Abstract
A range of locally available liming materials of different geological origin, particle size, and application rate were investigated in 15 field sites over a period of 8 years. At 5 sites, lime addition to soils caused average yield losses of 7%. Concentrations of Mn and Cu but not of boron and zinc in grains declined in limed plots. Decline in concentrations of Mn and Cu were significantly correlated with increasing soil pH-values. Crop data indicated that concentrations of Mn and Cu in grains reached low, critical levels. Yields declined at threshold values amounting to 15 mg Mn kg−1 for wheat and barley, 25 mg Mn kg−1 for rye, 30 mg Mn kg−1 for oat, and 3 mg Cu kg−1 for the four cereals.
Introduction
Liming of agricultural soils is effective to correct soil chemical constraints such as acidification and aluminum(III) dissolution. In general, lime has a positive effect on crop production and can increase crop yields by up to 70% (e.g., Farhoodi & Coventry, Citation2008). Concerning micronutrients, liming increases the solubility of molybdenum but the solubility of manganese, zinc, iron, boron, and copper is reduced (Lindsay, Citation1974; Fageria et al., Citation2002). Consequently, trace-metal concentrations in crops decrease at increasing soil pH-values (e.g., Öborn et al., Citation1995). Therefore, lime additions may reduce plant availability of micronutrients, which also can result in lower crop yields. Negative effects of lime applications to soil on crop yields have been ascribed to mineral imbalances or deficiency. The following mechanisms have been reported (a) calcium–magnesium imbalance (Carran, Citation1991), (b) iron deficiency due to high bicarbonate levels blocking iron(III) reduction in the root (Mengel & Kirkby, Citation2001), (c) zinc deficiency (Hylander, Citation1995), and (d) manganese deficiency (Kowalenko et al., Citation1980). Although micronutrient disorders in calcareous soils of the Mediterranean region are well documented (Rashid & Ryan, Citation2004), reports on yield losses caused by micronutrient deficiency after lime addition are relatively scarce.
The hypothesis tested in this paper is whether yield losses upon liming could be attributed to a decrease in micronutrient availability in soil as plant availability of micronutrients with the exception of Mo decreases at higher soil pH-values. Concentrations of micronutrients in cereal grains were measured from field sites where yield losses were observed. The aim was to find out (a) if liming resulted in diminishing micronutrient concentrations in grains, and (b) if micronutrients may reach critical levels affecting crop yields.
Material and methods
The study is based on field trials run from 2000 to 2003 at 15 sites testing 14 commercial Swedish lime products. Samples of cereal grains from treatments showing negative yield responses after lime application were used for the investigation. Treatments included limestones from different geological origin both powdered and crushed as well as dolomite, lime waste from sugar production, calcium hydroxide, and converter slag. Initial soil pH-values (in water) at the sites ranged from 4.8 to 6.3. Lime was applied to achieve a base saturation of 70–85% and ≥100%, respectively. Crops grown included winter and spring cereals, potatoes, peas, oil seed rape, and grass/clover forage. Textures at the sites varied from sand to clay including one organic soil. Recommended rates of inorganic fertilization were applied based on soil P and K analysis and crop type differing between sites.
Grain samples from limed and non-limed sites were analysed on boron, zinc, copper, and manganese to find out if low uptake of these micronutrients may explain yield reductions. Samples were analysed upon digestion in concentrated nitric acid and the micronutrients were determined on an inductively coupled plasma–mass spectrometer (Elan 6100 ICP-MS, Perkin Elmer SCIEX instruments). Metal concentrations are expressed in mg per kg grain dry weight (dm). In addition, soil pH-values (measured in water) were determined. Data were analysed with the statistics provided with Microsoft Excel (Microsoft Corp., Redmond, WA). Curve fits and correlations were made using Sigma Plot (SPSS Inc., Chicago, IL).
Results
At five out of the 15 sites, yields of crops declined after lime addition at a number of occasions as compared with control treatments including cereals, potatoes, peas, and oil seed rape (). However, no deficiency symptoms have been observed at any occasion. Furthermore, yield losses were only statistically significant for some of the crops. Large yield declines of up to 25% were rare and losses of less than 10% were most frequent. Liming soils to 70–85% of their base saturation resulted in an average yield loss of 6%, and liming up to or above 100% base saturation to an average reduction of 8%. Only cereal crops were used for further evaluation.
Table I. Soil and yield response upon liming at five field sites during the years 2000–2003.Figures in parenthesis indicate relative yields as compared with the control.
In order to find out if lime additions reduced the plant availability of micronutrients in soil, concentrations in cereal grains were plotted against soil pH-values. Such analysis showed that lime additions had no significant effect on boron and zinc concentrations in grains (data not shown). Boron concentrations in grains remained around 5 mg kg−1 dm independent of soil pH, which are actually higher than values reported for cereals from different parts of the world (Kabata-Pendias, Citation2001). Also zinc concentrations in grains remained around 30 mg kg−1 dm, which is close to mean values measured in grains (Eriksson et al., Citation2000). Shortage of boron or zinc in crops due to liming was excluded.
In contrast, plotting Mn and Cu concentrations in grains versus soil pH-values showed significant relationships. Concentrations in grains declined with increasing pH-values (). For example, liming soils to pH-values of 6.5 and above resulted in a decline of Mn in wheat grain to around 10 mg kg−1 dm, which is very low, representing less than 5% of wheat samples in a Swedish survey (Eriksson et al., Citation2000). Similarly, Mn concentrations in oat grain declined at pH-values between 6 and 7 to approximately 30 mg kg−1 dm, which is far below the average of 47 mg Mn kg−1 dm reported by Eriksson et al. (Citation2000). The same trend was found for Mn in barley grain, reaching 10 mg kg−1 dm at soil pH-values of 6.5 as compared with the average value of 17 mg Mn kg−1 dm for Swedish barley grain (Eriksson et al., Citation2000). Concerning copper, liming caused a significant decline of concentrations in grains from ∼ 4.5 mg kg−1 dm at a soil pH of 6 to a minimum between 2.5 and 3 mg Cu kg−1 dm at a pH of 7 (). In contrast to Mn, mean Cu concentrations did not deviate greatly in the different cereal species (3.0 for wheat, 4.1 for oat, 3.8 for barley, and 2.3 mg Cu kg−1 dm for rye).
Figure 1. Dry-matter concentrations of manganese and copper in grain in relation to soil pH-values. Data refer to sites, crops and treatments shown in but not all soil pH-values were available. Manganese in rye was not plotted due to too few soil pH-values being available.
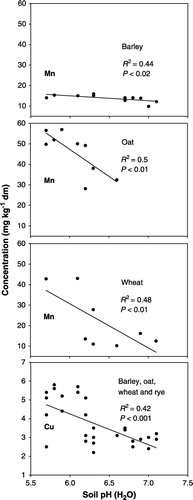
The question arises as to whether declines of Cu and Mn in grains correlate with yield losses. Alloway (Citation2005) pointed out that moderate yield losses of up to 10% as observed in these trials could be due to low levels of micronutrients in crops.
Plotting metal concentrations in grains against relative yields (whereby the maximum yield from each site was set to 100) showed that yields declined with decreasing concentration at certain levels (). A sigmoidal function, Hill-equation with 3 parameters (Hunt, Citation1982) was used to fit Mn and Cu concentrations in grains against yields. For barley, oat, and rye, yields declined significantly (P<0.05) with decreasing Mn concentration in grains. The same was found for copper and crop yield.
Figure 2. Dry-matter concentrations of Mn and Cu in grain related to crop yield. Data refer to sites, crops and treatments shown in .
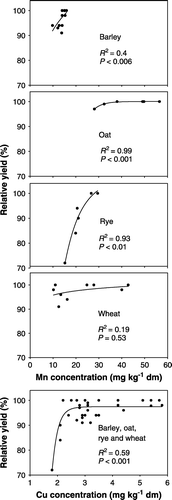
Data in were also used to examine concentrations in grains below which yield declines can be expected. For manganese, concentrations in barley and wheat of less than 15 mg kg−1, in rye concentrations of less than 25 mg kg−1, and in oat of less than 30 mg kg−1 were identified as critical threshold values. Concerning copper, concentrations less than 3 mg kg−1 were identified for the four types of cereals. Further investigations are required to corroborate our observations.
Discussion
Critical concentrations of micronutrients in plant analysis most often refer to growing tops and leaves. Still, grain analyses have also been used in the interpretation of sufficient concentrations. Alloway (Citation2005) compiled critical Cu concentrations in soils and crops and came up with a concentration of 2 mg kg−1 dm in cereal grains. Data in show that the critical threshold Cu concentrations reported in the literature is somewhat lower than our data but in good agreement with rapidly declining yield data measured in the trials.
However, concerning manganese, lowest critical concentrations in grains for the different cereal species have not been reported, with the exception of barley amounting to 12–14 mg Mn kg−1 dm (Hannam & Riggs, Citation1987). Owing to lack of data on critical manganese concentrations for cereal grains, we used the lowest range of data (5% percentile) reported by Eriksson et al. (Citation2000) as a comparison. Lowest concentrations of manganese amounted to 10 mg in barley, 23 mg in oat, and 15 mg kg−1 dm in wheat, which is similar to the critical concentrations identified. It is therefore likely that both low Mn and Cu concentrations may have caused the yield decline.
Why were yield losses observed in five but not all sites where lime was applied? Different soil textures seem to provide the most plausible explanation. The 10 sites where no yield losses were observed after lime application have textures varying from silt loam to clay. However, four out of the five sites showing a yield decline are sandy soils. It is well known that native copper contents are lower in sandy soil. Furthermore, plant availability of manganese is reduced in sandy soils due to favourable oxidation conditions. The findings may therefore be mainly relevant for soils with a light texture.
One can conclude that lime addition induced yield declines at 5 out of 15 sites, which are dominated by a sandy texture. Concentrations of Mn and Cu but not zinc or boron in grains declined with increasing pH in soil. Copper and manganese reached low, critical concentrations in grains after lime addition. Low concentrations of both Mn and Cu in grains were assumed to be responsible for the yield declines observed.
References
- Alloway , B.J. 2005 . Copper-deficient soils in Europe . International Copper Association Ltd. , NY, , USA 129
- Carran , R.A. 1991 . Calcium magnesium imbalance in clovers: A cause of negative yield response to liming . Plant and Soil , 134 : 107 – 114 .
- Eriksson , J. , Stenberg , B. , Andersson , A. Andersson , R. 2000 . Tillståndet i svensk åkermark och spannmålsgröda . Swedish Environmental Protection Agency, Report 5062 (In Swedish) .
- Fageria , N.K. , Baligar , C. and Clark , R.B. 2002 . Micronutrients in crop production . Advances in Agronomy , 77 : 186 – 268 .
- Farhoodi , A. and Coventry , D.R. 2008 . Field crop responses to lime in the mid-north region of South Australia . Field Crops Research , 108 : 45 – 53 .
- Hannam , R.J. and Riggs , J.L. 1987 . The critical concentrations of manganese in barley . Journal of Plant Nutrition , 10 : 2039 – 2048 .
- Hunt , R. 1982 . Plant growth curves – the functional approach to plant growth analysis Arnold Limited , London, , England , 248
- Hylander , L. 1995 . Effects of lime, phosphorus, manganese, copper and zinc on plant mineral composition, yield of barley and level of extractable nutrients for an acid Swedish mineral soil . Communications in Soil Science and Plant Analysis , 26 : 2913 – 2928 .
- Kabata-Pendias , A. 2001 . Trace elements in soils and plants . CRC Press Boca Raton, FL, , USA , 413
- Kowalenko , C.G. , Maas , E.F. and Vanlaerhoven , C.I. 1980 . Residual effects of high rates of limestone, P, K and Mg applications: Evidence of induced Mn and Zn deficiency in oats . Canadian Journal of Soil Science , 60 : 757 – 761 .
- Lindsay , W.L. 1974 . Chemical equilibria in soils . John Wiley and Sons , NY, , USA , 449
- Mengel , K. Kirkby , E.A. 2001 . Principles of Plant Nutrition , 5th edn Kluwer Academic Publishers , Dordrecht, , the Netherlands , 849
- Öborn , I. , Jansson , G. and Johansson , L. 1995 . A field study on the influence of soil pH on trace element levels in spring wheat (Triticum aestivum), potatoes (Solanum tuberosum) and carrots (Daucus carota) . Water Air and Soil Pollution , 85 : 835 – 840 .
- Rashid , A. and Ryan , J. 2004 . Micronutrient constraints to crop production in soils with Mediterranean-type characteristics: A review . Journal of Plant Nutrition , 27 : 959 – 975 .