Abstract
Plant-available P was first extracted in soils 114 years ago and a number of different analytical methods have since been developed, but for good reasons none of these methods has been adopted as a standard for all soils. With increasing cooperation within research, there is a need to harmonise the interpretation of analytical data for fertiliser recommendations, research, and environmental control. This paper evaluates the compatibility of the Swedish standard ammonium lactate (PAL) method and the widely used Olson's sodium bicarbonate (POls) method in 82 topsoil samples taken from Swedish long-term soil fertility field trials. The PAL-values were usually larger than POls, with a mean PAL/POls quotient of 2.30±1.04 (0.67–6.58). The PAL and POls means and ranges were 126±89 (5–360) and 55.1±33 (3.1–122.8) mg P kg−1 dry soil, respectively. Stepwise multiple linear regression analysis was used to evaluate the relationship between PAL and POls and how this relationship was affected by clay content, pH, and soil organic carbon content (SOC). After statistical transformation, it emerged that the square root of clay content (17.4%±13.82, range 1–54.4) and pH (6.45±0.54, range 5.5–7.7) significantly affected the relationship at partial R 2-values of 2 and 12%, respectively, while ln(SOC) (2.54%±1.21, range 1–6.03) did not, apparently due the narrow range. The regressions of predicted vs. measured values explained 95 and 94% of the variation in PAL and POls, respectively. The mean deviation of predicted compared with measured values was 21.3 and 8.3 mg P kg−1 dry soil for PAL and POls, respectively, corresponding to 20 or 19% of the measured values. We conclude that a data set consisting of PAL-values can be converted into POls-values and vice versa with reasonably high accuracy when accounting for clay content and pH.
Introduction
There is a general consensus that the complexity of soil phosphorus (P) forms of different solubility coupled with diverse soil mineralogy and properties render this element one of the most difficult nutrients to extract accurately in forms available to plants. The diversity of soil P disposition explains the different approaches for its extraction (e.g., Williams et al., Citation1967; Olsen & Sommer, Citation1982; Turner et al., Citation2005). The first attempt to extract plant-available P from soils was probably made by Dyer (Citation1894) using a 2% CH3COOH solution to obtain what he termed ‘plant mineral food’. Since then, a number of different methods have been developed and the search for new methods or modifications of existing method continues. However, none of these methods has been adopted as a standard for all soils.
As research cooperation is widening, there is also need to harmonise the interpretation of different soil P tests for fertiliser recommendations, research, and environmental control. For example, this need was highlighted in the EU COST Action 832 (EU COST832, Citation2000), which noted the need for a common action plan based on P test data for management of agricultural P to minimise eutrophication in EU member states. The questions arising here are whether soil P tests are compatible and whether analytical data obtained using one method can be translated accurately into those of another method. Theoretically, it is not possible to establish an exact relationship between analytical datasets obtained using different methods. However, the general relationship between the methods can be determined and can enable comparison and interpretation of results collected in different countries.
The ammonium lactate (AL) solution (0.1 M NH4-lactate+0.4 M HOAc, adjusted to pH 3.75) was developed by Egnér et al. (Citation1960) to extract plant-available P in soils. The method is currently standard in some European countries including Sweden, Norway, Belgium, Portugal, the former USSR Baltic states, and some Balkan states, though shaking time defers. In some countries soil samples are shaken for two hours, while in others the shaking time is 90 minutes. The P in AL extracts are hereafter designated PAL. The AL solution also provides an estimate of plant-available K and Mg content in the soil. However, in addition to accessing available P (Karlsson & Jansson, Citation1959; Semb, Citation1984), AL also dissolves insoluble Ca-P compounds, such as apatite (Semb, Citation1984) and Ca3(PO4)2 in limed and calcareous soils (Hahlin & Eriksson, Citation1981; Fernandes et al., Citation1999; Ivarsson & Gustafsson, Citation2001) and soils fertilised with phosphate rock (da Silva & Van Raij, Citation1999). Evidently, PAL is an overestimation of available P in such soils.
The reason why the PAL method remains the official method in Sweden is that cultivated soils have been regularly analysed using this method and there is wealth of data on PAL, pH, clay, and soil organic carbon (SOC) contents from the mid-1950s until now. Moreover, Swedish advisory authorities and farmers are conversant with PAL and can interpret it for soil fertility assessment and management. In addition, PAL is a valuable tool in research, teaching, and soil classification (Kirchmann et al., Citation1999).
The weakly alkaline sodium bicarbonate (SB) solution (0.5 M NaHCO3, pH 8.5) proposed by Olsen et al. (Citation1954) is widely used to complement established national standard methods in the USA and several European countries. The P extracted is hereafter termed POls. This method was originally designed to measure plant-available P content in alkaline soils, but has also predicted the P requirement for rice grown in submerged soils (Dobermann et al., 1996) as well as some acid soils (Bationo et al., Citation1991). In Denmark PAL is still included in routine analysis, but about two decades ago POls became the standard method. Similarly, in Norway, where PAL is still the official method, analysis of POls is recommended for sedimentary clay soils with a high pH (Riley & Steenberg, Citation1985). In Croatia, where PAL is widely used in soil fertility assessment, POls is also attracting interest (Lončarič et al., Citation2006). Both PAL and POls have been used as tools for risk assessment of P-leaching losses (Sharpley & Rekolainen, Citation1997; Hesketh & Brookes, Citation2000; Bechmann et al., Citation2003; Börling, Citation2003; Djodjic et al., Citation2004).
This study was therefore undertaken to determine and evaluate the compatibility of PAL and POls and to develop a model that can used to convert their data sets into each other. Because of high analytical costs, only those two methods were compared.
Materials and methods
Sites and soil analysis
Eighty-two topsoil (0–20 cm) samples were taken from Swedish long-term fertility trials located in 14 provinces (). The samples were air-dried, crushed and passed through a sieve (≤2 mm). The pH of duplicate samples was measured in a 1:5 suspension (1 g dry soil suspended in 5 mL deionised water) on a pH meter. Two replicates were analysed for PAL (1 g shaken with 20 mL AL solution for 90 min) and another two replicates were analysed for POls (1 g shaken with 20 mL SB solution for 30 min). Both AL- and SB-extracts were filtered through Whatman no. 42 paper. Phosphorus concentrations in the extracts were measured calorimetrically on a spectrophotometer. For PAL, the stannous chloride–molybdate procedure (Egnér et al., Citation1960) was used and absorbance was measured at 882 nm. For POls, the ascorbic acid–molybdate procedure was used and absorbance was measured at 882 nm (Murphy & Riley, Citation1962). Soil organic carbon was determined by dry combustion and infrared gas analysis on LECO CNS-2000 equipment. Samples from one calcareous soil were first treated with HCl (0.5 M) to remove carbonates. Clay was determined as described by Day (Citation1965). Details of the sites, soil properties, soil classification, and analytical procedures have been published elsewhere (Kirchmann et al., Citation1999; Börling et al., Citation2001, Citation2004).
Table I. Sites and treatments (n=82 soil samples).
Statistical analysis
Stepwise multiple linear regression analysis was used (SAS Institute, Citation1999–2000) to evaluate the relationship between PAL and POls and how this relationship is affected by the measured independent variables pH and contents of SOC and clay. Only variables significant at the 5% probability level were included in the resulting regression models. However, where it was deemed necessary, statistical transformation was performed to fulfil the requirements of normal distributions according to the tests (procedure univariate) implemented in the statistical software. For model evaluation, we regressed measured on predicted data according to Piñeiro et al. (Citation2008) and estimated the root mean squared deviation (RMSD) representing the mean deviation of predicted values with respect to those measured (Kobayashi & Salam, Citation2000) by means of Equation (Equation1):
Results
Selected soil properties
Of the 82 soils investigated, 51 had a pH range 6.0–6.9, 15 had a pH range 5.5–5.9, and 12 had pH range 7.0–7.7 (). Mean and range of soil pH was 6.45±0.54 (5.5–7.7), representing the pH range commonly found in Swedish cultivated mineral soils. One soil had pH 7.7 and contained about 3.5% CaCO3 (Börling et al., Citation2004). Clay and SOC contents also varied widely in the soils. The majority of Swedish cultivated soils are in PAL Classes IV and V (Mattsson & Carlgren, 2001; Mattsson, Citation2002). In this present study, the soils generally were in Classes III–V ().
Table II. Available P status and selected soil properties in the 82 soils.
Table III. Soil classification into Classes I–V according to the standard Swedish PAL test.
The PAL-values were usually larger than POls, with a mean PAL/POls quotient of 2.30±1.04 (range 0.67–6.58). Relationships among soil properties and P tests based on Pearson's linear correlation coefficient (r) are presented in . The P tests were significantly and positively correlated with each other, negatively with clay content, positively with SOC, and weakly with pH.
Table IV. Correlations (Pearson's r-values) between soil properties and soil P tests.
Discussion
Relationship between PAL and POls
Of the soil properties determined, pH and clay could be used in prediction regression equations. Rather surprisingly, the pH-values weakly correlated with the two P tests (). When untransformed data were used to predict the P tests, the results strongly diverged from the corresponding analytical values, especially when soil content of PAL was ≤40 mg kg−1, and predicted POls also markedly diverged from analytical values when PAL content was above about 250 mg kg−1. Among the three transformed independent variables tested, i.e. ln(SOC) and the square root of clay content and pH, only the latter two significantly affected the relationship between PAL and POls, with partial R 2-values of 2 and 12%, respectively. The resulting regression models [Equations (Equation2) and (Equation3)] accounted for between 95 and 94% of the total variation in the dataset. After raising both sides to the second power, the resulting equations become:
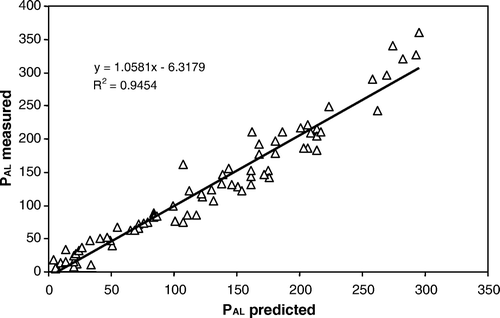
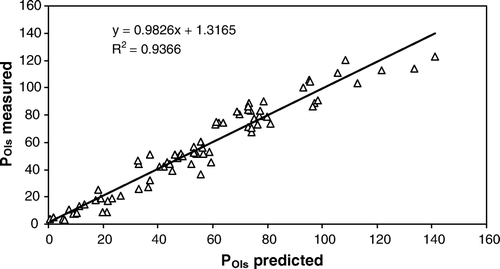
Plots of measured PAL against predicted PAL are depicted in , while plots of measured POls against predicted POls are depicted in . The mean deviation of predicted compared with measured values was 21.3 and 8.3 mg P kg−1 dry soil for PAL and POls, respectively, corresponding to 20 or 19% of the measured values. Hence, a data set consisting of PAL-values can be converted into POls-values and vice versa with reasonably high accuracy when accounting for clay content and pH.
The strong relationship () obtained between PAL and POls suggests that these two P tests accessed similar soil P. Strong relationships between the methods have also been reported elsewhere (Börling, Citation2003; Lončarič et al., Citation2006). However, the AL and SB solutions are different in composition, strength, and buffering. The AL solution is a strong solution buffered at pH 3.75, whereas the SB solution is a weak alkaline solution buffered at pH 8.5. Evidently, the two methods differ in the mechanisms by which they extract soil P. Owing to its acidic pH, AL can also hydrolyse P in insoluble A1-humic-P substances in soils (McDowell & Sharpley, Citation2001) and dissolves insoluble Ca-P compounds (Semb, Citation1984; Fernandes et al., Citation1999; da Silva et al., 1999). Moreover, the AL solution can liberate P from the Al-P and Fe-P compounds by chelation of the metals, and P can also be exchanged by the anion (COO)− present in the solution. Similarly, SB solution can displace P from the exchange sites by anion-exchange reactions promoted by the HCO3 −, CO3 2−, and OH− anions present in the SB solution. Probably by virtue of its slightly alkaline reaction (pH 8.5), the SB solution can also hydrolyse Al(OH)3, producing [Al(OH)4]− anion thus: [Al(OH)3]o+HOH ↔ [Al (OH)4]−+H+. The [Al(OH)4]− anion can displace (H2PO4)−. Moreover, P can be released by hydrolysis of Al- and Fe-P thus: AlPO4+3HOH ↔ Al(OH)3+H3PO4. Mobilisation of P via hydrolysis reactions could probably be important in tropical soils rich in Al and Fe oxides. Indeed, SB has predicted the P requirement for rice grown in some tropical acid soils (Bationo et al., Citation1991). These differences in the P stocks extracted by the two P tests are reflected in & 2. In , most of the points generally fell along the regression line, with some predicted PAL tending to be either underestimated or overestimated. Similar trends were noted for POls (), where some points clustered below or above the regression line, R 2=0.94 compared with R 2=0.95 for the PAL regression line. According to the regression models, the results from these two P-extraction methods are directly comparable. We conclude that a data set consisting of PAL-values can be converted into POls-values and vice versa with reasonably high accuracy when accounting for clay content and pH.
Acknowledgements
Financial support was provided by the Swedish Board of Soil Fertility and Plant Nutrition, Royal Academy of Forestry and Agriculture (KSLA).
References
- Bationo , A. , Baethegen , W.E. , Christianson , C.B. and Mokwunye , A.U. 1991 . Comparison of five soil testing methods to establish phosphorus sufficiency levels in soil fertilized with water-soluble and sparingly soluble-P sources . Fertiliser Research , 28 : 271 – 279 .
- Bechmann , M.E. , Krogstad , T. , & Sharpley , A.N. 2003 . A phosphorus index for Norway: Justification of factors . Proceedings of the Diffuse Pollution Conference Dublin 2003. 3H: Agriculture. 3-163 .
- Börling , K. 2003 . Phosphorus Sorption, Accumulation and Leaching: Effects of Long-term Inorganic Fertilization of Cultivated Soils . Acta Universitatis Agriculturae Sueciae. Agraria 428 (PhD Thesis). Swedish University of Agricultural Sciences, Department of Soil Sciences. P.O. Box 7014, SE-750 07 Uppsala, Sweden .
- Börling , K. , Otabbong , E. and Barberis , E. 2001 . Phosphorus in relation to soil properties in some cultivated Swedish soils . Nutrient Cycling in Agroecosystems , 59 : 39 – 46 .
- Börling , K. , Otabbong , E. and Barberis , E. 2004 . Soil variables for predicting potential phosphorus release in Swedish non-calcareous soils . Journal of Environmental Quality , 33 : 99 – 106 .
- Day , P.R. 1965 . “ Particle fractionation and particle-size analysis ” . In Methods of Soil Analysis. Part 1. Physical and mineralogical properties, including statistics of measurement and sampling , Edited by: Black , C.A. 545 – 568 . Madison, Wisconsin, , USA : American Society of Agronomy, Inc. .
- Djodjic , F. , Börling , K. and Bergström , L. 2004 . Phosphorus leaching in relation to soil type and soil phosphorus content . Journal of Environmental Quality , 33 : 678 – 684 .
- Dyer , B. 1894 . On the analytical determination of probably available “mineral” plant food in soil . Transactions of the Chemical Society , 65 : 115 – 167 .
- Egnér , H. , Riehm , H. , & Domingo , W.R. 1960 . Untersuchungen über die chemische Bodenanalyse als Grundlage für die Beurteilung des Nährstoffzustandes der Böden. II Chemische Extrationsmethoden zur Phosphor und Kaliumbestimmung . Kungliga Lantbrukshögskolans Annaler , 26 , 199 – 215 . (In German) .
- EU COST832 2000 . COST Action 832: Quantifying the Agricultural Contribution to Eutrophication. European Cooperation in the Field of Scientific and Technical Research, Agriculture and Biotechnology . Annual Report , 2000 .
- Fernandes , M.L.V. , Indiati , R. , Coutinho , J. and Buondonno , A. 1999 . Soil properties affecting phosphorus extraction from Portuguese soils by conventional and innovative methods . Communication in Soil Science and Plant Analysis , 30 : 921 – 936 .
- Hahlin , M. , & Ericsson , J. 1981 . Fosfor och fosforgödsling . Aktuellt från Lantbruksuniversitetet , 294. Mark.Växt. Uppsala, Sweden. (In Swedish) .
- Hesketh , N. and Brookes , P.C. 2000 . Development of an indicator for risk of phosphorus leaching Journal of Environmental Quality , 29 : 105 – 110 .
- Ivarsson , K. , & Gustafsson , K. 2001 . Extended interpretation of labile P for soil and yield related P fertilization . Report , 202. Department of Soil Sciences, Division of Soil Fertility and Plant Nutrition, Swedish University of Agricultural Sciences, P.O. Box 7014, SE-750 07 Uppsala, Sweden .
- Karlsson , N. , & Jansson , E. 1959 . Om sambandet mellan resultat från jordanalyser enligt AL-metoden och den finska metoden samt mellan AL-metoden och AL-metoden utan laktat, beträffande lättlöslig fosforsyra och lättlösligt kali. Satens lantbrukskemiska kontrollanstalt . Meddelande , 20 , bilagor V. (In Swedish) .
- Kirchmann , H. , Eriksson , J. and Snäll , S. 1999 . Properties and classification of soils of the Swedish long-term fertility experiments. IV. Site at Ekebo and Fjärdingslöv . Acta Agriculturae Scandinavica, Section B, Soil and Plant Science , 49 : 25 – 38 .
- Kobayashi , K. and Salam , M.U. 2000 . Comparing simulated and measured values using mean squared deviation and its components . Agronomy Journal , 92 : 345 – 352 .
- Lon(ari( , Z. , Popovi( , B. , Tekli( , T. , Engler , M. and Karali( , K. 2006 . Comparison of two soil phosphorus analytical methods in Croatia . Communications in Soil Science and Plant Analysis , 37 : 2867 – 2881 .
- Mattsson , L. 2002 . Exploiting P in heavy P-dressed fields in Sweden . Archiv für Acker- und Pflanzenbau und Bodenkunde , 48 : 577 – 583 .
- Mattsson , L. , & Carlgren , K. 2002 . Växtnäringsförsök 2000. Skörderesultat med växt-och jordanalyser . Rapport 11. Swedish University of Agricultural Sciences, Department of Soil Sciences, P.O. Box 7014, 750 07 Uppsala, Sweden. (In Swedish) .
- McDowell , R.W. and Sharpley , A.N. 2001 . Soil phosphorus fractions in solution: influence of fertiliser and manure, filtration and method of determination . Chemosphere , 45 : 737 – 748 .
- Murphy , J. and Riley , J.P. 1962 . A modified single solution method for determination of phosphate in neutral waters . Analytica Chemica Acta , 27 : 31 – 36 .
- Olsen , S.R. , Cole , C.V. , Watanabe , F.S. , & Dean , L.A. 1954 . Estimation of available phosphorus in soils by extraction with sodium bicarbonate. USDA Circular, 939 . US Government Printing Office , Washington, DC .
- Olsen , S.R. & Sommers , L.E. 1982 . Phosphorus . In A.L. Page , R.H. Miller and D.R. Keeney Methods of Soil Analysis. Part 2 Chemical and Microbiological Properties , 2nd edition , pp. 403 – 430 . Agronomy : American Society of Agronomy, Inc. Soil Science of America, Inc. Madison, Wisconsin, USA .
- Piñeiro , G. , Perelman , S. , Guerschman , J.P. and Paruelo , J.M. 2008 . How to evaluate models: Observed vs. predicted or predicted vs. observed? . Ecological Modelling , 216 : 316 – 322 .
- Riley , H. and Steenberg , K. 1985 . Fosfor til korn på bakkeplanert leirjord . Forskning og Fors⊘ i Landbruket , 36 : 177 – 183 . (In Norwegian)
- SAS Institute 1999–2000 . SAS Language Version 6.0 . SAS Institute Inc. , Cary, NC, , USA .
- Semb , G. 1984 . Sammenligning av AL- og natriumbikarbonatl⊘selig fosfor i jord med pH over 6.6 . Jordunders⊘kelsens Särtrykk 350, Särtrykk av Jord og Myr 5 . (In Norwegian) .
- Sharpley , A.N. and Rekolainen , S. 1997 . “ Phosphorus in agriculture and its environmental implications ” . In Phosphorus Loss from Soil to Water , Edited by: Tunney , H. , Carton , O.T. , Brookes , P.C. and Johnston , A.E. 1 – 53 . Wallingford, Oxon OX10 8DE : CAB International .
- da Silva , F.C. and Van Raij , B. 1999 . Phosphorus availability in soils determined by different extracting procedures . Pesquisa-Agropecuária-Brasileira , 34 : 267 – 288 .
- Turner , B.L. , Cade-Menum , B.J. , Condron , L.M. , & Newman , S. 2005 . Extraction of soil organic phosphorus . Talanta , 66 , 294 – 306 . www.elsevier.com/locate/talanta ( accessed 2008-07-14 ).
- Williams , J.D.H. , Syers , J.K. and Walker , T.W. 1967 . Fractionation of soil inorganic phosphate by a modification of Chang and Jackson's procedure . Soil Science Society of American Journal , 31 : 736 – 739 .