Abstract
The aim of the present study was to estimate the influence of different rates of soil-applied nitrogen on leaf N and chlorophyll content and photosynthesis in ‘Golden Delicious’ apple trees. Three different treatments were included: the trees were either fertilized with 80 kg N ha−1 (N-80), 250 kg N ha−1 (N-250) or left unfertilized (CON). Fertilization increased leaf nitrogen content, with a more prominent effect in high N application level treatment. In all treatments, a slight seasonal decrease in leaf nitrogen content was observed. N-250 treatment resulted in higher chlorophyll content; a similar effect was found late in the season for N-80 treatment. Measurements of A-C i curves, performed on spur leaves, revealed a higher CO2 saturated photosynthetic rate in N-250 trees compared with low application level fertilized or unfertilized trees. No effect of N fertilization on carboxylation efficiency was found, as revealed by comparisons of the initial slopes of A-C i curves. The lack of positive effect is rather surprising, since the leaf N content was efficiently increased with application of fertilizer. Obviously, the existing pool of leaf nitrogen in non-fertilized trees does not limit Rubisco activity and efficiency.
Keywords:
Introduction
Environmental factors and technological measures are key elements in the production of abundant, quality crops. For crop species such as apples characterized by high yields, nutrient uptake from the soil has to be adequate to meet the nutrient demand (Mengel, Citation2002). In the case of nitrogen, enzyme-based metabolic processes leading to increases in vegetative and reproductive growth and yield are totally dependent on its adequate supply (Lawlor, Citation2002). Both field and laboratory investigations have demonstrated that increasing the supply of nitrogen fertilizer increases growth and photosynthesis (Cheng & Fuchigami, 2000a, 2000b).
The photosynthetic decrease under nitrogen (N) deficiency can be partly related to enzymatic and non-enzymatic components of the photosynthetic complex. Studies have shown that nitrogen deficiency significantly decreases CO2 assimilation capacity. The decrease in CO2 assimilation capacity is associated with a decrease in Rubisco (Ribulose-1,5-bisphosphate carboxylase/oxygenase) content as well as with a decrease in synthesis of several other key enzymes involved in the Calvin cycle (Lu & Zhang, Citation2000). Rubisco is generally assumed to control photosynthesis in the initial region of the CO2-response curve (Stitt & Schulze, Citation1994) as well as under saturating irradiance (Woodrow & Berry, Citation1988). This means that the formation of RuBP is limited by the maximum rate of the whole chain electron transport, or co-limited by the rate of the whole chain electron transport and enzymes in the regenerative phase of the Calvin cycle (Von Caemmerer, Citation2000). The relationship between Rubisco and N content is studied with respect to changes in environmental factors. Regardless of irradiance, temperature or high CO2 conditions during growth, the ratio of Rubisco to total leaf N appears to be determined only by the amount of N. In other words, Rubisco content is thought to be due to total leaf N content (Nakano et al., Citation1997). Lower rates of photosynthesis under conditions of nitrogen limitation can also often be attributed to the reduction of chlorophyll content (Verhoeven, Citation1997; Toth, Citation2002).
Similar relations can also be confirmed for apple. Fallahi et al. (Citation2001) found that ‘Fuji’ trees with less N had lower levels of leaf photosynthesis than those with higher rates of N. Cheng and Fuchigami (2000b) also noticed that total Rubisco activity increased linearly with leaf N content. In ‘Fuji’, the chlorophyll content decreased with decreasing leaf N content (Cheng, Citation2003). Leaf nitrogen status affects both the capacity of light absorption by antenna pigments and the capacity of light utilization by photosynthetic electron transport.
The effects of nitrogen amendment to photosynthesis can largely depend on environmental conditions (soil, climate), cultivar and developmental stage of the plant. The objectives of this study were to determinate the response of photosynthesis to nitrogen application in ‘Golden Delicious’. In contrast to other studies where nitrogen was applied as a nutrient solution (Cheng & Fuchigami, Citation2000a), we decided to use soil amendment, which is the conventional method of fertilization in orchards. The aim of our research was to test the effect of fertilization application levels – one close to the N-input limit level suggested for this fruit growing region, and second exceeding it by ca. three-fold. We assumed that fertilization would increase leaf N content, and that it would consequently influence chlorophyll content and photosynthesis at the level of carboxylation efficiency and maximal photosynthetic rates.
Materials and methods
Plant materials
The measurements were carried out in summer 2004 on 16-year-old 'Golden Delicious’ apple trees from M9 rootstock, grown in the experimental orchard of the University of Ljubljana, central Slovenia. Soil analysis (data not shown) revealed that nutrients were in the optimal range; pH was slightly alkaline (7.2) and organic matter above the required 3% for orchards. Weather conditions from April to September 2004 were as follows: temperature was above the long-term average by up to 1.6°C; precipitation and solar radiation were close to the long-term average. Different rates of nitrogen (calciumammonium nitrate, 27% N, Agroruše d.o.o., Slovenia) were added in two equal portions (22 April and 5 May 2004). Treatments were the following: N-80 = 80 kg N ha−1; N-250 = 250 kg N ha−1; CON = 0 kg N ha−1 (control). Full bloom was on 27 April, and on 29 September ‘Golden Delicious’ was in technological maturity. All technological measures in the orchard, with the exception of nitrogen fertilization, were in accordance with the integrated fruit production guidelines.
Measurements and analyses
The samples for leaf N analysis were taken five times (1, 5, 6 June, 7 July and 29 September 2004) for ‘Golden Delicious’. Ten fully developed spur leaves per tree were sampled. The leaves were washed with distilled water to remove the remaining pesticides which could contain N. The foliar samples were dried (at 40°C) to constant weight, ground to a powder and analysed for N concentration using a modified Kjeldahl method. NUE (nitrogen use efficiency) was calculated with the NUE = A/N formula: (A = net photosynthesis, N = nitrogen expressed as % of leaf dry weight).
Determination of the photosynthetic pigments in the leaves
The field measurements of leaf chlorophyll were made with a chlorophyll meter SPAD-502 (Minolta Corporation, Japan) on three randomly chosen leaves of each tree without tearing them off. This was carried out five times throughout the season, at the same time as sampling of leaves for the nitrogen content analysis.
For the spectrophotometrical analyses of leaf pigments, leaf discs (4 mm in diameter) were sampled from the part of the leaves where SPAD readings were made. The leaf discs were placed in labelled paper bags and immediately transferred to the laboratory, where they were shock frozen in liquid nitrogen and stored at −20°C until analysis. For the extraction of chlorophyll and carotenoids, DMSO – dimethyl sulphoxide (Merck, Germany) was used. In micro centrifuge tubes, 0.5 ml DMSO was poured, and the leaf disc was added together with magnesium hydroxide carbonate crystals to assure a sufficient concentration of Mg2 + ions in the solution. Another 0.5 ml DMSO was added to each tube. The samples were incubated in a water bath at 65°C in the dark for 2 h. After the extraction, the samples were cooled down to room temperature and decanted into cuvettes. Immediately afterwards, the absorptions were measured by spectrophotometer (Lambda Bio 20, Perkin Elmer, USA) at a wavelength of 480 nm (carotenoids), 649 nm (chlorophyll b) and 665 nm (chlorophyll a). Concentrations of single photosynthetic pigments (chlorophyll a and b, and carotenoids) in the extract were calculated according to Wellburn (Citation1994).
Gas exchange measurements
Net photosynthesis (A) of apple leaves was measured with the LI-6400 portable photosynthesis system (LI-COR, Lincoln, USA.). We measured photosynthetic dependency on intercellular CO2 concentration (C i) by setting reference CO2 to 400, 200, 100, 50, 0, 400, 800, 1600 µmol mol−1 CO2. A-C i curves were plotted by using a non-linear regression model equation: A=a*(1 − exp (−b*(C i−c))), where a is CO2 saturated photosynthesis (µmol CO2 m−2 s−1), b is the initial, linear slope of the curve corresponding to the carboxylation efficiency (µmol CO2 m−2 s−1), and c is the CO2 compensation point (µmol mol−1, A=0). Measurements were made under saturating light conditions (PPFD = 1000 µmol m−2 s−1, 6400-02B light source). Other conditions were set as follows: leaf temperature around 25–26°C and relative humidity in the chamber 40±1%. The measurements were carried out on fully developed spur leaves on four plants per treatment. Measurements were carried out on 15 and 17 June. These were typical, cloudless, sunny days.
Statistical method
The results were statistically analysed with the Statgraphics plus 4.0 program (Statistical Graphics Corp., Manugistics Inc., USA) using analysis of variance (ANOVA). Differences between the treatments were estimated with a multiple range test using the least significant difference (LSD) or Duncan test at α < 0.05.
For three different treatments, the photosynthetic parameters derived from the A-C i curves – carboxylation efficiency, CO2 saturated photosynthesis, and CO2 compensation points were compared by the t-test.
Results and discussion
Nitrogen content in leaves
In all treatments on ‘Golden Delicious’ apple trees, the nitrogen concentration in the leaves was lower at harvest than at the first sampling (). This is in agreement with Aichner and Stimpfl (Citation2002), who noticed that nitrogen content in the apple leaves slightly decreases between the second week after full bloom and harvest. Also Neilsen et al. (Citation1995) reported the seasonal decrease of leaf nitrogen content in four different apple cultivars. These seasonal changes can be explained by the high use of nitrogen for vegetative growth and for fruit development. Towards the end of the season, nitrogen allocation to the root and trunk, where its function is to relieve wintering and then to serve for spring growth and development, could also be a reason for decreased leaf N (Millard et al., Citation1998; Stepien et al., Citation1994).
Figure 1. Leaf nitrogen content in % DW (average±standard error) of ‘Golden Delicious’ apple fertilized with different rations of nitrogen. CON = non-fertilized trees, N-80 = fertilized with 80 kg N ha−1; N-250 = fertilized with 250 kg N ha−1. Different letters indicate significantly different values at α < 0.05.
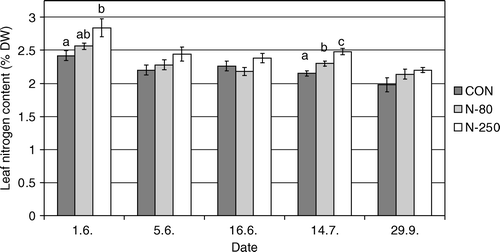
At the first and the fourth sampling of ‘Golden Delicious’ leaves, the nitrogen content was statistically lower in the CON treatment compared to the N-250 treatment. A similar trend was indicated at other sampling dates. In the middle of July, significantly higher amounts of nitrogen in leaves were also achieved by N-80 fertilization treatment compared to the un-fertilized control (). Our results are in agreement with the results obtained by Klein (Citation2002) and Fallahi et al. (2001). These authors report that higher N supply results in higher N content in the leaves. In contrast, low or high N application levels did not influence leaf nitrogen in the study by Neilsen et al. (1995). Clearly, leaf N content is less responsive to fertilization when there is high availability of N in the soil. In our experiment, also, this could be the reason for limited increases of leaf N. On the basis of high soil organic matter and weather conditions that favoured mineralization, we can presume that a substantial amount of N had been released from the organic N pool.
According to the literature, various authors suggest different optimal concentrations of nitrogen in the leaves. Fallahi et al. (2001) recommend an optimal concentration of nitrogen in the leaves of ‘Fuji’ of 2.30% of dry weight (DW), while Klein (2002) recommends 2.40% DW in ‘Golden Delicious’ and ‘Granny Smith’. We compared the nitrogen content measured in our study with Aichner's (Citation2002) recommendations, where optimal concentrations in the 20-day period after full bloom in ‘Golden Delicious’ apple were 2.55–3.00% DW, and in July and August 2.30–2.60% DW. In our experiment, the unfertilized ‘Golden Delicious’ trees showed suboptimal leaf nitrogen concentrations throughout the season. The N-250 treatment produced optimal nitrogen content throughout the season, while the N-80 treatment was slightly low in nitrogen content in June ().
Chlorophyll content in the leaves
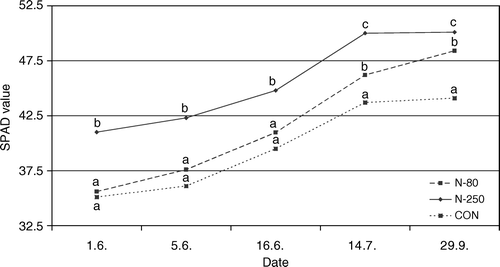
Throughout the season, the chlorophyll content was significantly higher in the N-250 treated ‘Golden Delicious’ than in the control treatment. At the mid-July and late September samplings, the same effect of fertilization could be observed for the lower nitrogen application level (N-80) (). The SPAD values in all treatments were increasing until the middle of July, and stagnating afterwards with the N-250 and CON treatments (). Nielsen et al. (1995) achieved similar seasonal results in their experiment on four different apple cultivars. The authors reported that the increasing SPAD values do not depend only on nitrogen fertilization but also on leaf thickness, which increases during the growing season; the SPAD values and chlorophyll content in the leaves were higher when leaf thickness increased. Because of the limitations of the SPAD technique, we decided to include parallel spectrophotometric measurements of pigments.
Figure 3. SPAD values in leaves (average) of ‘Golden Delicious’ apple fertilized with different rations of nitrogen. CON = non-fertilized trees, N-80 = fertilized with 80 kg N ha−1, N-250 = fertilized with 250 kg N ha−1. Different letters indicate significantly different values at α < 0.05.
As with SPAD measurements, this analysis revealed that chlorophyll a increased until the middle of July (, ). On the other hand, amounts of chlorophyll b and carotenoids had not changed during the growing season. In general, the content of all pigments was always highest in leaves of N-250 trees compared to low application level fertilized (N-80) or non-fertilized trees. A similar but lower increase in pigments was found with the N-80 treatment. At the autumn sampling, there was no significant difference in the effects of the two N application levels. Since both fertilization treatments resulted in a substantially higher content of photosynthetic pigments late in the season, we can assume that the N application contributed to delayed leaf senescence.
Figure 2. Content of chlorophyll a + b (µg mm−2) in leaves (average) of ‘Golden Delicious’ apple fertilized with different rations of nitrogen. CON = non-fertilized trees, N-80 = fertilized with 80 kg N ha−1; N-250 = fertilized with 250 kg N ha−1. Different letters indicate significantly different values at α < 0.05.
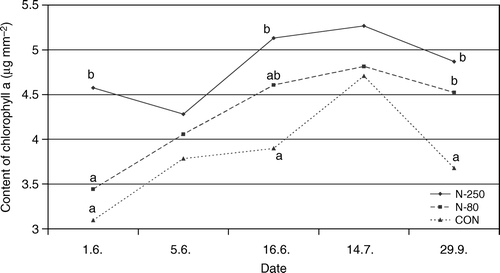
Table I. Concentrations of chlorophyll a, chlorophyll b and carotenoids (µg mm−2) in leaves (average±standard error) of ‘Golden Delicious’ apple fertilized with different rations of nitrogen: CON = non-fertilized trees, N-80 = fertilized with 80 kg N ha−1; N-250 = fertilized with 250 kg N ha−1. Values in columns followed by different letters are significantly different at α < 0.05 for the given sampling date.
Gas exchange measurement
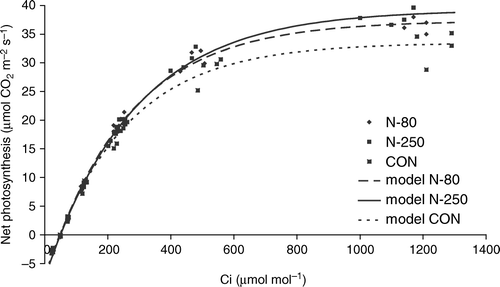
Different application levels of nitrogen resulted in changed photosynthetic activity of fully developed spur leaves of ‘Golden Delicious’ when measured at the saturating light intensity and at different CO2 concentrations (). High N application level fertilized trees (N-250) had a higher CO2 saturated photosynthetic rate than (N-80) fertilized or unfertilized (CON) trees. The increase of photosynthetic capacity was slightly lower in N-80 trees. No effect of N fertilization on carboxylation efficiency was found, as revealed by the comparisons of initial slopes of the A-C i curves calculated for the tree treatments (). The lack of positive effect is rather surprising, since the leaf N content was efficiently increased with the application of fertilizer. Obviously, the existing pool of leaf nitrogen in unfertilized trees does not limit Rubisco activity and efficiency. This would correspond to the fact that a limited effect of extra N on photosynthesis can be expected in plants that do not suffer N deficiency (Sinclair & Horie, Citation1989). In their studies on ‘Fuji’, Cheng and Fuchigami (Citation2000a, Citationb) found a curvilinear relationship between leaf N and light-saturated CO2 assimilation. This indicates that CO2 assimilation is less limited by nitrogen as leaf N content increases. In our case, the limited response of ‘Golden Delicious’ to N application can also be underlined by similar values for nitrogen use efficiency for three different treatments (7.06, 7.29, 8.00 for CON, N-80 and N-250, respectively).
Figure 4. A-C i curves of ‘Golden Delicious’ apple fertilized with different rations of nitrogen. CON = non-fertilized trees, N-80 = fertilized with 80 kg N ha−1, N-250 = fertilized with 250 kg N ha−1. CON model: A=33.50*(1 − EXP(0.0040*(Ci-47.82))); N-80 model: A=37.34*(1 − EXP(0.0038*(Ci-49.07))); N-250 model: A=39.2613*(1 − EXP(0.003512*(Ci-49.6558))).
The results of our study can also be partially related to the age of the apple trees. In general, N fertilizer makes a much smaller contribution to leaf growth in mature trees than in young ones (Sanchez et al., Citation1992; Millard, Citation1996). This can be explained by a larger storage pool and also by stronger competition from a larger fruit sink in mature trees (Weinbaum et al., Citation1987).
In our study we have proved that there was no or little effect on physiological parameters when the treatment with 80 kg N per ha was compared to control treatment (without any soil amendments). This application level of nitrogen is near to the maximal allowed application level of nitrogen (90 kg per ha) in growing conditions typical for the studied area in integrated apple production. Only the highest level of applied nitrogen showed significant influence on most parameters. Based on our results measured for ‘Golden Delicious’ we can conclude that N-input limits should be re-evaluated with respect to the cultivar N demands and growing conditions, taking into account different components of N balance in orchards systems.
Acknowledgements
This work is a part of the Horticulture No P4-0013-0481 program granted by the Slovenian Ministry of Higher Education, Science and Technology.
References
- Aichner , M. and Stimpfl , E. 2002 . Seasonal pattern and interpretation of mineral nutrition concentrations in apple leaves . Acta Horticulturae , 594 : 377 – 382 .
- Aichner , M. 2002 . “ Methoden zur Ermittlung des Düngenbedarfs ” . In Lucas Anleitung zum Obstbau. 32. Auflage , Edited by: Link , H . 235 – 243 . Stuttgart, Deutschland. (In German) : Eugen Ulmer GmbH & Co .
- Cheng , L. and Fuchigami , LH. 2000a . CO2 assimilation in relation to nitrogen in apple leaves . Journal of Horticultural Science Biotechnology , 75 : 383 – 387 .
- Cheng , L. and Fuchigami , LH. 2000b . Rubisco activation state decreases with increasing nitrogen content in apple leaves . Journal of Experimental Botany , 51 : 1687 – 1694 .
- Cheng , L. 2003 . Xanthophyll cycle pool size and composition in relation to the nitrogen content of apple leaves . Journal of Experimental Botany , 54 : 385 – 393 .
- Fallahi , E. , Colt , W.M. and Fallahi , B. 2001 . Optimum ranges of leaf nitrogen for yield fruit quality, and photosynthesis in ‘BC-2 Fuji’ apple . Journal of the American Pomological Society , 55 : 68 – 75 .
- Klein , I. 2002 . Nitrogen pool enrichment in fruit trees for specific target requirement . Acta Horticulturae , 594 : 131 – 137 .
- Lawlor , D.W. 2002 . Carbon and nitrogen assimilation in relation to yield: mechanisms are the key to understanding production systems . Journal of Experimental Botany , 53 : 773 – 787 .
- Lu , C.M. and Zhang , J.H. 2000 . Photosynthetic CO2 assimilation, chlorophyll fluorescence and photoinhibition as affected by nitrogen deficiency in maize plants . Plant Science , 151 : 135 – 143 .
- Mengel , K. 2002 . Alternative or complementary role of foliar supply in mineral nutrition . Acta Horticulturae , 594 : 33 – 47 .
- Millard , P. 1996 . Ecophysiology on internal cycling of nitrogen for tree growth . Journal of Plant Nutrition and Soil Science , 159 : 1 – 10 .
- Millard , P. , Wendler , R. , Hepburn , A. and Smith , A. 1998 . Variations in the amino acid composition of xylem sap of Betula pendula (Roth.) trees due to remobilization of stored N in the spring . Plant Cell Environ , 21 : 715 – 722 .
- Nakano , H. , Makino , A. and Mae , T. 1997 . The effect of elevated partial pressures of CO2 on the relationship between photosynthetic capacity and N content in rice leaves . Plant Physiology , 115 : 191 – 198 .
- Neilsen , D. , Hogue , E.J. , Neilsen , G.H. and Parachomchuk , P. 1995 . Using SPAD-205 values to assess the nitrogen status and of apple trees . Hortscience , 30 : 508 – 512 .
- Sanchez , E.E. , Righetti , T.L. , Sugar , D. and Lombard , P.B. 1992 . Effects of timing of nitrogen application on nitrogen partitioning between vegetative, reproductive, and structural components of mature ‘Comice’ pears . Journal of Horticultural Science , 67 : 51 – 58 .
- Sinclair , T.R. and Horie , T. 1989 . Leaf nitrogen, photosynthesis and crop radiation use efficiency: a review . Crop Science , 29 : 90 – 98 .
- Stepien , V. , Sauter , J.J. and Martin , F. 1994 . Vegetative storage proteins in woody plants . Plant Physiology and Biochemistry , 32 : 185 – 192 .
- Stitt , M. and Schulze , D. 1994 . Does Rubisco control the rate of photosynthesis and plant growth, an exercise in molecular ecophysiology . Plant Cell and Environment , 17 : 465 – 87 .
- Toth , V.R. , Meszkaros , I. , Veres , S. and Nagy , J. 2002 . Effects of the available nitrogen on the photosynthetic activity and xanthophyll cycle pool of maize in field . Journal of Plant Physiology , 159 : 627 – 634 .
- Von Caemmerer S. ( 2000 ). Biochemical models of leaf photosynthesis . CSIRO Publishing .
- Weinbaum , S.A. , Klein , I. and Muraoka , T.T. 1987 . Use of nitrogen isotopes and a light-textured soil to assess annual contributions of nitrogen from soil and storage pools in mature almond trees . Journal of the American Society for Horticultural Science , 112 : 526 – 529 .
- Wellburn , A.R. 1994 . The spectral determination of chlorophyll a and chlorophyll b, as well as total carotenoids, using various solvents with spectrophotometer of different resolution . Journal of Plant Physiology , 144 : 307 – 313 .
- Woodrow , I.E. and Berry , JA. 1988 . Enzymatic regulation of photosynthetic CO2 fixation in C3 plants. Annual Review . Plant Physiology Plant Molecular Biology , 39 : 533 – 94 .
- Verhoeven , A.S. , Demmig-Adams , B. and Adams , W.W. 1997 . Enhanced employment of the xanthophyll cycle and thermal energy dissipation in spinach exposed to high light and N stress . Plant Physiology , 113 : 817 – 824 .