Abstract
Shoot radiation-use efficiency (RUE) and nitrogen-uptake efficiency (UPE) of monocultures of perennial sow-thistle (Sonchus arvensis L.) and spring barley (Hordeum distichon L.) were quantified to assess the significance of these traits for the relative performance of the two species. RUE and UPE were derived for shoot growth and N uptake by calibrating a mechanistic model to above-ground biomass and N observations in an outdoor box experiment, conducted during two years at two soil nitrogen levels in Central Sweden. The model, which is driven by climate variables, predicts above-ground biomass and nitrogen increment as a function of intercepted radiation, temperature, and nitrogen availability. Observed values of leaf area and root development are used as input. Shoot RUE in S. arvensis was only 56% of the RUE in spring barley (1.35 and 2.40 g dry weight MJ−1, respectively). On the other hand, shoot UPE in S. arvensis at low N supply during early season was seven times higher than in barley (0.07 and 0.01 d−1, respectively). For S. arvensis, UPE was higher at the low soil nitrogen level than at high level, while the reverse was found for barley, at a given amount of biomass per area unit. We suggest that the higher shoot UPE in S. arvensis at low nitrogen supply, in comparison with the low UPE of annual small grain crops at low soil nitrogen levels, is a contributing cause for the observed increase in S. arvensis in organic farming.
Introduction
The occurrence of perennial sow-thistle (Sonchus arvensis L.) has increased in recent years in Scandinavia, not only in conventional (Fykse, Citation1974; Hallgren, Citation1996; Reintam & Koster, Citation2002; Vanhala et al., Citation2006), but particularly in organic farming (Lundkvist, Citation1998; Salonen et al., Citation2001; Ericson, Citation2003). The increase of S. arvensis in organic farming is likely caused by absence of herbicides, but also due to traits in S. arvensis giving this species a competitive advantage over crops under current Scandinavian conditions.
Traits of importance for a potential relative competitive advantage primarily deal with the acquisition of the resources water, nutrients, and light. While the availability of water usually is not limiting under Scandinavian conditions in spring, soil mineral N levels are low due to temperature and quality-limited N mineralization from organic fertilizers (Eltun et al., Citation2002), and may interact with specific traits for nitrogen acquisition in determining the relative performance of S. arvensis in organic farming. Therefore, we concentrate on the other eco-physiological properties which govern a species field response, and further can be summarized in terms of radiation-use efficiency and nitrogen-uptake efficiency.
The processes of nitrogen uptake and radiation interception and their utilization have attracted considerable interest in recent years for evaluation of cereal species- and variety-production properties. For this purpose radiation-use and N-uptake efficiencies have been determined by direct measurements in field crops (Eckersten & Nilsson, Citation1990; Delogu et al., Citation1998; Gouis et al., Citation1999; Kemanian et al., Citation2004; Sinebo et al., Citation2004; Muurinen & Peltonen-Sainio, Citation2006; Muurinen et al., Citation2006 ,Citation2007), or by calibration of cereal-production models (Legg et al., Citation1979; Scotter et al., Citation1984; Eckersten & Jansson, Citation1991; Jamieson et al., Citation1995; Ritchie et al., Citation1998; Korsaeth et al., Citation2001; Bingham et al., Citation2007; Eckersten et al., Citation2007; Beaudoin et al., Citation2008).
Although there is an extensive literature describing S. arvensis biology (Håkansson, Citation1969; Håkansson & Wallgren, Citation1972a ,Citationb; Lemna & Messerschmith, Citation1990; Champion, Citation2001), physiology (Fykse, Citation1974; Zollinger & Kells, Citation1991; Grondal et al., Citation2003; Vanhala et al., Citation2004), and control (Boström & Fogelfors, Citation1999; Darwent et al., Citation1999; Blackshaw et al., Citation2001; Streit et al., Citation2003; Thomas et al., Citation2004; Vanhala et al., Citation2006), no report has been found on the quantification and significance of radiation-use and N-uptake efficiencies of S. arvensis.
In this study, we set out to determine and compare the shoot radiation-use (RUE) and nitrogen-uptake (UPE) efficiencies of S. arvensis and spring barley in monocultures in relation to nitrogen supply by means of a modelling approach. These characteristics have been instrumental in the increased occurrence of perennial sow-thistle in recent years. Our hypothesis is that S. arvensis has higher shoot nitrogen-uptake efficiency and/or higher shoot radiation-use efficiency than does barley, and that this is the case irrespective of soil nitrogen level.
Material and methods
Experimental data
Two outdoor box experiments were conducted during 2006 and 2007 at Ultuna, close to Uppsala, Sweden (59°48′N, 17°39′E). The boxes were rectangular, 80×80 cm2 and 20 cm deep and filled with clay soil (Kirchmann, Citation1991). Before planting, Biofer 7-9-0 was used as nitrogen fertilizer and was mixed with the clay soil at low (5 g m−2 N) and high (15 g m−2 N) nitrogen rates. In 2007, only the low level of nitrogen was used. The boxes were then irrigated just before planting.
The plant material used was perennial sow-thistle (Sonchus arvensis L.) and spring barley (Hordeum distichon, var Gustav). Sonchus arvensis root runners were collected in the autumn of 2005 from weed-infested fields at Sala (59° 40′N, 16° 40′E) and stored in buckets over winter in the dark at +2 − 4°C. Before the start of the experiments, the S. arvensis roots were conditioned for about seven days indoors at +20 °C. The roots were cut into five-cm pieces with at least two adventitious buds on each piece and were then distributed into three thickness classes:<2, 2–4, and > 4 mm in diameter. To generate even weed stands, the material was sorted so that the number of root cuttings from each thickness class was the same in all boxes (Håkansson, Citation1969).
In 2006, a fully randomized design was established, containing nine replicates of monocultures of S. arvensis and of barley, all at two nitrogen levels (5 and 15 g N m−2), giving 36 boxes in total (3 samplings with 3 replicates for 2 N rates and 2 species). In 2007, a fully randomized design was employed at a single nitrogen level (5 g N m−2) with monocultures of S. arvensis and barley, each with six replicates, giving 12 boxes in total (3 samplings with 2 replicates for 2 species).
Planting dates were chosen such that emergence was intended to occur at a similar time in the two species (). Barley was sown in eight rows and the S. arvensis root cuttings were placed evenly in each of the boxes to establish comparable plant stands. After planting, the grains and root pieces were covered with 2–3 cm of planting soil with a low nutrient content (Hasselfors såjord, Hasselfors Garden AB, Hasselfors) and the boxes were again irrigated. During the whole growing season, the soil was kept moist by means of irrigation.
Table I. Dates for planting, emergence and sampling, and planting density in 2006 and 2007 (5 cm root cuttings or grains).
At each sampling occasion, three times per year (), the central area of 0.7*0.7 m−2 (2006) or 0.6*0.6 m−2 (2007) was harvested in three (2006) or two (2007) replicates of each of the treatments. At all sampling occasions, above-ground and root dry weight were determined for both species, except for barley in 2006, when only above-ground dry matter was sampled. For all harvest occasions and per replicate of each of the species, leaf area was measured with a leaf area meter (Type AAM-5, Hayashi Denko Co Ltd., Tokyo, Japan). The plant material was dried at 50°C and weighed and the total nitrogen content of above- and below-ground dry-matter material was determined by a combustion method (LECO Corporation, USA).
Model description
The model is based on processes describing growth and biomass accumulation as well as N availability and uptake by plant. Its structure is basically similar to those of commonly used models for simulating biomass and N dynamics (Eckersten & Jansson Citation1991; Eckersten et al., Citation2007; Torssell et al., Citation2007). However, the current model is simpler and adjusted to cover both main growth and nitrogen dynamics and to be applicable for a test against observations, while not introducing any new functional relationships. Therefore only the equations related to RUE and UPE are described below, whereas the full set of equations (denoted with an A) is given in Appendix I. For literature references to different parts of the model, we refer to former applications of more detailed models, e.g. Eckersten et al. (Citation2004 ,Citation2007). The shoot is represented by one pool for above-ground biomass and one for above-ground N. The available nitrogen for shoot uptake is represented by one pool, and soil organic N by one pool. The time step of the model is one day and the basic area unit is one square metre.
Biomass
Daily shoot growth (ΔW Shoot/Δt) was proportional to the radiation-use efficiency (RUE) and intercepted solar radiation (R Int), but limited by indices of temperature (f TPlant) and shoot nitrogen concentration (f N) [Equation (Equation1)].
Nitrogen
The available nitrogen is increased by mineralization that is a fraction of the soil organic N and a temperature function [Equations (EquationA4a–Equationb), Appendix I], and decreased by the uptake to the shoot [Equation (EquationA4c), Appendix I].
There are two flows determining daily changes in shoot nitrogen; uptake from the available N and losses by litter [Equation (EquationA5), Appendix I]. When there is sufficient available nitrogen to cover the shoot demand for nitrogen, the uptake is driven by the demand. The demand is determined by the maximum nitrogen concentration times the daily growth of shoots, although limited by an internal deficiency of nitrogen within the plant [NDemand; Equation (EquationA6a), Appendix I]. This deficiency is defined by a shoot nitrogen maximum concentration that decreases with shoot biomass, and is assumed to reflect the influence of structural biomass and self shading [Equation (EquationA6c), Appendix I].
When nitrogen availability is lower than the N demand, nitrogen uptake is limited by the ability of shoots to take up the available N (NAvailable) that is defined as the sum of soil mineral nitrogen and N stored in roots and root turnover [Equation (EquationA4c), Appendix I]. This ability is expressed in terms of the shoot nitrogen-uptake efficiency (UPE), i.e. the fraction of NAvailable that can be taken up per day. The actual N uptake of the shoots during a single day is determined by which of the factors is limiting, either the available amount or the plant nitrogen demand [Equation (Equation2)].
The shoot nitrogen-uptake efficiency increases proportionally (UPEo) to root biomass (W r) to the power of ¾ (Rastetter & Ågren, Citation2002; Knecht Billberger, Citation2006), up to a maximum value (UPEMax) [Equation (Equation3)].
Model inputs
Variables and initial states
Daily values of mean air temperature and global radiation sum were obtained from the Ultuna meteorological station (59.8°N, 17.7°E), close to Uppsala near the experimental location (Anonymous, Citation2008). Botanical observations used as targets for calibration were above-ground yield of dry matter (DM) and nitrogen. Observed leaf area was used as a boundary variable for calculation of radiation interception [Equation (EquationA1), Appendix I]. A linear relation between observed above-ground DM and root DM for S. arvensis was used to estimate root biomass [for calculation of the nitrogen-uptake efficiency; Equation (Equation3)]. For S. arvensis the relationship was derived from the current data set, whereas for barley the data obtained by Didon (Citation2002) from another experiment under similar conditions were used.
Simulation of soil dynamics started at time of emergence. Initial state of the available N pool was set the same for both species and equal to 1 g N m−2 plus the fertilisation supply (5 and 15 g N m−2, respectively). The initial value of the soil organic N pool was estimated by calibration (see below) to be 500 g N m−2. Simulation of plant dynamics started at the first sampling time, two weeks after emergence.
Parameter calibration
The calibration was conducted by a stepwise procedure. RUE was first calibrated under nonlimiting nitrogen conditions (high nitrogen level) by setting the nitrogen response factor equal to 1.0 [f N in Equation (Equation1)]. The first sampling time was used for generation of initial values for the plant simulation and the second and third samplings as calibration targets. The value of RUE obtained was then used to calibrate the growth nitrogen response factor [parameter a in Equation (EquationA3b), Appendix I] for the low-nitrogen treatments in 2006 and 2007. The nitrogen mineralization and uptake were calibrated with observed above-ground nitrogen as targets. The specific mineralization rate [k Mineral; Equation (EquationA4a), Appendix I] and initial soil organic N [NSoilOrg(t=0); Equation (EquationA4a), Appendix I] were adjusted to fit the accumulated seasonal nitrogen uptake in the low-nitrogen treatment and the maximum nitrogen concentration was [by reducing W Max in Equation (EquationA6c), Appendix I] adjusted to fit individual observations of above-ground nitrogen in the high-nitrogen treatment. The nitrogen-uptake model was applied only to 2006, and only to the first two sampling times since a nitrogen loss, that was not accounted for in the model, occurred in the final S. arvensis observation in 2006 and in all of the observations in 2007.
Statistical analysis
Model fit was estimated by calculating the regression between observed and simulated yields of above-ground DM. Results are given in terms of the coefficient of determination (R 2) and the slope of the regression line.
Results
Measurements
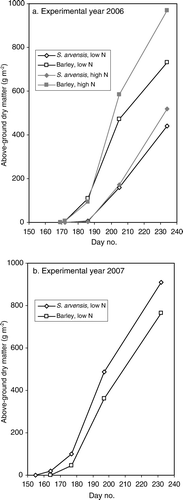
After initial emergence, density (number of plants/m2) of barley remained constant in the experiments, while in S. arvensis shoot density increased throughout the season. In 2006 barley emerged 3 days ahead of the first shoots of S. arvensis () and above-ground DM at final sampling was almost twice as high in barley as in S. arvensis, at high N supply. Under nitrogen-limiting conditions above-ground biomass was reduced by 30% in barley and 20% in S. arvensis (). In 2007, the first shoots of S. arvensis emerged 8 days prior to barley and DM production (at low nitrogen) was higher in S. arvensis than in barley, opposite to the observations in 2006.
Figure 1. Above-ground dry matter content (g m−2) of S. arvensis and barley in monocultures at low and high nitrogen supply in experimental year (a) 2006 and (b) 2007.
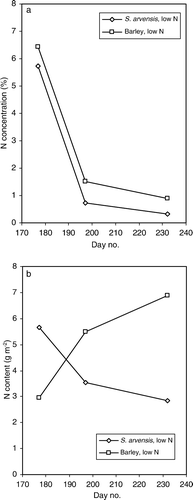
Root DM allocation at the first and second sampling times, in terms of the root-to-shoot DW ratio, was higher at the lower nitrogen supply. At the last sampling time in 2006, there was no significant difference in root DM allocation between nitrogen levels. Note that these data were used only in Equation (Equation3).
Nitrogen concentration in 2006 was higher in S. arvensis than in barley, by the second sampling time almost three times as high (a). However, yield of nitrogen (b) was the higher in barley as was DM production. In barley, a gradual senescence of the whole plant occurred (turning yellow), but no leaf fall was observed. In S. arvensis, there was a significant leaf fall but all shedded leaves were included in the harvest and are thereby accounted for in the presented above-ground biomass data.
Figure 2. (a) Nitrogen concentration in above-ground dry matter (%), and (b) nitrogen content (g N m−2) of S. arvensis and barley in monocultures at low and high nitrogen supply in experimental year 2006.
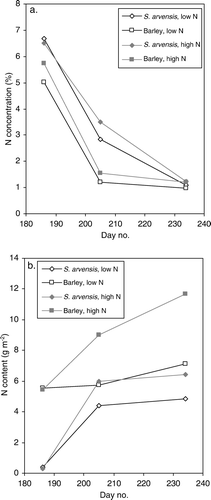
Nitrogen concentrations during 2007 (a) displayed the same declining pattern over time as in 2006 for both species at low soil nitrogen. In contrast to 2006 the N-concentration of S. arvensis in 2007 remained below that of barley. Nitrogen yield (b) of barley at low N increased considerably with time during 2007 whilst in 2006 the increase was slight. In S. arvensis there was a decline over time in 2007 and a clear increase in 2006. At the first sampling, N-concentration in S. arvensis at low N was of the same order of magnitude in 2006 and 2007. Thus the somewhat higher N yield at the first sampling in 2007 was due to a higher biomass amount of S. arvensis. Also, the low N-concentration in S. arvensis at the second and third samplings in 2007 was probably due to the fact that the weed was sampled in a more advanced phenological stage compared with 2006. The temperature sum (T>0°C) from emergence to the two sampling times was 365 and 865 day-degrees in 2006. The corresponding values for 2007 were 513 and 1113 day-degrees, respectively.
Calibrated model parameters
The model application by means of calibration against specific targets resulted in different parameter values for S. arvensis, barley, and low N and high N treatments, respectively. The calibration detected three main differences between barley and S. arvensis (). First, the shoot radiation-use efficiency was consistently higher in barley than in S. arvensis. Second, maximum shoot nitrogen-uptake efficiency (UPEMax) early in the season was higher in S. arvensis than in barley. This superiority was greater at low than at high nitrogen treatment. S. arvensis reached its value of maximum nitrogen-uptake efficiency (UPEMax) at lower above-ground biomass than did barley (higher value of UPEo in ; ). Third, nitrogen demand decreased more with increasing biomass in S. arvensis than in barley, shown by lower values of W Max [; Equation (EquationA6c) in Appendix I].
Figure 4. Nitrogen uptake efficiency (UPE; d−1) in relation to above-ground dry matter (g m−2) in monocultures of S. arvensis and barley, at high and low nitrogen supply in experimental year 2006.
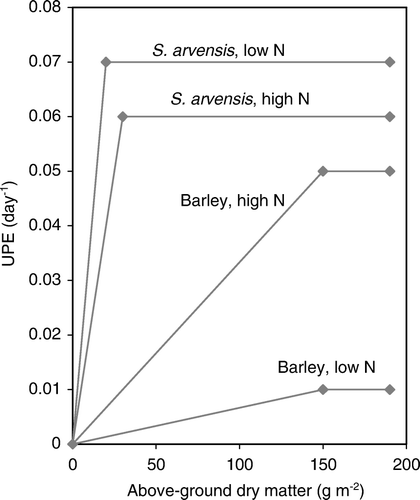
Table II. Parameter values in the model application to S. arvensis and barley monocultures for high and low nitrogen treatments in 2006. All parameters except m are determined by calibration (see Materials and methods section). RUE is the shoot radiation-use efficiency; UPEMax the maximum shoot N-uptake efficiency; UPEo a coefficient of the UPE response to root biomass; a the minimum n Shoot (shoot N concentration) of non-N-limited growth; n Max0 the maximum n Shoot; W Max a coefficient of maximum n Shoot decrease with shoot biomass; m the relative mortality rate (the value is set arbitrarily); and k Mineral the specific mineralization rate of soil organic matter.
The simulated average N-response (f N) was by definition unity (i.e.,=1) for the high-nitrogen treatment. For the low-nitrogen treatment, f N was in S. arvensis reduced to 0.8 in 2006, but only to 0.95 in 2007. For barley the reduction was stronger, with f N-values equal to 0.4 and 0.5 in 2006 and 2007, respectively.
When comparing the calibration results against mean values of yield observations with calibration against minimum and maximum values derived from the replicates, no consistent differences in model parameters were found. The precision of the model calibration was high, i.e. both R 2 and slope were close to unity (y=1.02x−6.94; R 2=0.99; n=12).
Discussion
We postulated the hypothesis that S. arvensis has a higher shoot nitrogen-uptake efficiency and/or a higher shoot radiation-use efficiency than does barley, irrespective of soil nitrogen level, and that differences between species with regard to these traits may be playing a role in the current increase of S. arvensis in, especially, organic farming in Scandinavia.
The results of model calibrations on experimental growth data of monocultures of S. arvensis, in comparison with barley, showed that S. arvensis at low N supply was superior to barley with regard to early nitrogen acquisition (UPE of 0.07 and 0.01 d−1, respectively), while the radiation-use efficiency in S. arvensis was only 56% of the RUE in barley (1.35 and 2.40 g dry weight MJ−1, respectively). The reduced superiority of S. arvensis in UPE at the higher nitrogen level strengthens the argument that high UPE of the weed is an important factor in its success in organic farming. At high N, the species were more equal in UPE (0.06 and 0.05 d−1, respectively). This is consistent with observations by Håkansson (Citation2003) that S. arvensis competes more successfully with barley at low N supply than at high.
The high shoot nitrogen-uptake efficiency (UPE) of S. arvensis at low nitrogen levels may be seen as an adaptation of a weed to survive on low-fertility sites. It is likely to be a significant factor in the success of S. arvensis in organic farming, as soil mineral N levels in Scandinavia are low during spring due to temperature and quality-limited N mineralization from organic fertilizers. Also in cereals, UPE is found to be a significant factor in nitrogen-use efficiency (Sinebo et al., Citation2004; Muurinen et al., Citation2006) and associated with high nitrogen harvest index (Gouis et al., Citation1999). The superior UPE of S. arvensis could then be an important factor for the competitiveness of this weed. In the case of S. arvensis the nitrogen harvest index is, though, not a relevant term as nitrogen basically seems to be allocated to the greatly expanding roots. The relatively low nitrogen demand (W Max) of S. arvensis, compared with barley, may also be seen as an adaptation to successful growth under low-nitrogen conditions.
In field experiments, growth might vary with nitrogen application (Eckersten et al., Citation2004; Muurinen & Peltonen-Sainio, Citation2006), drought (Legg et al., Citation1979), evaporative demand (Goyne et al., Citation1993; Kemanian et al., Citation2004) and temperature (Goyne et al., Citation1993), and a growth model is needed to account for the nonlinear relations between these factors. The results obtained in our study agree with the principle that the nitrogen stimulation of growth is due to both increased leaf area expansion and assimilation per unit of leaf area. The consistently higher RUE in barley than in S. arvensis may be seen as a result of long-term adaptation of an annual species rather than as a result of plant breeding, since modern varieties are not always superior to old land races in this respect (Muurinen & Peltonen-Sainio, Citation2006).
To realise the growth potential associated with a high RUE, an annual crop such as barley needs to develop a functional leaf canopy, which only can result from an adequate period of nitrogen acquisition and allocation to leaves. However, when an annual crop with high RUE is competing with a weed such as S. arvensis, which may be effective in pre-emptying a common N-resource, a poor canopy development may result in the crop, which thereby cannot make use of its inherently high RUE. The N-resource pre-emption by the weed thereby leads to a weed canopy which, although having a lower RUE, will not be shaded to a greater extent by its competitors.
Another factor that may play a role in the relative performance of S. arvensis and barley is the emergence time. During 2006, barley emerged 3 days before S. arvensis and reached a much higher yield. During 2007, S. arvensis emerged 8 days prior to barley, and attained the highest yield figures.
Barley and S. arvensis also differed with respect to temporal leaf dynamics. In S. arvensis, leaves were continuously shed and new shoots and leaves continued to emerge over time, while leaves in barley developed over a shorter time and then gradually turned yellow. This difference in leaf dynamics is likely to be associated with the different life cycles of the two species, one being an annual for which its survival depends on its capacity to allocate its resources to grains, the other being a perennial, which uses its roots as a store for overwintering and next year's performance.
The variations in RUE and UPE, as investigated in this study, are not solely dependent on the plant's ability to capture the resources but also to the allocation between shoots and roots. The higher UPE of S. arvensis in the low-N treatment might be a consequence of a higher N availability in the transplanted roots compared with the N availability in the barley seeds. Similarly, the lower RUE of S. arvensis might be due to higher allocation of total growth to roots compared with barley, and a higher value of UPE might be associated with a lower value of RUE. However, approximate estimates of the N content in transplanted root cuttings and seeds were less than 0.3 g N m−2 (less for the root cuttings), which is less than 5% of observed shoot N content at the first sampling, suggesting that the shoot N originates from soil mineral N, and that the assumption of a similar initial N availability for both species was reasonable. It remains to investigate the values of UPE and RUE as defined for the whole plant to evaluate the effect of allocation versus the plant ability to assimilate N and carbon dioxide. UPE and RUE defined for the whole plant might be more similar for the two species than would be UPE and RUE defined for the shoots only.
The results suggest, though, that the higher shoot nitrogen-uptake efficiency in S. arvensis at low nitrogen supply, in comparison with the low UPE of an annual small grain crop at low soil nitrogen levels, might play an important role for the observed increase in S. arvensis in organic farming, and that the presented quantitative evaluations of both traits can be used to model the competition for light and nitrogen between the species.
Acknowledgements
The studies were financially supported by the Swedish Research Council Formas. We also want to thank Professor Theo Verwijst for his very valuable comments and suggestions on the manuscript.
References
- Anonymous 2008 . Ultuna Meteorological Station . Swedish University of Agricultural Sciences , Uppsala, , Sweden . http://grodden.evp.slu.se/slu_klimat/index.html accessed on
- Beaudoin , N. , Launay , M. , Sauboua , E. , Ponsardin , G. and Mary , B. 2008 . Evaluation of the soil crop model STICS over 8 years against the “on farm” database of Bruyeres catchment . European Journal of Agronomy , 29 : 46 – 57 .
- Bingham , I.J. , Blake , J. , Foulkes , M.J. and Spink , J. 2007 . Is barley yield in the UK sink limited? I. Post-anthesis radiation interception, radiation-use efficiency and source-sink balance . Field Crops Research , 101 : 198 – 211 .
- Blackshaw , R.E. , Moyer , J.R. , Doram , R.C. and Boswell , A.L. 2001 . Yellow sweet clover, green manure, and its residues effectively suppress weeds during fallow . Weed Science , 49 : 406 – 413 .
- Boström , U. and Fogelfors , H. 1999 . Type and time of autumn tillage with and without herbicides at reduced rates in southern Sweden: 2. Weed flora and diversity . Soil & Tillage Research , 50 : 283 – 293 .
- Champion , G. 2001 . Weed biology series-thistles . British Sugar Beet Review , 69 : 35 – 37 .
- Darwent , A.L. , Harker , K.N. , Broatch , J.M. and Mills , P. 1999 . Evaluation of the “fall rosette” procedure for perennial sow thistle control in fallow . Canadian Journal of Plant Science , 79 : 633 – 637 .
- Delogu , G. , Cattivelli , L. , Pecchioni , N. , de Falcis , D. , Maggiore , T. and Stanca , A.M. 1998 . Uptake and agronomic efficiency of nitrogen in winter barley and winter wheat . European Journal of Agronomy , 9 : 11 – 20 .
- Didon , U.M.E. 2002 . Growth and development of barley cultivars in relation to weed competition . Acta Universitatis Agriculturae Sueciae. Agraria 332 Swedish University of Agricultural Sciences Uppsala, , Sweden .
- Eckersten , H. and Jansson , P.-E. 1991 . Modelling water flow, nitrogen uptake and production for wheat . Fertilization Research , 27 : 313 – 329 .
- Eckersten , H. Nilsson , L.O. 1990 . Light absorption and willow production in southern Sweden, a case study In J. Burley IUFRO XIX World Congress 5–11 August 1990, Proceedings, Division 2 59 67 . Montreal , Canada .
- Eckersten , H. , Torssell , B. , Kornher , A. Olson , U. 2004 Department of Ecology and Crop Production Science, Swedish University of Agricultural Sciences . Modeling radiation use and regrowth in grass and red clover swards Report 5 Uppsala , Sweden .
- Eckersten , H. , Torssell , B. , Kornher , A. and Boström , U. 2007 . Modelling biomass, water and nitrogen in grass ley: Estimation of N uptake parameters . European Journal of Agronomy , 27 : 89 – 101 .
- Eltun , R. , Korsaeth , A. and Nordheim , O. 2002 . A comparison of environmental, soil fertility, yield, and economic effects in six cropping systems based on an 8-years experiment in Norway. Agriculture . Ecosystems & Environment , 90 : 155 – 168 .
- Ericson , L. 2003 . How do you control thistle? Ekologisk odling, 4. Nytt från institutionen för norrländsk jordbruksvetenskap , Sveriges lantbruksuniversitet (SLU) , Umeå , Sverige (in Swedish) .
- Fykse , H . 1974 . Research into Sonchus arvensis L.: Distribution in Norway, growth and dormancy in comparison with similar species . Forskning og forsøk i landbruket , 25 , 389 412 in Norwegian .
- Gastal , F. and Lemaire , G. 2002 . N uptake and distribution in crops: an agricultural and ecophysiological perspective . Journal of Experimental Botany , 53 : 789 – 799 .
- Gouis le , J. , Delebarre , O. , Beghin , D. , Heumez , E. and Pluchard , P. 1999 . Nitrogen uptake and utilisation efficiency of two-row and six-row winter barley cultivars grown at two N levels . European Journal of Agronomy , 10 : 73 – 79 .
- Goyne , P.J. , Milroy , S.P. , Lilley , J.M. and Hare , J.M. 1993 . Radiation interception, radiation use efficiency and growth of barley cultivars . Australian Journal of Agricultural Research , 44 : 1351 – 1366 .
- Grondal , H. , Graglia , E. Jensen , R.K. 2003 . Root bud dormancy of Canada Thistle and Perennial Sowthistle? DJF Rapport – Markbrug , 89 , 357 358 (in Danish) .
- Håkansson , S. 1969 . Experiments with Sonchus arvensis L. I. Develpment and growth, and response to burial and defoliation in different developmental stages . Lantbrukshögskolans annaler , 35 : 989 – 1030 .
- Håkansson , S. 2003 . Weeds and weed management on arable land. An ecological approach , Wallingford, , UK : CABI Publishing .
- Håkansson , S. and Wallgren , B. 1972a . Experiments with Sonchus arvensis L. II. Reproduction, plant development and response to mechanical disturbance . Swedish Journal of Agricultural Research , 2 : 15 – 26 .
- Håkansson , S. and Wallgren , B. 1972b . Experiments with Sonchus arvensis L. III. The development from reproductive root cuts into different lengths and planted at different depths, with and without competition from barley . Swedish Journal of Agricultural Research , 2 : 3 – 14 .
- Hallgren , E. 1996 . Do the weed flora and effect of a herbicide change with time? In H. Brown , G.W. Cussans , M.D. Devine , S.O. Duke , C. Fernandez-Quintanilla A. Helwig , R.E. Labrada , M. Landes , P. Kudsk J.C. Streibig Proceedings of the Second International Weed Control Congress , 25–28 June 1996 1355 1368 . Copenhagen , Denmark .
- Jamieson , P.D. , Martin , R.J. , Francis , G.S. and Wilson , D.R. 1995 . Drought effects on biomass production and radiation-use efficiency in barley . Field Crops Research , 43 : 77 – 86 .
- Kemanian , A.R. , Stockle , C.O. and Huggins , D.R. 2004 . Variability of barley radiation-use efficiency . Crop Science , 44 : 1662 – 1672 .
- Kirchmann , H. 1991 . Properties and classification of soils of the Swedish long term fertility experiments. Sites at Fors and Kungsängen . Acta Agricultuae Scandinavica, Section B-Soil and Plant Science , 41 , 227 242 .
- Knecht Billberger , M.F. 2006 . Plant Growth – Stoichiometry and Competition. Theory Development in Ecosystem Ecology . Doctorial Thesis No. 2006:24. Faculty of Natural Resources and Agricultural Sciences, Swedish University of Agricultural Sciences , Uppsala , Sweden . http://diss-epsilon.slu.se/archive/00001059/01/KnechtBillberger.pdf . (accessed 2008-10-27) .
- Korsaeth , A. , Molstad , L. and Bakken , L.R. 2001 . Modelling the competition for nitrogen between plants and microflora as a function of soil heterogeneity . Soil Biology & Biochemistry , 33 : 215 – 226 .
- Legg , B.J. , Day , W. , Lawlor , D.W. and Parkinson , K.J. 1979 . The effects of drought on barley growth: models and measurements showing the relative importance of leaf area and photosynthetic rate . Journal of Agricultural Science , 92 : 703 – 707 .
- Lemna , W.K. and Messerschmith , C.G. 1990 . The biology of Canadian weeds. 94. Sonchus arvensis L . Canadian Journal of Plant Science , 70 : 509 – 532 .
- Lundkvist , A. 1998 . Weed control in ecological farming. A questionnaire . Växtskyddsnotiser , 62 , 23 26 (in Swedish with an English Summary) .
- Muurinen , S. and Peltonen-Sainio , P. 2006 . Radiation use efficiency of modern and old spring cereal cultivars and its response to nitrogen in northern growing conditions . Field Crops Research , 96 : 363 – 373 .
- Muurinen , S. , Slafer , G.A. and Peltonen-Sainio , P. 2006 . Breeding effects on nitrogen use efficiency of spring cereals under northern conditions . Crop Science , 46 : 561 – 568 .
- Muurinen , S. , Kleemola , J. and Peltonen-Sainio , P. 2007 . Accumulation and translocation of nitrogen in spring cereal cultivars differing in nitrogen use efficiency . Agronomy Journal , 99 : 441 – 449 .
- Rastetter , E.B. and Ågren , G.I. 2002 . Changes in individual allometry can lead to species coexistence without niche separation . Ecosystems , 5 : 789 – 801 .
- Reintam , E. Koster , T. 2002 . Biodiversity and nutrient cycling in natural and cultural ecosystems . In: J.L. Rubio , R.P.C. Morgan , S. Asins V. Andreu Proceedings of the Third International Congress of the European Society for Soil Conservation , 28 March–1 April 2000 607 619 . Valencia , Spain .
- Ritchie , J.T. , Singh , U. , Godwin , D.C. and Bowen , W.T. 1998 . “ Cereal growth, development and yield ” . In Understanding options for agricultural production , Edited by: Tsuji , G.Y. , Hoogenboom , G. and Thornton , P.K. 79 – 98 . Heidelberg, , Germany : Springer .
- Salonen , J. , Hyvonen , T. and Jalli , H. 2001 . Weed flora in organically grown spring cereals in Finland . Agricultural and Food Science in Finland , 10 : 231 – 242 .
- Scotter , D.R. , Mohammed , I.H. and Gregg , P.E.H. 1984 . The short-term fate of urea applied to barley in a humid climate. II. A simple model . Australian Journal of Agricultural Research , 22 : 181 – 190 .
- Sinebo , W. , Gretzmacher , R. and Edelbauer , A. 2004 . Genotypic variation for nitrogen use efficiency in Ethiopian barley . Field Crops Research , 85 : 43 – 60 .
- Streit , B. , Rieger , S.B. , Stamp , P. and Richner , W. 2003 . Weed populations in winter wheat as affected by crop sequence, intensity of tillage and time of herbicide application in a cool and humid climate . Weed Research , 43 : 20 – 32 .
- Thomas , A.G. , Derksen , D.A. , Blackshaw , R.E. , van Acker , R.C. , Legere , A. , Watson , P.R. and Turnbull , G.C. 2004 . A multistudy approach to understanding weed population shifts in medium-to long-term tillage systems . Weed Science , 52 : 874 – 880 .
- Torssell , B. , Eckersten , H. , Kornher , A. , Nyman , P. and Boström , U. 2007 . Modelling carbon dynamics in mixed grass-red clover swards . Agricultural Systems , 94 : 273 – 280 .
- Vanhala , P. , Salonen , J. and Lötjönen , T. 2004 . Emergence and growth of Sonchus arvensis in different crop stands under organic farming . Zeitschrift fur Planzenkrankheiten und Pflanzenschutz , 19 : 511 – 516 .
- Vanhala , P. , Lötjönen , T. , Hurme , T. and Salonen , J. 2006 . Managing Sonchus arvensis using mechanical and cultural methods . Agricultural and Food Science , 15 : 444 – 458 .
- Zollinger , R.K. and Kells , J.J. 1991 . Effect of soil pH, soil water, light intensity and temperature on perennial sow-thistle (Sonchus arvensis L.) . Weed Science , 39 : 376 – 384 .
Appendix I
The intercepted radiation (R sInt) was calculated from the global radiation (R s), the leaf area (LAI) and the radiation extinction coefficient (k) in Beer′s law (set to 0.5) [Equation (EquationA1)].
Available nitrogen
The model of available nitrogen is based on processes of nitrogen mineralization and shoot nitrogen uptake. The model simulated nitrogen dynamics on a daily basis. Loss of soil organic nitrogen (ΔNSoilOrg/Δt) equalled the N mineralized from the soil organic matter according to [Equation (EquationA4a)]:
Shoot nitrogen
There are two flows determining daily changes in shoot nitrogen: uptake from the soil [N Avail→Shoot; Equation (Equation2)] and losses assumed to be proportional (m) to the shoot nitrogen [Equation (EquationA5)].