Abstract
Screening of natural biodiversity for the variation in quality traits is of prime importance for quality-breeding programmes. The objective of this investigation was to select candidate accession of hot pepper having high concentrations of ascorbic acid, capsaicin, β-carotene, and total phenols for use as parents in breeding for these compounds. Forty-two accessions of pepper (Capsicum annuum) were field grown and their mature fruits were analysed for their functional and nutritional composition. Wide variations were observed in most of the measurements, e.g. ascorbic acid (25–217mg per 100g), total phenolics (38.4–188.1mg per 100g catechol eq.), and capsaicin (0.08–0.67%), suggesting that there are considerable levels of genetic diversity. Across all accessions the concentration of ascorbic acid was negatively correlated with that of β-carotene (r=−0.33, p <0.05). Concentrations of L-ascorbic acid were significantly greater in VLC 22-I-2-1, VLC-29-II-1-1, VLC-30-I-1, and Janjeera Mirch compared with other accessions analysed. Total capsaicin concentrations were greatest (0.67%) in VLC-30-II-1 and lowest (0.08%) in VLC-32-3. Four factors were computed by principal-component analysis to explain 67% of the variation in the traits. The great variability for these phytochemicals suggests that these selected accessions may be useful as parents in breeding programmes to produce fruits with value-added traits.
Introduction
Exploring natural biodiversity as a source of novel alleles to improve the productivity, adaptation, quality and nutritional value of crops, is of prime importance in 21st century breeding programmes (Fernie et al., Citation2006). Efforts are going on to improve the quality of not only grains but also vegetable crops (Romer et al., Citation2000).
Pepper is an important agricultural crop, not only because of its economic importance, but also for the nutritional value of its fruits, mainly due to the fact that vegetables are an excellent source of natural colours and antioxidant compounds (Lee et al., Citation1995; Howard et al., Citation2000). It is an excellent source of vital micronutrients such as vitamin C and vitamin E (Minguez-Mosquera & Hornero-Mendez, Citation1994; Daood et al., Citation1996; Gnayfeed et al., Citation2001). This high-value crop is commercially cultivated in Mexico, China, Korea, the East Indies, USA, and many countries of the Indian sub-continent (DeWitt & Bosland, Citation1993). Total world production of hot pepper has been estimated to be 14–15 million tonnes a year (Weiss, Citation2002).
Capsaicinoids (heat principle) have aroused great interest lately owing to their biological and therapeutic importance (Govindarajan & Sathyanarayana, Citation1991; Surh & Lee, Citation1996; Szolcsanyi, Citation2004). Capsaicinoids are alkaloids specific for Capsicum spp.
A wide spectrum of antioxidant compounds is present in pepper fruits. It includes antioxidant vitamins (vitamin E, vitamin C, and vitamin A), phenolic compounds, carotenoids, and xanthophylls. One of the important antioxidant groups is phenolic compounds, which occur in peppers in connection with sugars (Materska et al., Citation2003a, Citationb). The level of antioxidants differs between accessions; generally, hot peppers are a better source than are the sweet ones (Daood et al., Citation1996; Gnayfeed et al., Citation2001). Phenolic compounds retard or inhibit lipid autoxidation by acting as radical scavengers (Namiki, Citation1990) and, consequently, are essential antioxidants that protect against propagation of the oxidative chain. Another important antioxidant, vitamin C, an important compound of pepper fruits, chelates heavy-metal ions (Namiki, Citation1990), reacts with singlet oxygen and other free radicals, and suppresses peroxidation, reducing the risk of arteriosclerosis, cardiovascular diseases, and some forms of cancer (Harris, Citation1996). Carotenoid pigment, vitamin E, and vitamin C are located in the pericarp of pepper fruit, whereas capsaicinoids are distributed in different parts.
There is a lack of studies evaluating available genetic variability, especially for quality in given sets of germplasm. Studies related to pepper as a functional food or as a source of micronutrient are not meagre. However, a systematic screening study for the quality evaluation of hot pepper and use of this information for the development of better varieties is lacking. Therefore, the present study was undertaken to evaluate hot-pepper accessions for breeding better quality hot-pepper varieties.
Materials and methods
Plant material
Pepper accessions (Capsicum sp.) (42) were grown in a field experiment in kharif season (June–September) 2007, at an experimental farm in Hawalbagh (29°36″ N, 79°40″ E; 1250 m above msl). All accessions received similar water and fertilizer treatments. Peppers were harvested at mature stage. Three replicates of 42 peppers were analysed for different functional and nutritional attributes. Accessions were VLC 21-II-3-1, VLC 21-II-3-2, VLC 22-I-2-1, VLC 22-I-2-2, VLC-32-1, VLC-32-3, VLC-32-4, VLC-34-2, VLC-16-I-1, VLC-16-I-2, VLC-16-II-1, VLC-16-II-3, VLC-20-I-2, VLC-20-II-1, VLC-22-1, VLC-25-1, VLC-28-II-1, VLC-29-1, VLC-29-II-1-1, VLC-29-II-1-2, VLC-30-II-1, VLC-30-II-1, VLC-31-I-1, VLC-18-1-1, VLC-18-1-2, VLC-22-IV-1, VLC-22-V-1-1, VLC-22-V-1-2, VLC-22-VII-1, VLC-23-I-1-1, VLC-23-II-2-1, VLC-23-II-2-2, VLC-24-I-1, VLC-28-II-1, VLC-28-III-1, VLC-30-I-1, P. Jwala, Lakhori Mirch, Janjeera Mirch, Berry Mirch, Lal Mirch, and Sadabahar.
Chemicals and reagents
All chemicals and reagents were procured from Merck, India Ltd. Double-distilled water was used throughout the analysis.
Chromatographic conditions
For the estimation of β-carotene and ascorbic acid content in pepper berries, an HPLC system (Shimadzu, Japan) was used. The HPLC system was operated on a Shimadzu HPLC system equipped with an Hitachi pump (L-7100) and UV-Vis detector (L-7400) controlled by Win Chrom chromatographic software. The HPLC column was a Purospher®, RP- C18 (4.6×250 mm I.D; 5 µ). Samples were injected in 20-µl volumes at ambient temperature. Quantification of β-carotene/ascorbic acid present in the samples was achieved by comparing each peak's retention time and area with those of the standard.
Chemical analysis
Determination of ascorbic acid content
L-Ascorbic acid (LAA) was extracted and quantified by HPLC as described by Abdulnabi et al. (Citation1997) with minor modifications. The sample (10 g) was homogenized with a solution (10 ml) containing metaphosphoric acid (0.3 M) and acetic acid (1.4 M) for 15 minutes at room temperature. The mixture was filtered through Whatman No. 4 paper to obtain a clear extract. All samples were extracted in triplicate. Mobile phase was acetonitrile–methanol–tetrahydrofuran (45:50:5; v/v/v) at a flow rate of 1.0 ml min−1, and LAA was detected at 254 nm.
Determination of β-carotene content
β-Carotene in the pepper samples was extracted according to the method of Ismail & Fun (Citation2003) with minor modifications. The β-carotene standard ( in hexane) was obtained from Sigma Chemical Co. (St. Louis, MO). Pepper samples (10 g) were extracted with a mixture of 40 ml of ethanol (99.8%) and 10 ml of 100% (w/v) potassium hydroxide and homogenized for three minutes. The mixture was saponified by heating for 30 minutes. Then, the mixture was partitioned thrice in n-hexane, followed by washing with distilled water and then passed through sodium sulfate. Hexane was removed under reduced pressure at 45 °C using a rotary evaporator. The standards and pepper isolates were dissolved in 10 ml of hexane prior to HPLC analysis. A mobile phase ran at 0.8 ml min−1 and consisted of water containing 0.01% formic acid–acetonitrile (95:5; v/v). β-Carotene was detected at 450 nm using a UV-Vis detector. The column was equilibrated to the original mobile phase concentration prior to the next sample injection.
Determination of total phenolics content
Total phenolics content of the methanol extracts was determined by the Folin–Ciocalteu assay and catechol was used as standard (Singleton & Rossi, Citation1965). A sample (500 mg) was weighed into 50-ml plastic extraction tubes and vortexed with 25 ml of extraction solvent (80% ethanol). Then, the sample with the extraction solvent was heated at 60 °C (water-bath) for 1 h, allowed to cool to room temperature, and homogenized for 30 s with a sonicator. Two hundred and fifty microlitres (three replicates) were introduced into screw-cap test tubes; 1.0 ml of Folin–Ciocalteu's reagent and 1.0 ml of aq. sodium carbonate (7.5%) were added. The tubes were vortexed and heated for 15 minutes at 45 °C. The absorption at 765 nm was measured (Model U 2001, Hitachi UV/Vis spectrophotometer) and the total phenolic content was expressed as catechol equivalents in mg per 100 g of dry material.
Determination of capsaicin content
Capsaicin content was determined using a spectrophotometer. A sample (500 mg) was weighed into a 50-ml plastic extraction tubes and extracted with 10 ml of extraction solvent (acetone) for 3 h. Then, the combined sample and extraction solvent was centrifuged and a 1-ml extract was taken in a beaker and evaporated. The residue was dissolved in 0.4% aq. sodium hydroxide (5 ml) and 3 ml of 3% phosphomolybdic acid was added. The mixture was kept for 1 h and absorbance was taken at 650 nm after centrifugation. The capsaicin content was expressed as per cent dry material.
Determination of nutritional attributes
Peppers were analysed for nutrient parameters after di-acid digestion (HNO3–HClO4; 10:4 v/v). The K content was determined by flame photometry, while Fe, Zn, Cu, and Mn contents were analysed by using an atomic absorption spectrophotometer. Phosphorcus (P) was estimated photometrically via development of the phosphomolybdate complex (Taussky & Shorr, Citation1953).
Statistical analysis
Data represent the mean of three replicate samples for each pepper type and maturity stage. The genotypic mean value of each parameter was used for statistical analysis using the SPSS program (Version 10; SPSS Inc., Chicago, Illinois, USA). Correlation analysis, Principal-Component Analysis (PCA; Brereton, Citation2003), and cluster analysis were performed using SPSS.
Results and discussion
HPLC Methodology
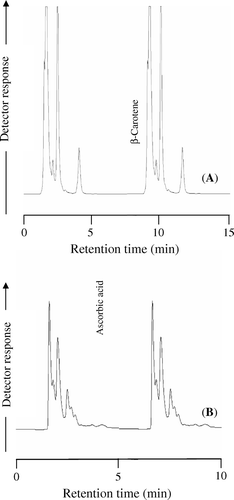
Saponification prior to HPLC analysis has been recommended to remove chlorophyll and to hydrolyse carotenol esters (Scott, Citation1992). The lack of saponification in peppers prior to analysis can result in underestimation of carotenoid values because these compounds are esterified to fatty acids in immature fruit (Minguez-Mosquera & Hornero-Mendez, Citation1994; Hart & Scott, Citation1995; Minguez-Mosquera & Perez-Galvez, Citation1998). Several investigators have noted greater carotenoid extraction at elevated saponification temperatures, but usually at the expense of xanthophyll recovery due to thermal degradation (Khachik et al., 1986; Scott, Citation1992). In our study, preliminary work determined that 40 °C was the optimal saponification temperature that maximized the retention of both xanthophyll and non-oxygenated carotenoids. A chromatogram of β-carotene in a typical pepper extract is shown in part A of .
The polar or lipophobic extract of pepper contains ascorbic acid, the major precursor of vitamin-C, and capsaicinoids, the pungency principle. A chromatogram of L-ascorbic acid in a typical pepper extract is shown in part B of .
Variations in pepper properties
Wide variation was observed in most of the evaluated attributes (). This is due to the wide genetic basis of the tested pepper genotypes. Wide variation (0.20–0.36 mg per 100 g) was observed in β-carotene content in evaluated pepper accessions, suggesting that there are considerable levels of genetic diversity. Our report was not consistent with the report by Gnayfeed et al. (Citation2001), where it was reported that paprika red pepper contains 171–250 µg g−1 β-carotene, but it was consistent with the report by Howard et al. (Citation2000). The latter reported 337–800 µg per 100 g β-carotene in Capsicum annuum fresh fruit. Ascorbic acid content in pepper accessions ranged from 25–217 mg per 100 g, consistent with the reports by Howard et al. (Citation2000) and Gnayfeed et al. (Citation2001). L-Ascorbic acid content in Capsicum annuum fruit was reported to be 102–202 mg per 100 g fresh fruit (Howard et al., Citation2000). Total phenolics ranged between 38.4–188.1 mg per 100 g catechol equivalent. Howard et al. (2001) reported 285–571 mg per 100 g chlorogenic acid equivalent, whereas Conforti et al. (Citation2007) reported 43.2 mg g−1 in Capsicum annuum fruit at maturity. Capsaicin content ranged between 0.08–0.67%, which is far below that reported by Contreras-Padilla & Yahia (Citation1998). It was reported that paprika accession contains 383–1075 µg g−1 (Gnayfeed et al., Citation2001) and 997–1240 µg g−1 (Ayuso et al., Citation2008) dry matter capsaicinoids. Phosphorus and potassium content in pepper ranged between 0.33–0.59 and 2.9–6.7% (dry-matter basis). It was reported that physiologically mature peppers are richer in mineral content than are green ones (Jadczak & Grzeszczuk, Citation2004). Iron and zinc content ranged from 146–317 µg g−1 and 11.4–32.6 µg g−1, respectively.
Table I. Basic statistics for properties of 42 tested pepper accessions.
Frequency distribution of pepper accessions
Forty-two accessions used for the study included nine traits, comprising three functional and six nutritional traits. Frequency distribution for potassium and phosphorus content amongst the accessions was also classified into four groups (B). Similarly, micronutrients were also grouped into four classes (C). For iron, the first two groups contributed maximum accessions. Frequency distribution for zinc is also presented in C. Group 2 contributed more than 60% out of 42 tested accessions. The first, third, and fourth groups comprised 5, 9, and 1 pepper accessions.
Figure 2. Frequency distribution of (A) β-carotene, ascorbic acid, total phenolics, and capsaicin content, (B) potassium and phosphorus, (C) zinc, copper, iron, and manganese content for the 42 hot pepper accessions.
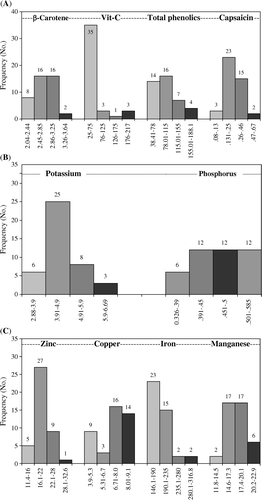
Frequency distribution for β-carotene amongst the accessions studied averaged 0.28 mg per 100 g, which was classified into four groups with 8, 16, 16, and 2 accessions in each group respectively (A). For ascorbic acid and total phenolics, content was also classified into four groups. In the case of ascorbic acid, the first group comprised more than 80% of total accessions, whereas in the case of total phenolics, the four groups comprised 14, 16, 7, and 4 accessions. For capsaicin content pepper accessions were classified into four groups. The second and third groups comprised 90% of the total accessions, which accounted for 38 out of 42.
Correlation between textural and functional/nutritional attributes
Few significant correlation coefficients among traits from –0.37 (copper versus ascorbic acid) to 0.66 (e.g., phosphorus versus potassium) were observed, but most values were low (). Manganese was correlated with four other nutritional attributes. It was highly correlated with potassium and phosphorus (r = 0.50, 0.45, p < 0.01). Interestingly, ascorbic acid was negatively correlated with both β-carotene and copper content (r =−0.33, −0.37, p < 0.01). Total phenolics was significantly correlated with iron content. Capsaicin content was not correlated with any other attributes.
Table II. Correlation coefficients of functional and nutritional attributes.
Statistical procedure for classification
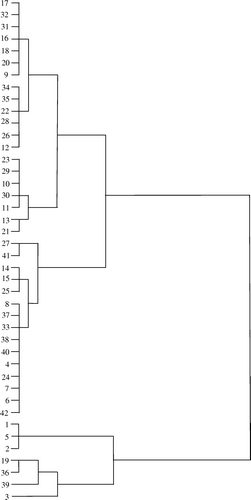
In order to see patterns of clustering between the hot pepper accessions studied, hierarchical cluster analysis was used. The data matrix included as objects each of the nine attributes analysed for the forty-two accessions. The variables were the attributes described in the Experimental section. Pearson correlation was used as similarity criterion and furthest neighbour as a clustering method (). Using a similarity level 42 pepper accessions were classified into mainly four groups (). An important conclusion is obtained from this: based on composition, the differences between the accessions studied are still great enough to classify them correctly, on the basis of the variables introduced in the present analysis.
Figure 3. Dendogram showing the relationship among 42 pepper accessions based on the ten quality attributes.
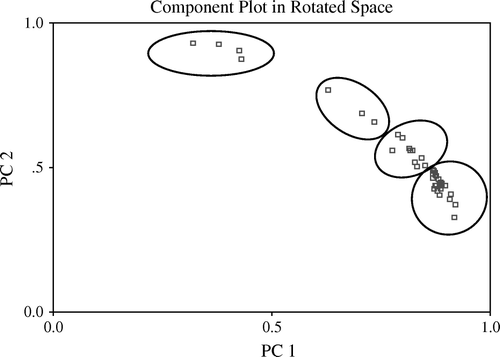
The complex relationship among the traits measured indicates the multifactor aspect of pepper quality (). To summarize the information of the data matrix briefly, factor analysis was applied using PCA as the method from extraction of factors. After orthogonal transformation four components (PC) were obtained with Kaiser criterion (taking eigenvalues greater than unity) that explained 66.5% of the variance (). Rotated Varimax solutions () show that PC1 is related mainly to potassium content, PC2 to L-ascorbic acid content, PC3 to zinc content, and PC4 to total phenolics. When the scores of each pepper sample were represented in the plane generated by PC1 and PC2 (potassium and ascorbic acid, ) the following information was obtained:
Table III. Matrix of varimax rotated components. Significant factor loadings are markedin bold.
Table IV. Variance explained by principal components.
Accessions with low potassium and high ascorbic acid content: VLC 22-I-2-1 (3), VLC-29-II-1-1 (19), VLC-30-I-1 (36), Janjeera Mirch (39); accessions with medium potassium and medium ascorbic acid content: VLC 21-II-3-1, VLC 21-II-3-2, VLC-32-1; accessions with high potassium and low ascorbic acid content: rest of the accessions.
Factor 1 explained the largest proportion of the variance (22.8%), and other four factors also explained similar levels of variance (10.2–19.6%). Important variables integrated by the first factor were potassium, phosphorus, and manganese content (). The second factor was correlated mainly with L-ascorbic acid, β-carotene, and copper. Ascorbic acid negatively influenced the factor, whereas β-carotene influenced positively. Again, this matches the correlation between L-ascorbic acid and β-carotene (). Factor 3 was zinc and iron, explaining 13.8% of the variance. Factor 4 explains the least percentage (10.2%) of variability as compared with the other three factors. It consisted of the total phenolics and capsaicin.
Using similarity level, pepper accessions were classified into four groups (). The lowest dissimilarity was exhibited by three accessions (1 vs 2 vs 5), indicating low level of genetic diversity. The dendrogram of 42 pepper accessions showed 4 clusters (). Cluster 1 consisted of 20 genotypes [starting from top (17) to 21], all of which were advance breeding lines developed by the breeders. Cluster 2 comprised a mixture of 15 landrace genotypes (numbers 27 to 42 in the Figure) collected from different areas of India with the exception of five commercial cultivars. Three accessions (numbers 1, 5, and 2) formed cluster 3. Cluster 4 consisted of 4 genotypes (numbers 19, 36, 39, and 2).
All these important traits may be used in the breeding programmes to increase variability for different functional/nutritional characteristics and to make suitable selections that are acceptable to consumers. The variability in different quality traits will be useful to obtain appreciable responses to selection for these traits. These results are also potentially useful in the efficient conservation of an important part of the agricultural biodiversity of India.
Acknowledgements
The senior author thanks Dr R.S. Rawal and Mr Harish Andola, GBPIHED, Kosi-Katarmal, Almora for assisting in the HPLC analysis.
References
- Abdulnabi , A.A. , Emhemed , A.H. , Hussein , G.D. and Biacs , P.A. 1997 . Determination of antioxidant vitamins in tomatoes . Food Chemistry , 60 : 207 – 212 .
- Ayuso , M.C. , Bernalte , M.J. , Lozano , M. , Garcia , M.I. , Perez , M.M , Hernandez , M.T. and Somogyi , N. 2008 . Quality characteristics of different red pepper accessions (Capsicum annuum L.) for hot paprika production . European Food Research Technology , 227 : 557 – 563 .
- Brereton , R.G. 2003 . Chemometrics. Data Analysis for the Laboratory and Chemical Plant , Chichester, , England : John Wiley & Sons .
- Conforti , F. , Giancarlo , A.S. and Menichini , F. 2007 . Chemical and biological variability of hot pepper fruits (Capsicum annuum var. acuminatum L.) in relation to maturity stage . Food Chemistry , 102 : 1096 – 1104 .
- Contreras-Padilla , M. and Yahia , E.M. 1998 . Changes in capsaicinoids during development, maturation, and senescence of chile peppers and relation with peroxidase activity . Journal of Agricultural and Food Chemistry , 46 : 2075 – 2079 .
- Daood , H.G. , Vinkler , M. , Markus , F. , Hebshi , E.A. and Biacs , P.A. 1996 . Antioxidant vitamin content of spice red pepper (paprika) as affected by technological and varietal factors . Food Chemistry , 55 : 365 – 372 .
- DeWitt , D. and Bosland , P.W. 1993 . The Pepper Garden , Berkeley, CA : Ten Speed Press .
- Fernie , A.R. , Tadmor , Y. and Zamir , D. 2006 . Natural genetic variation for improving crop quality . Current Opinion in Plant Biology , 9 : 196 – 202 .
- Gnayfeed , M.H. , Daood , H.G. , Biacs , P.A. and Alcaraz , C.F. 2001 . Content of bioactive components in pungent spice red pepper (paprika) as affected by ripening and maturity . Journal of the Science of Food and Agriculture , 81 : 1580 – 1585 .
- Govindarajan , V.S. and Sathyanarayana , M.N. 1991 . Capsicum-production, technology, chemistry and quality. Part V. Impact on physiology, pharmacology, nutrition and metabolism: structure, pungency, pain and desensitization sequences . CRC Critical Reviews in Food Science and Nutrition , 29 : 435 – 474 .
- Harris , J.R. 1996 . Subcellular biochemistry, ascorbic acid: biochemistry and biomedical cell biology , Vol. 25 , New York : Plenum .
- Hart , D.J. and Scott , K.J. 1995 . Development and evaluation of an HPLC method for the analysis of carotenoids in foods, and the measurement of the carotenoid content of vegetables and fruits commonly consumed in the UK . Food Chemistry , 54 : 101 – 111 .
- Howard , L.R. , Talcott , S.T. , Brenes , C.H. and Villalon , B. 2000 . Changes in Phytochemical and Antioxidant Activity of Selected Pepper Accessions (Capsicum Species) As Influenced by Maturity . Journal of Agricultural and Food Chemistry , 48 : 1713 – 1720 .
- Ismail , A. and Fun , C.S. 2003 . Determination of vitamin C, β-carotene and riboflavin contents in five green vegetables organically and conventionally grown . Malasian Journal of Nutrition , 9 : 31 – 39 .
- Jadczak , D. and Grzeszczuk , M. 2004 . Determination of content of mineral components in fruits of hot and semi-hot pepper accessions . Journal of Elementology , 9 : 15 – 23 .
- Khachik , F. , Beecher , G.R. and Whittaker , N.F. 1995 . Separation, identification, and quantification of the major carotenoid and chlorophyll constituents in extracts of several green vegetables by liquid chromatography . Journal of Agricultural and Food Chemistry , 34 : 603 – 616 .
- Lee , Y. , Howard , L.R. and Villalon , B. 1995 . Flavonoids and antioxidant activity of fresh pepper (Capsicum annuum) accessions . Journal of Food Science , 60 : 473 – 476 .
- Materska , M. , Perucka , I. , Stochmal , A. , Piacente , S. and Oleszek , W. 2003a . Quantitative and qualitative determination of flavonoids and phenolic acid derivatives from pericarp of hot pepper fruit cv. Bronowicka Ostra . Polish Journal of Food and Nutrition Science , 12/53 : 72 – 76 .
- Materska , M. , Piacente , S. , Stochmal , A. , Pizza , C. , Oleszek , W. and Perucka , I. 2003b . Isolation and structure elucidation of flavonoid and phenolic acid glycosides from pericarp of hot pepper fruit Capsicum annuum L . Phytochemistry , 63 : 893 – 898 .
- Minguez-Mosquera , M.I. and Hornero-Mendez , D. 1994 . Formation and transformation of pigments during the fruit ripening of Capsicum annuum cv. Bola . Journal of Agricultural and Food Chemistry , 42 : 38 – 44 .
- Minguez-Mosquera , M.I. and Perez-Galvez , A. 1998 . A study of lability and kinetics of the main carotenoid pigments of red pepper in the de-esterification reaction . Journal of Agricultural and Food Chemistry , 46 : 566 – 569 .
- Namiki , M. 1990 . Antioxidants/antimutagens in food . CRC Critical Reviews in Food Science and Nutrition , 29 : 273 – 300 .
- Romer , S. , Fraser , P.D. , Kiano , J.W. , Shipton , C.A. , Misawa , N. , Schuch , W. and Bramley , P.M. 2000 . Elevation of the provitamin A content of transgenic tomato plants . Nature Biotechnology , 18 : 666 – 669 .
- Scott , K.J. 1992 . Observations on some of the problems associated with the analysis of carotenoids in foods by HPLC . Food Chemistry , 45 : 357 – 364 .
- Singleton , V.L. and Rossi , J.A. 1965 . Colorimetry of total phenolics with phosphotungstic–phosphomolybdic acid reagents . American Journal of Enology and Viticulture , 16 : 144 – 157 .
- Surh , Y. and Lee , S.S. 1996 . Capsaicin in hot chilli pepper: carcinogen, co-carcinogen or anti-carcinogen? . Food Chemistry and Toxicology , 34 : 313 – 316 .
- Szolcsanyi , J. 2004 . Forty years in capsaicin research for sensory pharmacology and physiology . Neuropeptides , 38 : 377 – 384 .
- Taussky , H.H. and Shorr , E. 1953 . A microcolorimetric method for the determination of inorganic phosphorus . Journal of Biological Chemistry , 202 : 675 – 682 .
- Weiss , E.A. 2002 . World Production and Trade , Wallingford, , UK : CABI Publishing, CAB International .