six acid soils . Journal of Soil Science , 25 : 51 – 62 .
- Ashraf , M. and Waheed , A. 1993 . Response of some genetically diverse lines of chickpea (Cicer arietinum L.) to salt . Plant and Soil , 154 : 257 – 266 .
- Barrow , N.J. 1983 . A mechanistic model for describing the sorption and desorption of phosphate by soil . Journal of Soil Science , 34 : 733 – 750 .
- Barrow , N.J. and Shaw , T.C. 1979 . Effect of ionic strength and nature of the cation on desorption of phosphate from soil . Journal of Soil Science , 30 : 53 – 65 .
- Bernstein , L. , Francois , L.E. and Clark , R.A. 1974 . Interactive effects of salinity and fertility on yields of grains and vegetables . Agronomy Journal , 66 : 412 – 421 .
- Bowman , R.A. and Cole , C.V. 1978 . Transformations of organic phosphorus substrates in soils evaluated by NaHCO3 extraction . Soil Science , 125 : 49 – 54 .
- Cerda , A. and Bingham , F.T. 1978 . Yield, mineral composition, and salt tolerance of tomato and wheat as affected by NaCl and P nutrition . Agrochimica , 22 : 140 – 149 .
- Champagnol , F. 1979 . Relationships between PO4 nutrition of plants and salt toxicity . Phosphorus in Agriculture , 76 : 35 – 43 .
- Chien , S.H. and Clayton , W.R. 1980 . Application of Elovich equation to the kinetics of phosphate release and sorption in soils . Soil Science Society of American Journal , 44 : 265 – 268 .
- Couto , W. , Lathwell , D.J. and Boulden , D.R. 1979 . Sulphate sorption by two oxisols and an alfisol of the tropics . Soil Science , 127 : 108 – 116 .
- Dormaar , J.F. and Chang , C. 1995 . Effect of 20 annual applications of excess feedlot manure on labile soil phosphorus . Canadian Journal of Soil Science , 75 : 507 – 512 .
- Elias-Azar , K. , Laag , A.E. and Pratt , P.F. 1980 . Bicarbonate-extractable phosphorus in fresh and composted dairy manures . Soil Science Society of American Journal , 44 : 435 – 437 .
- Gale , P.M. , Mullen , M.D. , Cieslik , C. , Tyler , D.D. , Dcuk , B.N. , Kirchner , N. and McClure , J. 2000 . Phosphorus distribution and availability in response to dairy manure applications . Communication in Soil Science and Plant Analysis , 31 : 553 – 565 .
- Garg , B.K. and Gupta , I.C. 1997 . Saline Wastelands Environment and Plant Growth , Jodhpur, , India : Scientific Publishers .
- Gee , G.W . & Bauder , J.W . (1986) . Particle-size analysis . In A Klute Method of Soil Analysis, Part 1 (pp. 383 – 411 ). Agronomy series No. 9. American Society of Agronomy and Soil Science Society of America, Inc. Publ. Madison, WI .
- Gibson , T.S. 1988 . Carbohydrate metabolism and phosphorus/salinity interactions in wheat (Triticum aestivum L.) . Plant and Soil , 111 : 25 – 35 .
- Grattan , S.R. and Grieve , C.M. 1992 . Mineral element acquisition and growth response of plants grown in saline environments . Agriculture Ecosystems and Environment , 38 : 275 – 300 .
- Günes , A. , Inal , A. , Alpaslan , M. and Cykyly , Y. 1999 . Effect of salinity on P induced Zn deficiency in pepper plants . Turkish Journal of Agriculture and Forestry , 23 : 459 – 464 .
- Harsharn , S.G ., Shane , N. & Peter , C. (2004) . Subsoil salts affect root function, shoot growth and ionic balance of wheat plants . Fourth International Crop Science Congress held at Brisbane; 2004 26 September–1 October, Australia. [Cited 2005 Aug. 25; Available from: http://www.cropscience.org.au/ics2004/poster/index.htm ]
- Kafkafi , U. , Bar-Yosef , B. , Rosenberg , R. and Sposito , G. 1988 . Phosphorus adsorption by kaolinite and montmorillonite. II. Organic anion competition . Soil Science Society of American Journal , 52 : 1585 – 1589 .
- Kamprath , E.J. , Nelson , W.L. and Fitts , J.W. 1956 . The effect of pH, sulphate and phosphate concentrations on the adsorption of sulphate by soils . Soil Science Society of America Proceedings , 28 : 463 – 466 .
- Kuo , S. and Lotse , E.G. 1974 . Kinetics of phosphate adsorption and desorption by haematite and gibbsite . Soil Science , 116 : 400 – 406 .
- Laboski , C.A.M. and Lamb , J.A. 2003 . Changes in soil test phosphorus concentration after application of manure or fertilizer . Soil Science Society of American Journal , 67 : 544 – 554 .
- Liu , F. , He , J. , Colombo , C. and Violante , A. 1999 . Competitive adsorption of sulfate and oxalate on goethite in the absence or presence of phosphate . Soil Science , 164 : 180 – 189 .
- McLaughlin , J.R. , Ryden , J.C. and Syers , J.K. 1977 . Development and evaluation of a kinetic model to describe phosphate sorption by hydrous ferric oxide gels . Geoderma , 18 : 295 – 307 .
- Manchanda , H.R. , Sharma , S.K. and Bhandari , D.K. 1982 . Response of barley and wheat to phosphorus in the presence of chloride and sulphate salinity . Plant and Soil , 66 : 233 – 241 .
- Meek , B.D. , Graham , L.E. , Donovan , T.J. and Mayberry , K.S. 1979 . Phosphorus availability in a calcareous soil after high loading rates of animal manure . Soil Science Society of American Journal , 43 : 741 – 743 .
- Mer , R.K. , Prajith , P.K. , Pandya , D.H. and Pandey , A.N. 2000 . Effect of salts on germination of seeds and growth of young plants of Hordeum vulgare, Triticum aestivum, Cicer arietinum and Brassica juncea . Journal of Agronomy and Crop Science , 185 : 209 – 217 .
- Parfitt , R.L. 1977 . Adsorption on hydrous oxides. I. oxalate and benzoate on goethite . Journal of Soil Science , 28 : 289 – 296 .
- Parfitt , R.L. , Farmer , V.C. and Russell , J.D. 1980 . “ Chemical properties of variable charge soils ” . In Soils with variable charge , Edited by: Theng , BKG. 167 – 194 . Lower Hutt, NZ : New Zealand Society of Soil Science .
- Pasricha , N.S. and Fox , R.I. 1993 . Plant nutrient sulphur in the tropics and subtropics . Advances in Agronomy , 50 : 209 – 269 .
- Pessarakli , M. and Tucker , T.C. 1988 . Dry matter yield and nitrogen-15 uptake by tomatoes under sodium and chloride stress . Soil Science Society of American Journal , 52 : 698 – 700 .
- Ramoliya , P.J. and Pandey , A.N. 2003 . Effect of salinization of soil on emergence, growth and survival of seedlings of Cordia rothii . Forest Ecology and Management , 176 : 185 – 194 .
- Ravikovitch , S. and Yoles , D. 1971 . The influence of phosphorus and nitrogen on millet and clover growing in soils affected by salinity. II. Plant composition . Plant and Soil , 35 : 569 – 588 .
- Reddy , D.D. , Rao , A.S. and Takkar , P.N. 1999 . Effect of repeated manure and fertilizer phosphorus additions on soil phosphorus dynamics under a soybean wheat rotation . Biology and Fertility of Soils , 28 : 150 – 155 .
- Ryden , J.C. , McLaughlin , J.R. and Syers , J.K. 1977 . Mechanisms of phosphate sorption of soils and hydrous ferric oxide gel . Journal of Soil Science , 28 : 72 – 92 .
- SAS . 1999 . StatView for windows. Version 5.0.1 , NY, , USA : SAS Institute Inc. Car. .
- Schlege , A.J. 1992 . Effect of composted manure on soil chemical properties and nitrogen use by grain sorghum . Journal of Production Agriculture , 5 : 153 – 157 .
- Schofield , R.K. 1955 . Can a precise meaning be given to available phosphorus . Soils and Fertilizers , 18 : 373 – 5 .
- Shalhevet , J. and Hsiao , T.C. 1986 . Salinity and drought . Irrigation Science , 7 : 677 – 678 .
- Shannon , M. 1984 . “ Breeding, selection and genetics of salt tolerance ” . In Salinity Tolerance in Plants, Strategies for Crop Improvement , Edited by: Stables , RC and Toenniesen , GH . NY : John Willey and Sons .
- Shapiro , R.E. and Fried , M. 1959 . Relative release and retentiveness of soil phosphates . Soil Science Society of America Proceedings , 23 : 195 – 198 .
- Sharpley , A.N. , Ahuja , L.R. , Yamamoto , M. and Menzel , R.G. 1981 . The kinetics of phosphorus desorption from soil . Soil Science Society of American Journal , 45 : 493 – 496 .
- Sharpley , A.N. 1995 . Soil phosphorus dynamics, agronomic and environmental impacts . Ecological Engineering , 5 : 261 – 279 .
- Sharpley , A.N. and Sisak , I. 1997 . Differential availability of manure and inorganic sources of phosphorus in soil . Soil Science Society of American Journal , 61 : 1503 – 1508 .
- Sonneveld , C. and De-Kreij , C. 1999 . Response of cucumber (Cucumis sativus L.) to an unequal distribution of salt in the root environment . Plant and Soil , 209 : 47 – 56 .
- Struthers , P.H. and Sieling , D.H. 1950 . Effect of organic anions on phosphate precipitation by iron and aluminum as influenced by pH . Soil Science , 69 : 205 – 513 .
- Syers , J.K. , Murdock , J.T. and Williams , J.D.H. 1970 . Adsorption and desorption of phosphate by soils . Soil Science and Plant Analysis , 1 : 57 – 62 .
- United Soil Classification System of Japan (2002) . The fourth committee for soil classification and nomenclature . The Japanese Society of Pedology .
- Tarafdar , J.C. and Claassen , N. 2003 . Organic phosphorus utilization by wheat plants under sterile conditions . Biology and Fertility of Soils , 39 : 25 – 29 .
- Thomas , G.W . (1982) . Exchangeable cations . In AL Page , RH Miller & DR Keeney Methods of soil Analysis, Part 2 (pp. 159 – 165 ). Agronomy series No. 9 . American Society of Agronomy and Soil Science Society of America, Inc . Publ. Madison, WI .
- Toor , G.S. and Bahl , G.S. 1997 . Effect of solitary and integrated use of poultry manure and fertilizer phosphorus on the dynamics of P availability in different soils . Bioresource Technologies , 62 : 25 – 28 .
- Vivekananda , M. and Fixen , P.E. 1990 . Effect of large manure applications on soil P intensity . Communication in Soil Science and Plant Analysis , 21 : 287 – 297 .
- Williams , J.D.H. , Syers , J.K. , Harris , R.F. and Armstrong , D.E. 1971 . Fractionation of inorganic phosphate in calcareous lake sediments . Soil Science Society of America Proceedings , 35 : 250 – 255 .
- Williams , J.D.H. , Mayers , T. and Nriagu , J.O. 1980 . Extractability of phosphorus from phosphate minerals common in soils and sediment . Soil Science Society of American Journal , 44 : 462 – 465 .
- Woodruff , J.R. and Kamprath , E.J. 1965 . Phosphorus adsorption maximum as measured by the Langmuir isotherm and its relationship to phosphorus availability . Soil Science Society of America Proceeding , 29 : 148 – 50 .
- Yeo , A.R. 1983 . Salinity resistance; Physiologies and prices . Physiologia Plantarum , 58 : 214 – 222 .
- Zahoor , A ., Faridullah , El-Sharkawi , H. , Irshad , M. , Monna , T. , Yamamoto , S. & Al-Busaidi , A.S. (2006) . Changes in water-extractability of soil inorganic phosphate induced by chloride and sulphate salts . Environmental Science and Pollution Research OnlineFirst <DOI: http://dx.doi.org/10.1065/espr2006.06.309>.
- Zekri , M. and Parsons , L.R. 1992 . Salinity tolerance of citrus rootstocks, Effect of salt on root and leaf mineral concentrations . Plant and Soil , 147 : 171 – 181 .