Abstract
A perennial forage legume species tolerant to water stress would be useful to improve pasture and animal productivity in the central zone of Chile. The growth, dry-matter (DM) production, and drought tolerance of twelve accessions of Lotus tenuis Waldst & Kit, Syn. L. glaber, naturalized in Chile were evaluated with the objective to select contrasting genotypes, tolerant and sensitive to drought, for future breeding programmes. The accessions were sown in pots of 1.3 L containing a volcanic soil (Andisol). A completely randomized design with all combinations of Lotus accessions and four water treatments [100, 70, 40, and 10% of soil water availability (SWA)] was used. The relative rate of stem elongation (RRSE), the shoots and roots DM production, the relative water content (RWC), and the specific leaf area (SLA) were measured. A drought-sensitivity index (DSI) and the interaction genotype×environment were calculated. The RRSE, the DM production, RWC, and SLA all showed a significant reduction (P<0.05) in the treatment with the higher water restriction (10% SWA). There were significant differences (P<0.05) within RRSE and DM production genotype means, but the RWC and SLA did not differ among populations. The DSI varied broadly among genotypes, from 0.49 to 1.34, and was correlated negatively with DM production under water stress (10% SWA). It was concluded that the Lotus tenuis populations showed water-stress tolerance genetic variability, with accessions Lt14 and Lt4 the most contrasting. These findings will permit us to identify chromosomal regions associated with drought-tolerant genotypes which will allow us to accelerate the development of cultivars adapted to water-restricted environments.
Introduction
The growing demand for good quality soils for the expansion of crops or the establishment of more profitable productive systems (fruits and vineyards) is displacing forage crops to marginal environments where there are often soils with low fertility, poor drainage and problems with periods of drought, different degrees of acidity, and thermal imbalance (Baum et al., Citation2003; Passiura, Citation2007). As a result, forage species are increasingly being cultivated to the limit of adaptation, in areas where the capacity to tolerate environmental stress has become an essential characteristic in determining success (Taleinsnik et al., Citation1998; Lucero et al., Citation2000; Striker et al., Citation2005; Teakle et al., Citation2007; Real et al., Citation2008). In consequence, developing vegetal genetic resources that have high yield potential and are adapted to marginal conditions is essential to the sustainability of any animal production system.
Drought and salinity are the abiotic stresses in marginal environments that most limit plant growth and crop productivity (Reynolds et al., Citation2001). Currently, the development of genotypes that are tolerant to drought and have greater water-use efficiency is a global challenge because of the continued growth of world human population and the reduction of water resources available for agriculture (Nguyen, Citation1999).
The response of plants to water deficit can be studied through the systematic identification of morphological, physiological, and biochemical characteristics that confer the ability to tolerate water stress (Reynolds et al., Citation1999; Araus et al., Citation2002; Richards et al., Citation2002; Condon et al., Citation2004; Poormohammad et al., Citation2007). To determine which characteristics confer adaptive advantages under conditions of water stress, it is necessary to know the environment in which these genotypes will be established. Donald (Citation1968) proposed determining selection criteria using the concept of a preconceived model plant or ideotype that should express phenotypic characteristics that confer adaptation to a specific environment. In regions with Mediterranean climate, like central Chile, the annual rainfall is concentrated in winter (approximately 80%) when temperature limits plant growth. On the other hand, during spring and summer, water limitations for perennial forage species are strong and affect their productivity and persistence. Therefore, genotypes tolerant to water shortage for long periods are required for these environments, where the water for irrigation is scarce and there is a competition for this resource between crops and pasture.
In this context, some species of the Lotus genus have been proposed as promising genetic resources given their high forage potential and good capacity of adaptation to marginal environments (Acuña, Citation1998; Carter et al., Citation1997; Striker et al., Citation2005; Teakle et al., Citation2007; Real et al., Citation2008). At present, three species of Lotus have been domesticated and genetically improved (Blumenthal & McGraw Citation1999): birdsfoot trefoil (L. corniculatus L.), the greater lotus (L. uliginosus Schkuhr), and the narrow-leaf trefoil (L. tenuis Waldst & Kit., syn. L. glaber Mill). These are characterized by producing good quality forage with high nutritive value, not producing bloat in ruminants, and improving protein use in the rumen (McMahon et al., Citation2000), owing to their concentration of condensed tannins (Acuña et al., Citation2008). As well, as legumes they have the capacity to fix atmospheric nitrogen (Graham & Vance, Citation2003; Udvardi et al., Citation2005). Despite the recognized forage attributes, only one cultivar of L. corniculatus has been released in Chile and neither of the other two species mentioned.
Lotus tenuis is a perennial species of European origin. It is found in Chile in a wide range of environments between the Valparaíso (32° S) and Bío Bío Regions (38° S), with a strong presence in areas with clay and volcanic soils of medium texture with problems of moisture retention (Acuña et al., Citation1997; Acuña & Cuevas, Citation1999). Eleven naturalized populations of L. tenuis were collected by Acuña et al. (Citation2002) in the central zone of Chile (between 33°39’ S and 37°09’ W). The differences between the environments, particularly in soil water availability during spring and summer, where the eleven populations were collected and the genetic diversity found by Acuña et al. (Citation2002) in previous studies of agronomic characterization of these materials allow us to suppose that also genetic variability in terms of water-stress tolerance exists.
This experiment was carried out as part of the project ‘Lotus adaptation and sustainability in South-American soils’ (LOTASSA; http://www.lotassa.org/online/site/) founded by EU, with the objective of evaluating the growth and plant water status of eleven populations of L. tenuis, naturalized in Chile, and a cultivar of Argentinean origin of the same species, cultivated in a greenhouse under different levels of soil-water availability, in order to select populations with contrasting tolerance to water stress for future breeding programmes.
Materials and methods
Plant material
Eleven populations of Lotus tenuis naturalized in Chile, collected in the summer of 1998 and 1999, and the cultivar Toba of Argentinean origin, were used. Details related to the collection sites and the agronomic characterization of these populations was presented in Acuña et al. (Citation2002). The identification and place of origin of these materials is as follows: Lt1 (Cabrero; 36°58’ S, 72°23’ W), Lt3 (Yumbel; 37°09’ S, 72°26’ W), Lt4 (San Javier; 35°39’ S, 71°48’ W), Lt 5 (Parral; 36°04’ S, 72°00’ W), Lt 6 (Parral; 36°07’ S, 71°52’ W), Lt7 (Cato; 36°32’ S, 71°54’ W), Lt8 (Coihueco; 36°37’ S, 71°48’ W), Lt11 (Itahue; 35°07’ S, 71°20’ W), Lt12 (Villa Alegre; 35°41’ S, 71°40’ W), Lt14 (Melipilla; 33°39’ S, 71°15’ W), and Lt15 (Las Cabras; 34°14’ S, 71°23’ W).
Growing environment and water-stress treatment
The experiment was carried out under greenhouse conditions during the spring of 2006 in the Centro Regional de Investigación Quilamapu, INIA, Chillán, Chile (36°03’ S, 72°07’ W). The temperature and relative humidity of the environment was controlled with a forced-air cooling system. These parameters were recorded at 15-minute intervals throughout the experimental period using an automatic sensor (Hobo pro series, Onset, USA). The greenhouse average temperature was of 23/16 °C (day/night) and the average relative humidity was 55/75% (day/night).
The experiment employed 1.3-L pots (upper diameter of 14 cm, lower diameter of 12 cm, and a height of 9.5 cm) containing as substrate soil derived from volcanic ash with a silt–loam texture (Andisol; medial, thermic Humic Haploxerands), 10% of organic matter, and high levels of available N, P, and K. No fertilizers were applied to the substrate and the seeds were not inoculated given that in the sector where the soil was obtained the species grows spontaneously and develops nodules. The sowing date was 16 August 2006 and ten plants per pot were established.
A physical analysis of the soil was made to determine its water content at tensions of −0.03 MPa (field capacity) and −1.5 MPa (permanent wilting point). The maximum capacity of available water retention (100% of soil water availability, SWA) was determined to generate four water treatments: 100, 70, 40, and 10% SWA. Finally, the weight of the pot + substrate was determined for each level of SWA, assuming that the soil was at the field capacity after flooding of the pot and leaving it to drain for 48 h. The pots were weighed daily throughout the period of application of the water treatments in order to maintain the desired SWA levels.
The application of water treatments was carried out in two periods. The first period began 43 days after sowing, with very young plants that, maintained without water restrictions, had reached an average height of six cm. In this experimental phase, which lasted 56 days, the relative rate of stem elongation (RRSE) was evaluated and the shoot DM growth (leaves + stems) was measured, cutting at two cm above the soil level.
The second period, with a duration of 22 days, began after 20 days of plant regrowth, keeping all the experimental pots with 100% SWA. In this phase shoot DM growth, root DM growth, the relative water content (RWC) in the leaves, the specific leaf area (SLA = leaf area/leaf DM), and evapotranspirated water (ET) were quantified.
Assessment and calculations
Dry-matter biomass was determined by drying the samples at 65 °C in a forced-air oven until reaching a constant weight. The sum of shoot DM growth in the two growth periods was considered as total shoot DM. This value served to calculate the ratio between the growth of roots and shoots (root:shoot ratio of DM).
The RRSE (cm cm−1 day−1) was calculated based on measurements of stem length made seven times during the first experimental period: 21, 25, 29, 32, 36, 42, and 47 days after the beginning of the water treatment application. Two stems per pot were marked and on each occasion their lengths were measured with a ruler graduated in mm. RRSE is defined as the r parameter in Equation (Equation1):
The Drought-Sensitivity Index (DSI) was calculated as proposed by Fischer & Maurer (Citation1978), which method estimates the relative reduction of the DM growth of a population [1−(Y
10%/Y
100%)] in relation to the measured relative reduction of all of the population [1 − ]. The DSI was calculated with the DM growth data obtained in the treatments of 10% SWA (Y
10%) and 100% SWA (Y
100%) during the second experimental period.
The results of shoot DM growth were used to estimate the genotype×environment interaction (G×E) of the twelve populations through the methodology proposed by Finlay & Wilkinson (Citation1963). The G×E interaction was calculated with a regression between the shoot DM growth of each population and the average shoot DM growth of all the populations in the same environment (Environmental Index). The G×E interaction is the regression coefficient (b) of this relationship. To calculate the environmental index, data of shoot DM growth obtained in the four water treatments (100, 70, 40, and 10% SWA) in the two evaluation periods were used. The deviation from the regression proposed by Finlay & Wilkinson (Citation1963), estimated using the mean squares (MS), is understood as an index of the stability of DM growth (Calderini & Dreccer Citation2002; Kraakman et al., Citation2004; Inostroza et al., Citation2007).
The relative water content in the leaves was calculated using Equation (Equation3) (Teulat et al., Citation2003):
Evapotranspirated water was measured during the second experimental period by weighing the pots before and after watering.
Experimental design and statistical analysis
A completely random design in a factorial arrangement (four levels of SWA×12 populations), with two replicates (96 pots in total), was used. The data were analysed using analysis of variance (ANOVA), comparison of means (Least Significant Difference, LSD), and lineal regression in SAS (SAS Institute Inc., Citation1999).
Results
The interactions among levels of soil water availability and populations of L. tenuis were not significant for any of the variables analysed.
Stem elongation and dry-matter growth
Stem elongation (RRSE) and biomass production (shoot DM growth) varied significantly (P<0.05) among populations and levels of soil water availability (). Relative rate of stem elongation in the severe water-stress treatment (10% SWA) was 56% lower than what was observed with 100% SWA, which resulted in a reduction in shoot DM growth of similar magnitude. Among the populations this rate fluctuated between 0.009 and 0.016 cm cm−1 day−1 and shoot DM growth fluctuated between 3.7 and 5.3 g pot−1. Three populations showed a level of shoot DM growth equal to or greater than that of the cultivar Toba.
Table I. Relative stem elongation rate (RRSE), shoot dry-matter (DM), and root:shoot DM ratio of the Lotus tenuis populations and water treatments. Means of the four levels of water availability and the eleven populations, respectively.
The root:shoot ratio of DM was significantly affected by the availability of water in the soil. When water was a limiting factor (10% SWA) this ratio increased by more than 20% () in comparison with the treatment without water stress (100% SWA). The variation among populations was significant (P<0.05), fluctuating between 0.40 and 0.54. The population Lt12 presented the highest value, while the accession Lt7 presented the lowest.
Response to water stress and tolerance to drought
Relative water content and SLA varied significantly among water treatments but not among populations (). The RWC was 87.3 and 72.8% in the treatments with 100 and 10% SWA, respectively, while the SLA in the treatment without water restrictions (100% SWA) was 20% higher than what was observed in the treatment with severe water stress (10% SWA).
Table II. Relative water content (RWC), specific leaf area (SLA), and evapotranspiration (ET) of the Lotus tenuis populations and water treatments. Means of the four levels of water availability and the eleven populations, respectively.
The shoot DM growth reached during the second experimental period increased lineally with an increase in the accumulated ET of each population (). Evapotranspiration varied significantly among populations and fluctuated between 883.5 (Lt5) and 652.4 g (Lt8). As well, it went down by close to 20% under conditions of water stress ().
Figure 1. Relationship between the DM production of twelve L. tenuis populations and evapotranspiration, during the second experimental period.
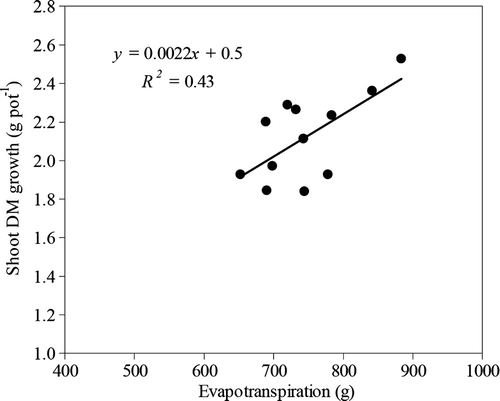
Tolerance to drought was assessed in the twelve populations of L. tenuis using the drought-sensitivity index (DSI) proposed by Fischer & Maurer (Citation1978). The DSI varied widely among the populations, from 0.49 (Lt4) to 1.34 (Toba) (). As a lower DSI-value indicates greater tolerance to drought, it can be inferred that the population Lt4 was the most tolerant, while Toba was the most sensitive.
Table III. Drought-sensitivity index (DSI), regression coefficient (b), and mean squares (MS) of the Finlay & Wilkinson methodology of twelve Lotus tenuis populations.
also presents the G×E interaction (b) and the stability of shoot DM growth (MS-values), calculated for the second period. The populations that showed the greatest response to changes in the hydric condition of soil (highest b-values) were the cultivar Toba and the accession Lt14, while that which had the least response was the accession Lt4. On the other hand, the stability of shoot DM growth (MS-values) varied between 0.001 (Lt4) and 0.106 (Lt11) ().
Discussion
Dry-matter growth
The RWC did not show differences among the populations, however, it was a good indicator of the water status of the plant and the effectiveness of the treatments of soil moisture, owing to which RWC decreased by more than 15% under conditions of water stress, which significantly affected all of the growth variables, namely RRSE, DM growth, and the root:shoot ratio of DM (). The RWC is the most commonly used expression to measure the level of water in the tissue, given that it is related to the water potential (ΨH), because the water potential and its components (Ψ H=Ψ P+Ψ π), the pressure potential (Ψ P) and the osmotic potential (Ψ π), are functions of the water volume of the protoplasm (Jones, Citation2007).
Körner (Citation1991) proposed that the root:shoot ratio of DM or the proportion between heterotrophic and autotrophic tissue is one of the most important determining facts in plant growth. Under ideal growing conditions, plants with a high root:shoot ratio of DM have a high energy cost of respiration. Nevertheless, under conditions restrictive for growth, a high value of this ratio is considered a characteristic that confers adaptability, which allows for more exploration of the soil to capture resources. In this experiment, the root:shoot ratio of DM varied significantly among populations (). The population Lt12 had the highest value but was one of the populations that showed a lower DM growth. This is due to what was stated by Körner (Citation1991), that is to say, genotypes with a high investment in heterotrophic tissue (roots) spend more energy in the process of cellular respiration. On the other hand, those genotypes that regulate the partition of photosynthates in a function of environmental conditions, in environments favorable for growth, invest less in heterotrophic tissue and more in autotrophic tissue, thus increasing photosynthetic capacity and growth. This could have occurred in population Lt14, which had one of the highest values of the root:shoot ratio of DM, RRSE, and shoot DM growth ().
Water is vital in the production of crops, given that the growth of vegetal cells is produced in large part through the action of water. The lack of this element induces less foliar area and a lower rate of photosynthesis, which results in lower biomass production. In this sense, SLA is a characteristic associated with the photosynthetic capacity of the leaves; plants with a high SLA generally have high nitrogen content and greater photosynthetic nitrogen-use efficiency (Poorter & Evans, Citation1998), which determines a higher plant growth. In this work, the SLA did not vary significantly among populations, because of which the differences observed in DM growth can be due to the activation of physiological mechanisms that regulate the loss of water from the plant and favor the growth under conditions of water stress (osmotic adjustment, stomatic regulation, water-use efficiency, among others) (Reynolds et al., Citation2001; Richards et al., Citation2002; Condon et al., Citation2004). As well, wide differences were observed in the ET rate of each population and the shoot DM growth increased lineally with increases in ET (), which corresponds with results obtained in other studies (Oweis et al., Citation2000; Angus & Herwaarden, Citation2001). This confirms the existence of physiological mechanisms that regulate water loss in the populations of Lotus tenuis.
Selection of populations tolerant to drought
The G×E interaction was estimated using the methodology proposed by Finlay & Wilkinson (Citation1963). This methodology considers a direct measurement of the response of a genotype to changes in the growing environment (favorable vs. unfavorable conditions). Those authors found various patterns of G×E interactions in their experiments and concluded that with the objective of selection, the best pattern is to choose genotypes that present a high average yield and a regression coefficient of the relationship between the yield of each genotype and environment index that was equal to one. shows the two contrasting populations in terms of their G×E interaction. The population Lt14 showed a regression coefficient (b) greater than one, which means that this population has a high response capacity to the environment. As growth conditions improve, the population Lt14 responds with increased shoot DM growth. On the other hand, the population Lt4 showed a b-value of less than one (0.51).
Figure 2. Response of contrasting L. tenuis populations to soil water treatments as calculated by the Finlay and Wilkinson methodology. Coefficients of regression (b) and determination (R 2 ).
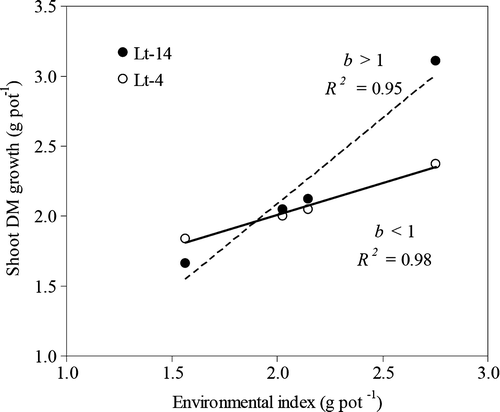
The stability of DM growth (MS-values) varied between 0.001 (Lt4) and 0.106 (Lt11) (). In high-production environments Calderini & Dreccer (Citation2002) recommended choosing genotypes that showed a high average production of DM, a high value of b, and a low value of MS. In contrast, for low-production environments, they recommend choosing genotypes that show high average DM, a low b-value, and a low MS-value.
The Lt4 population was the most tolerant to drought (lowest DSI-value), obtained the lowest b- and MS-values, and an intermediate level of DM growth, compared with the other populations (). This means that Lt4 has a low production potential, but shows good productive behavior under conditions of water stress. On the other hand, the Lt14 population showed a high DSI-value and a high b-value, which means that this population is strongly affected by water stress, but that under conditions more favorable for growth it is shows a high production potential.
The methodology developed in this study allowed us to preliminarily characterize the growth, DM production, and biomass partitioning of the Lotus tenuis germplasm naturalized in Chile. Additionally, it is a suitable way for characterizing a high number of genotypes in a short time. Despite the fact that RWC did not vary between populations, it was a good indicator of the plant-water status and allowed us to confirm the water-treatment effects. The interaction G×E estimated by the Finlay & Wilkinson methodology and the DSI, allowed us to select contrasting genotypes to drought tolerance. These results are relevant because the contrasting populations will be characterized, using molecular markers of simple sequence repeats (SSR) developed by the LOTASSA project (Hougaard et al, Citation2008), and subsequent genetic studies will identify chromosomal regions associated to genotypes tolerant or sensitive to drought. These findings will permit us to accelerate the development of drought-tolerant perennial forage plants, which will increase pastures’ productivity and enhance animal-production systems of the central zone of Chile.
In conclusion, drought tolerance genetic variability within the populations studied was found, which allowed us to select two contrasting populations, sensitive (Lt14) and tolerant (Lt4) to drought.
References
- Acuña , H. 1998 . Comparación de variedades de tres especies del género Lotus (L. corniculatus L., L. uliginosus Cav. y L. Tenuis Wald et Kit) . Agricultura Técnica , 58 , 7 14 in Spanish .
- Acuña , H. Cuevas , G. 1999 . Efecto de la altura y frecuencia de la defoliación, bajo corte y pastoreo, en el crecimiento y productividad de tres especies del género Lotus en suelos arcillosos . Agricultura Técnica , 59 , 296 308 in Spanish .
- Acuña , H. , Figueroa , M. Fuente , A. 1997 . Efecto de la profundidad de siembra y el contenido de agua del suelo en la germinación, desarrollo y crecimiento inicial de Lotus spp, en suelos arcillosos . Agro-Ciencia , 13 , 265 274 in Spanish .
- Acuña , H. , Figueroa , M. , Fuente , A. , Ortega , F. , Seguel , I. Mundana , R. 2002 . Caracterización agronómica de accesiones de Lotus glaber Mill y Lotus uliginosus Schkur. naturalizadas en Chile . Agro-Ciencia , 18 , 63 74 in Spanish .
- Acuña , H. , Concha , A. and Figueroa , M. 2008 . Condensed tannin concentrations of three Lotus species germplasm grown in different environments . Chilean Journal of Agricultural Research , 68 : 31 – 41 .
- Angus , J.F. and Herwaarden , A.F. 2001 . Increasing Water Use and Water Use Efficiency in Dryland Wheat . Agronomy Journal , 93 : 290 – 298 .
- Araus , J.L. , Slafer , G.A. , Reynolds , M. and Royo , C. 2002 . Plant breeding and drought in C3 cereals: What should we breed for? . Annals of Botany , 89 : 925 – 940 .
- Baum , M. , Grando , S. , Backes , G. , Jahoor , A. , Sabbagh , A. and Ceccarelli , S. 2003 . QTLs for agronomic traits in the Mediterranean environment identified in recombinant inbred lines of the cross ‘Arta’ x H. spontaneum 41-1 . Theoretical and Applied Genetics , 107 : 1215 – 1225 .
- Blumenthal , M.J. and McGraw , G.L. 1999 . “ Lotus adaptation, use, and management ” . In Trefoil: The Science and Technology of Lotus , Edited by: Beuselinck , P.R. 97 – 119 . Madison, Wisconsin, USA : American Society of Agronomy, Inc. & Crop Science Society of America, Inc .
- Calderini , D. and Dreccer , F. 2002 . “ Choosing genotype, sowing date, and plant density for malting barley ” . In Barley science. Recent advances from molecular biology to agronomy of yield and quality , Edited by: Slafer , G. , Molina , J. , Savin , R. , Araus , J. and Romagosa , I. 413 – 443 . New York, , USA : Food Products Press .
- Carter , E. , Theodorou , M. Morris , P. 1997 . Responses of Lotus corniculatus to environmental change I. Effects of elevated CO 2 , temperature and brought on growth and plant development . New Phytol , 136 , 245 253 .
- Condon , G. , Richards , R.A. , Rebetzke , G.J. and Farquhar , G.D. 2004 . Breeding for high water-use efficiency . Journal of Experimental Botany , 55 : 2447 – 2460 .
- Donald , C.M. 1968 . The breeding of crop ideotypes . Euphytica , 17 : 385 – 403 .
- Finlay , K. and Wilkinson , G. 1963 . The analysis of adaptation in a plant-breeding program . Australian Journal of Agricultural Research , 14 : 342 – 354 .
- Fischer , R. and Maurer , R. 1978 . Drought resistance in spring wheat cultivars. I. Grain yield responses . Australian Journal of Agricultural Research , 29 : 897 – 905 .
- Graham , P.H. and Vance , C.P. 2003 . Legumes: Importance and Constraints to Greater Use . Plant Physiology , 131 : 872 – 877 .
- Hougaard , B.K. , Madsen , L.H. , Sandal , N. , de Carvalho Moretzsohn , M. , Fredslund , J. , Schauser , L. , Nielsen , A.M. , Rohde , T. , Sato , S. , Tabata , S. , Bertioli , D.J. and Stougaard , J. 2008 . Legume Anchor Markers Link Syntenic Regions Between Phaseolus vulgaris, Lotus japonicus, Medicago truncatula and Arachis . Genetics , 179 : 2299 – 2312 .
- Hunt , R. 1990 : Basic growth analysis . Edward Arnold (Publishers) Limited .
- Inostroza , L. , del Pozo , A. , Matus , I. , Castillo , D. , Hayes , P. , Machado , S. Corey , A. 2008 . Association mapping of plant height, yield, and yield stability in recombinant chromosome substitution lines (RCSLs) using Hordeum vulgare subsp. spontaneum as a source of donor alleles in a Hordeum vulgare subsp. vulgare background . Molecular Breeding , 23 , 365 376 .
- Inostroza , L. , del Pozo , A. , Matus , I. and Hayes , P. 2007 . Drought tolerance in recombinant chromosome substitution lines (RCSLs) derived from the cross Hordeum vulgare subsp. spontaneum (Caesarea 26-24)×Hordeum. vulgare subsp. vulgare cv. Harrington . Chilean Journal of Agricultural Research , 67 : 253 – 261 .
- Jones , H.G. 2007 . Monitoring plant and soil water status: established and novel methods revisited and their relevance to studies of drought tolerance . Journal of Experimental Botany , 58 : 119 – 130 .
- Körner , C.H. 1991 . Some often overlooked plant characteristics as determinants of plant growth: a reconsideration . Functional Ecology , 5 : 162 – 173 .
- Kraakman , A.T.W. , Niks , R.E. , Van den Berg , P.M. , Stam , P. and Van Eeuwijk , FA. 2004 . Linkage disequilibrium mapping of yield and yield stability in modern spring barley cultivars . Genetics , 168 : 435 – 446 .
- Lucero , D.W. , Grieu , P. and Guckert , A. 2000 . Water deficit and plant competition effects on growth and water-use efficiency of white clover (Trifolium repens, L.) and ryegrass (Lolium perenne, L.) . Plant and Soil , 227 : 1 – 15 .
- McMahon , L.R. , McAllister , T.A. , Berg , B.P. , Majak , W. , Acharya , S.N. , Popp , J.D. , Coulman , B.E , Wang , Y. and Cheng , K.J. 2000 . A review of the effects of forage condensed tannins on ruminal fermentation and bloat in grazing cattle . Canadian Journal of Plant Science , 80 : 469 – 485 .
- Nguyen , H. 1999 . “ Molecular dissection of drought resistance in crop plants: from traits to genes ” . In Molecular Approaches for the Genetic Improvement of Cereals for Stable Production in Water-Limited Environments , Edited by: Ribaut , J.M. and Poland , D. 36 – 40 . Mexico D.F : CIMMYT .
- Oweis , T. , Zhang , H. and Pala , M. 2000 . Water Use Efficiency of Rainfed and Irrigated Bread Wheat in a Mediterranean Environment . Agronomy Journal , 92 : 231 – 238 .
- Passioura , J. 2007 . The drought environment: physical, biological and agricultural perspectives . Journal of Experimental Botany , 58 : 113 – 117 .
- Poormohammad , S. , Grieu , P. , Maury , P. , Hewezi , T. , Gentzbittel , L. and Sarrafi , A. 2007 . Genetic variability for physiological traits under drought conditions and differential expression of water stress-associated genes in sunflower (Helianthus annuus L.) . Theoretical and Applied Genetics , 114 : 193 – 207 .
- Poorter , H. and Evans , J.R. 1998 . Photosynthetic nitrogen-use efficiency of species that differ inherently in specific leaf area . Oecologia , 116 : 26 – 37 .
- Real , D. , Warden , J. , Sandral , G.A. and Colmer , T.D. 2008 . Waterlogging tolerance and recovery of 10 Lotus species . Australian Journal of Experimental Agriculture , 48 : 480 – 487 .
- Reynolds , M. , Rajaram , S. and Sayre , K. 1999 . Physiological and genetics changes of irrigated wheat in the post-green revolution period and approaches for meeting projected global demand . Crop Science , 39 : 1611 – 1621 .
- Reynolds , M.P. , Ortiz-Monasterio , J.I. McNab , A. 2001 . Application of Physiology in Wheat Breeding . CIMMYT. Mexico D.F .
- Richards , R.A. , Rebetzke , G.J. , Condon , A.G. and Herwaarden , A.F. 2002 . Breeding Opportunities for Increasing the Efficiency of Water Use and Crop Yield in Temperate Cereals . Crop Science , 42 : 111 – 121 .
- SAS Institute Inc 1999 . SAS OnlineDoc®, Version 8, Cary, NC, SAS Institute Inc.
- Striker , G. , Insausti , P. , Grimoldi , A. , Ploschuk , E. and Vasellati , V. 2005 . Physiological and Anatomical Basis of Differential Tolerance to Soil Flooding of Lotus corniculatus L. and Lotus glaber Mill . Plant and Soil , 276 : 301 – 311 .
- Taleisnik , E. , Pérez , H. , Córdoba , A. , Moreno , H. , García Seffino , L. , Arias , C. , Grunberg , K. , Bravo , S. and Zenoff , A. 1998 . Salinity effects on the early development stages of Panicum coloratum: cultivar differences . Grass & Forage Science , 53 : 270 – 278 .
- Teakle , N.L. , Flowers , T.J. , Real , D. and Colmer , T.D. 2007 . Lotus tenuis tolerates the interactive effects of salinity and waterlogging by ‘excluding’ Na+ and Cl- from the xylem . Journal of Experimental Botany , 58 : 2169 – 2180 .
- Teulat , B. , Zoumarou-Wallis , N. , Rotter , B. , Salem , M.B. , Bahri , H. and This , D. 2003 . QTL for relative water content in field-grown barley and their stability across Mediterranean environments . Theoretical and Applied Genetics , 108 : 181 – 188 .
- Udvardi , M.K. , Tabata , S. , Parniske , M. and Stougaard , J. 2005 . Lotus japonicus: legume research in the fast lane . Trends in Plant Science , 10 : 222 – 228 .