Abstract
The effects of riboflavin combined with α-naphthaleneacetic acid on the in vitro rooting of sour and trifoliate orange explants were studied. After four weeks in culture, there was a reduction in both the rooting percentage and root length of sour orange explants. When riboflavin was added to the nutrient medium containing 0.1 mg dm−3 α-naphthaleneacetic acid, the rooting percentage of sour orange explants was reduced. However, in the treatments containing 1.0 mg dm−3 α-naphthaleneacetic acid the root length increased. Rooting in trifoliate orange explants was not distinctly affected by riboflavin. Increasing riboflavin concentration in the nutrient medium leads to a) the rooting percentage was affected (reduced) only in sour orange explants treated with 0.1 mg dm−3 NAA; b) the root length either decreased (0.1 mg dm−3 NAA) or increased (1.0 mg dm−3 NAA); and c) the root number was reduced in most treatments. Finally, it is strongly recommended that riboflavin in vitro should be extensively studied, as it has immense economic significance for commercial laboratories when it is added to the nutrient medium with the aim to reduce exogenous auxin levels, rather than transferring cultures to a growth-regulator-free medium.
Introduction
Sour orange (Citrus aurantium L.) is one of the most commonly used Citrus rootstocks in the Mediterranean region (Georgiou & Gregoriou, Citation1999). Another is trifoliate orange (Poncirus trifoliata L.), which is a relative of the genus Citrus. Both are used extensively in citriculture and are propagated through different procedures.
Micropropagation protocols have been described for a number of Citrus species. There have been many attempts to improve rhizogenesis by adding growth regulators to the culture medium in various concentrations and combinations. Furthermore, there are a great number of possible growth-regulating substances which have not been extensively tested (George, Citation1996). Little research has been conducted concerning the effectiveness of vitamins on in vitro rooting of Citrus. Riboflavin, occasionally used in tissue culture media, has been found to stimulate rooting of Eucalyptus, papaya, and walnut explants (Bachelard & Stone, Citation1962; Drew & Miller, Citation1989; Gruselle & Boxus, Citation1990) or to inhibit root formation of papaya explants (Drew & Smith, Citation1986). Furthermore, the inclusion of α-naphthaleneacetic acid (NAA) in an in vitro medium was found to be beneficial for the rooting not only of Citrus (Al-Wasel, Citation2000; Bordon et al., Citation2000) but for other species as well (Moshen, Citation2004; Tian et al., Citation2007).
As the results for other species appear to be variable and contradictory, the present experiment was conducted in order to investigate the effects of riboflavin in combination with two NAA concentrations on the in vitro rooting and growth of sour and trifoliate orange explants.
Materials and methods
Apical shoot tips (10–15 mm) of sour and trifoliate orange were obtained from previous subcultures on a full-strength MS (Murashige & Skoog, Citation1962) medium and were transferred to 10 cm3 of the same rooting medium. Riboflavin was added in five concentrations: 0, 0.5, 1.0, 1.5, and 2.0 mg dm−3. All media contained 0.1 and 1.0 mg dm−3 NAA, sucrose 30 g dm−3, and agar Oxoid No. 3, 6 g dm−3. The pH of the media was adjusted to 5.8 prior to autoclaving at 121 oC for 20 min. Cultures were maintained at 26±1 oC under cool white fluorescent light (Phillips, 90 µmol m−2 s−1) with a 16 h photoperiod. After four weeks in culture, the microcuttings were separated into shoots and roots, counted, and weighed. Each treatment included fifteen replications (culture vessels) with one microcutting in each 25×100 mm test tube.
The ex vitro acclimatization procedure of the plantlets was similar to that applied by Shiyab et al. (Citation2003). At the end of three weeks, the survival percentage was recorded and plants were transferred to a greenhouse (T min 18 oC, T max 38 oC, T mean 30 oC) for further growth and development.
The experimental layout was completely randomized and the experiment was repeated twice. The reported data are the means of the two experiments. The means were subjected to analysis of variance (ANOVA) and compared by using the Duncan multiple-range test (P≤0.05).
Results and discussion
The addition of riboflavin to the nutrient medium containing 0.1 mg dm−3 NAA led to a decrease in the rooting percentage of sour orange explants, whereas there was no effect when NAA concentration was 1.0 mg dm−3 (A). However, the rooting percentage in trifoliate orange explants was affected (reduced) only by the presence of 1.0 mg dm−3 riboflavin, independently of NAA levels (A).
Figure 1. Effect of riboflavin and NAA (0.1 mg dm−3 □, 1.0 mg dm−3 ▪) on the rooting% (A), root number per explant (B), root fresh weight (C), and root length (D) of sour orange at the end of the experiment. Within the same NAA treatment, means followed by the same letter are not significantly different (Duncan multiple-range test, P≤0.05).
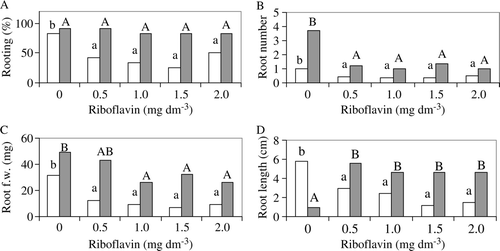
Figure 2. Effect of riboflavin and NAA (0.1 mg dm−3 □, 1.0 mg dm−3 ▪) on the rooting% (A), root number per explant (B), root fresh weight (C), and root length (D) of trifoliate orange at the end of the experiment. Within the same NAA treatment, means followed by the same letter are not significantly different (Duncan multiple-range test, P≤0.05).
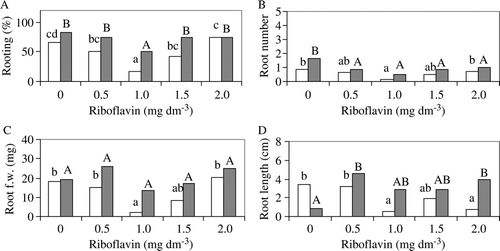
The root number per explant was reduced with the addition of riboflavin in the sour orange for both NAA treatments (B), and in the trifoliate orange treated with 1.0 mg dm−3 NAA (B). Root fresh weight was negatively affected with the addition of riboflavin in sour orange explants for both NAA levels (C), whereas in the trifoliate orange it was reduced only in treatment containing 1.0 mg dm−3 riboflavin and 0.1 mg dm−3 NAA (C). From ANOVA, riboflavin significantly affected root number, as well as the root fresh and dry weight of the trifoliate orange explants (data not shown). It was found that the addition of riboflavin reduced the root length of sour orange explants treated with 0.1 mg dm−3 NAA and increased it in the treatments containing 1.0 mg dm−3 NAA (D). The same trend was observed in half of the riboflavin-treated trifoliate orange explants (D). The greater root length observed in explants treated with 1.0 mg dm−3 in combination with the higher riboflavin concentrations could be attributed to the negative correlation between root number and root length. In all treatments, no calli developed, the microshoots of both species were green and vigorous, and new shoots appeared after their buds burst. Rooted plantlets (75–85%) were successfully acclimatized and there were no statistical differences in survival percentages among treatments (data not shown). The acclimatized plantlets were healthy and were successfully grown in the greenhouse.
Riboflavin, like other chemical compounds, is also used in tissue culture media (George, Citation1996) but is not a basic component of the MS media. In most of the treatments reported above, riboflavin either reduced or had no effect on root initiation and growth of sour orange and/or trifoliate orange explants. Our data are consistent with those reported by Antonopoulou et al. (Citation2005), who found that the effects of riboflavin were deleterious to root initiation for GFg677 (Prunus persica×Prunus amygdalus) rootstocks. In contrast, 1.0 mg dm−3 of riboflavin added to the Driver Kuniyuli Walnut (DKW) media promoted rooting of Juglans regia and Paradox microcuttings (Gruselle & Boxus, Citation1990). In addition, riboflavin promoted rooting of Eucalyptus explants (Bachelard & Stone, Citation1962; Trinidade, Citation1997). Unlike the findings of an experiment with GFg677 explants (Antonopoulou et al., Citation2005), in the present study the addition of riboflavin did not have a negative effect on shoot growth (data not shown). The fact that during rooting the shoots’ basal region was maintained under light could explain the negative effects of riboflavin. Riboflavin was found to catalyse auxin photooxidation (Drew et al., Citation1991). In treatments with 0.1 mg dm−3 NAA, riboflavin probably catalysed auxin photooxidation, explaining the low percentage of rooting. Alternatively, in the liquid MS medium with 0.1 mg dm−3 NAA, trifoliate orange explants presented a high number of roots as well as an increase in root length (Al-Wasel, Citation2000). The findings of Gorst et al. (Citation1983) are in line with ours, namely that the presence of riboflavin in the nutrient medium under conditions of exposure to light sensitizes the oxidation of the synthetic indole-3-butyric acid (IBA), reducing the amount of auxin in the medium. Even though NAA is more photooxidative tolerant than other auxins (Stasinopoulos & Hangarter, Citation1990), as regards the sour orange explants treated with 1.0 mg dm−3 NAA (part of which could be photooxidized), in our study a significant part remained stable and was high enough to stimulate rhizogenesis. Similarly, this same NAA concentration in the nutrient medium led to a high percentage of rooting in the in vitro cultures of sour orange (Bordon et al., Citation2000). A proposed technique to avoid any possible negative effects that riboflavin may have on rooting is to inject riboflavin onto the agar surface one day following the establishment of the culture on the rooting medium (Drew et al., Citation1993).
References
- Al-Wasel , A. 2000 . Micropropagation of trifoliate orange rootstock (Poncirus trifoliata Raf.) HortScience , 41 , 1052 .
- Antonopoulou , C. , Dimassi , K. , Therios , I. and Chatzissavvidis , C. 2005 . Inhibitory effects of riboflavin (Vitamin B2) on the in vitro rooting and nutrient concentration of explants of peach rootstock GF 677 (Prunus amygdalus x P. persica) . Scientia Horticulturae , 106 : 268 – 272 .
- Bachelard , E.P. and Stone , B.S. 1962 . A possible link between root initiation and anthocyanin formation . Nature , 194 : 209 – 210 .
- Bordon , Y. , Guardiola , J.L. and Garcia-Luis , A. 2000 . Genotype affects the morphogenic response in vitro of epicotyl segments of Citrus rootstocks . Annals of Botany , 86 : 159 – 166 .
- Drew , R.A. and Miller , R.M. 1989 . Nutritional and cultural factors affecting rooting of papaya (Carica papaya L.) in vitro . Journal of Horticultural Science , 64 : 767 – 773 .
- Drew , R.A. and Smith , N.G. 1986 . Growth of apical and lateral buds of papaya (Carica papaya L.) as affected by nutritional and hormonal factors . Journal of Horticultural Science , 61 : 535 – 543 .
- Drew , R.A. , Simpson , B.W. and Osborne , W.J. 1991 . Degradation of exogenous indole-3-butyric acid and riboflavin and their influence on rooting response of papaya in vitro . Plant Cell Tissue & Organ Culture , 26 : 29 – 34 .
- Drew , R.A. , McComb , J.A. and Considine , J.A. 1993 . Rhizogenesis and root growth of Carica papaya L. in vitro in relation to auxin sensitive phases and use of riboflavin . Plant Cell Tissue & Organ Culture , 33 : 1 – 7 .
- George , E.F. 1996 . Plant propagation by tissue culture in practice-Part I . Exegetics Limited Press , London, , UK .
- Georgiou , A. and Gregoriou , C. 1999 . Growth, yield and fruit quality of ‘Shamouti’ orange on fourteen rootstocks in Cyprus . Scientia Horticulturae , 80 : 113 – 121 .
- Gorst , J.R. , Slaytor , M. and De Fossard , R.A. 1983 . The effect of indole-3-butyric acid and riboflavin on the morphogenesis of adventitious roots of Eucalyptus ficifolia F. Meull. grown in vitro . Journal of Experimental Botany , 34 : 1503 – 1515 .
- Gruselle , R. and Boxus , P. 1990 . Walnut propagation . Acta Horticulturae , 284 : 45 – 52 .
- Moshen , Khe , 2004 . Comparison, determination and optimizing the conditions required for rhizome and shoot formation, and flowering of in vitro cultured calla explants . Scientia Horticulturae , 101 , 305 313 .
- Murashige , T. and Skoog , F. 1962 . A revised medium for rapid growth and bioassays with tobacco tissue cultures . Physiologia Plantarum , 15 : 473 – 497 .
- Shiyab , S.M. , Shibli , R.A. and Mohammad , M.M. 2003 . Influence of sodium chloride salt stress on growth and nutrient acquisition of sour orange in vitro . Journal of Plant Nutrition , 26 : 985 – 996 .
- Stasinopoulos , T.C. and Hangarter , R.P. 1990 . Preventing photochemistry in culture media by long-pass light filters alters growth of cultured tissues . Plant Physiology , 93 : 1365 – 1369 .
- Tian , L. , Sibbald , S. , Subramanian , J. and Svircev , A. 2007 . Characterization of Prunus domestica L. in vitro regeneration via hypocotyls . Scientia Horticulturae , 112 : 462 – 466 .
- Trinidade , H. 1997 . In vitro studies on Eucalyptus globulus rooting ability . In Vitro Cellular Developmental Biology-Plant , 33 : 1 – 5 .