Abstract
Alfalfa (Medicago sativa L.) is a forage legume of world-wide importance used in agriculture. The genetic relationship and distance among cultivars is of great interest for breeding programs. Random amplified polymorphic DNA (RAPD) was used in the current study to evaluate genetic variability of 7 alfalfa cultivars in Northwest China. A total of 132 discernible loci were obtained for all populations using 10 primers, and 88.64% of these loci were polymorphic, which indicated that a high diversity existed in the cultivars from Northwest China. Analysis of molecular variation (AMOVA) showed that the majority of the genetic variation was within cultivars (60.4%), with a relatively smaller proportion being due to the differences between cultivars (39.6%). The smallest genetic distance (0.0813) was estimated between cultivars Gannong-2 and Zhonglan-1, while the largest (0.2840) was between cultivars Gannong-1 and Tianshui. Cluster analysis using the UPGMA method based on Nei's similarity coefficient divided studied populations into five groups. The RAPD-derived diversity data were in correspondence with habitat heterogeneity of 7 alfalfa cultivars in Northwest China, which suggesting that alfalfa cultivars in Northwest China tended to be divergent to adapt to different stress environments.
Keywords:
Abbreviations
| AMOVA | = |
Analysis of molecular variance |
| RAPD | = |
Random amplified polymorphic DNA |
| UPGMA | = |
Unweighted pair-group method with arithmetic averages |
Introduction
Alfalfa (Medicago sativa L.) is one of the most important perennial legume crops and a superior source of forage due to its high nutritional quality and herbage yield (Riday & Brummer, Citation2002; Li & Brummer, Citation2009). Alfalfa contains high protein content, making it highly desirable as hay and pasture for livestock, especially dairy cows (Mertens, Citation2002). In addition, the ability of alfalfa to fix atmospheric nitrogen makes it valuable in crop rotations for higher productivity of crops (Barnes, Citation1993; Sandoval et al., Citation2007). Therefore, to improve alfalfa varieties adapted to different farming systems and/or growing environments is of paramount importance in applied pasture and agricultural research. Analysis of genetic variation both within and among elite breeding materials is of fundamental importance for alfalfa breeders as it provides an estimation of the extent of genetic variation in existing germplasms. It can also be used to predict potential genetic gains in different crosses (Moreno-Gonzalez & Cubero, Citation1993; Maureira-Butler et al., Citation2007). Current commercial perennial alfalfa cultivars are mostly synthetic populations formed from a large number of parents and thus have a broad genetic base. In most cases, identification of alfalfa cultivars is based on morphological characteristics and requires extensive observation for distinctness, uniformity, and stability (DUS) during growing seasons.
Alfalfa is an allogamous tetraploid species with polysomic inheritance and is not a simple system to apply molecular marker analysis (Osborn et al., Citation1998). However, molecular techniques in association with more powerful statistical models are exploited to reveal the genetic structure of this forage species. Molecular markers that detect different types of DNA-sequence polymorphisms have been used to estimate genetic diversity among various alfalfa germplasms, including restriction fragment-length polymorphism (RFLP) (Brummer et al., Citation1991; Kidwell et al., Citation1994; Pupilli et al., Citation2000), sequence-related amplified polymorphisms (SRAPs) (Ariss & Vandemark, Citation2007), random amplified polymorphic DNA (RAPD), simple sequence repeat (SSR), and amplified fragment-length polymorphism (AFLP) (Crochemore et al., Citation1996; Mengoni et al., Citation2000; Musial et al., Citation2002; Zaccardelli et al., Citation2003; Flajoulot et al., Citation2005; Julier, Citation2009). RAPD markers were used in this study because they allow a rapid analysis of the polymorphism of many individuals within a population (Iqbal et al., Citation2007; Rocco et al., Citation2007; Tucak et al., Citation2008). Experiments with alfalfa have demonstrated the potential for RAPD markers as a rapid and useful method for measuring genetic distance between allogamous populations of alfalfa (Ghérardi et al., Citation1998) and other grass species (Bolaric et al., Citation2005).
Alfalfa has been cultivated for over 3000 years in Northwest China, which is considered as one of its origin regions in China (Gen et al., Citation1995). In Northwest China, many adverse environmental conditions, including biotic stress conditions (e.g., disease and insect pests) and abiotic stress (e.g., drought, salinity, and freezing) which are present in this area, may produce great diversity in alfalfa germplasms. In order to fit the request for breeding new alfalfa cultivars to achieve maximum biomass and avoid inbreeding depression, it is necessary to investigate whether there is sufficient genetic diversity among various native cultivars for breeding progress. We tested the hypothesis that the abiotic stresses determine the diversity of alfalfa. The more divergent the habitats, the higher diversity of alfalfa cultivars were observed. To test this hypothesis, we investigated the degree and reproducibility of polymorphisms displayed by RAPDs in seven alfalfa populations in Northwest China as well as the genetic diversity among these populations.
Materials and methods
Plant materials
Three commercial cultivars and four local populations of alfalfa, from different regions in Northwest China, were used in this study (). Cultivars Gannong-1, Gannong-2, and Zhonglan-1 were all originally from Lanzhou. Gannong-1 was bred for its tolerance to cold stress. Gannong-2 had good tillering ability with high yield and developed root system. Zhonglan-1 was good at pest resistance. All of the 7 cultivars are registered by the Chinese Herbage Cultivar Registration Board (Citation1999). For each cultivar, we analysed one ‘population’ (which is meant by one accession of its cultivated form). One hundred seeds per cultivar selected were planted in plastic pots containing a soil mixture of loamy soil and quartz sand (1:1 v:v). The plants were cultured under 25±3 °C with natural sunlight and watered three times a week. After 8 weeks, 40 plants per population were randomly selected, and healthy leaves from each plant were collected for analysis.
Table I. Origin and habitat of the 7 alfalfa (Medicago sativa L.) cultivars used in this study.
DNA isolation
Genomic DNA was isolated using a CTAB (Hexadecyl trimethyl-ammonium bromide) method following Saghai-Maroof et al. (Citation1984) with some modifications: approximately 100 mg of leaves were ground to a fine powder in liquid nitrogen, re-suspended in 0.7 ml of CTAB buffer (2% CTAB, 0.1 M Tris-HCl (Tris (hydroxymethyl) aminomethane HCl), pH 8.0, 0.5 M EDTA (Ethylenediaminetetraacetate acid di-sodium salt), 5.0 M NaCl, 0.2%-mercaptoethanol) and incubated at 65 °C for 30 min. After chloroform:isoamyl alcohol (24:1) extraction, the aqueous phase was collected and the nucleic acid was precipitated with isopropanol (2-propanol) and 10 mM ammonium acetate, washed with 75% ethanol, and re-suspended in TE (Tris-EDTA) buffer. DNA was quantified spectrophotometrically and diluted to 50 ng DNA/µl in TE buffer.
RAPD reactions
To select suitable RAPD primers, we initially screened 40 primers (Promega, USA) using five individual plants from each of the seven populations. The primers which were polymorphic for all 35 individuals were subsequently tested for stability and reproducibility. Ten primers were selected for further screening (). The amount of polymorphism present in 7 populations of alfalfa was analysed in 40 plants for each population. Concentration and conditions of the polymerase chain reaction (PCR) were optimized by means of preliminary assays for random samples to give repeatable markers. Amplification reactions were performed in final volumes of 25 µl, containing 30 ng of template DNA, 0.2 µM primer, 1.5 U Taq DNA polymerase (Qiagen, Germany), 0.5 µM of each dNTP (Deoxyribonucleotide triphosphate), 3 mM MgCl2, and 10×PCR reaction buffer (Qiagen, Germany). The reactions were performed in a PTC-200 thermocycler (MJ Research Inc., USA) for 45 cycles in the following steps: 3 min at 94 °C (denaturation); 30 sec at 36 °C (annealing), and 1 min at 72 °C (extension), and a final 10-min elongation at 72 °C. Amplification products were separated by electrophoresis in 1.2% w/v agarose gels with 1×TAE (Tris-acetate EDTA) buffer, stained with ethidium bromide, visualized by illumination with ultraviolet light, and photographed (AlphaImager™ IS-3400, Germany) for analysis. The molecular weight of the fragments was estimated using a molecular marker ladder of 100 bp (Promega, USA).
Table II. Names and sequences of ten primers used in this study.
Data analysis
The bands in the RAPD profile were scored as either 0 (absent) or 1 (present). For each individual plant, a molecular binary phenotype by linear combination of the presence/absence of each marker was determined. Genetic diversity was estimated by calculating the average number of pairwise differences over each locus among RAPD binary phenotypes using Nei's original measures of genetic distance (Nei & Li, Citation1979). A UPGMA dendrogram was drawn using the software NTSYS-pc 2.02 (Rohlf, Citation1990) based on the Nei's genetic distance. Absence/present (0/1) vector matrices were used to compute Euclidean distance matrices; these matrices were then used to perform analysis of molecular variance (AMOVA) (Excoffier et al., Citation1992) to estimate intra- and inter-population variations. AMOVA was performed using the PREP-AMOVA 1.55 software (Gulhan et al., Citation2004).
Results
Degree of polymorphism
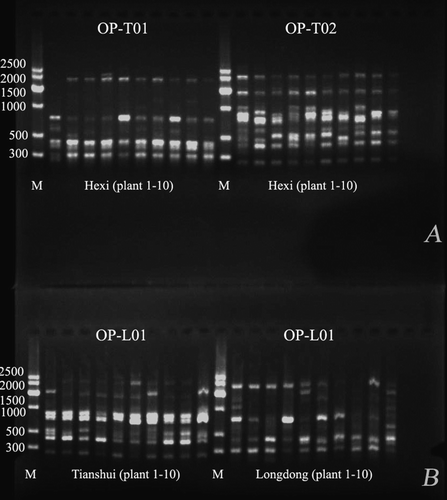
The distribution of markers was scored within the assayed plants using each of 10 random primers. The diversity in the banding profile of RAPD products obtained from amplification of individual plants within cultivars Hexi, Tianshui, and Longdong using primers OP-T01, OP-T02, and OP-L01 is demonstrated in . Based on the banding profile of RAPD, there was obvious diversity within cultivars as well as differences between cultivars, suggesting that the sampling strategy using individual plants within a single alfalfa cultivar was valuable to explore the in-depth genetic diversity. By pooling the ten primers, the results showed that Longzhong had 136 scorable bands (50% polymorphic bands), while Longdong had 136 (46.21%), Zhonglan-1 136 (46.21%), Hexi 135 (56.06%), Tianshui 134 (46.97%), Gannong-2 131 (50%), and Gannong-1 129 (53.79%), but none was found to be specific to a single cultivar. There were 117 polymorphic loci which accounted for 88.64% of the total markers. The average of polymorphic RAPD loci over all loci was 46–56%, which provides an adequate number of markers for assessment of genetic diversity among these populations.
Genetic distance and molecular variation
Mean genetic distances determined based on the results of RAPD markers are presented in . Nei's genetic distance among the studied populations varied from 0.0813 (between Gannong-2 and Zhonglan-1) to 0.2840 (between Tianshui and Gannong-1) with an average of 0.2235 ().
Table III. Nei's genetic distances between seven Alfalfa populations.
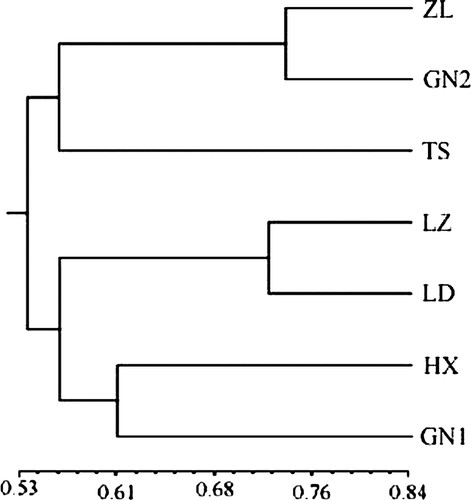
A UPGMA tree () was produced using polymorphic RAPD markers. As illustrated by the dendrogram, the populations were divided into five groups: 1) Zhonglan-1 and Gannong-2, 2) Tianshui, 3) Longzhong and Longdong, 4) Hexi, 5) Gannong-1, which are closely related to geographical distribution, annual rainfall, soil type, etc. For instance, Longdong and Longzhong were clustered together while Tianshui fell into another subgroup. The two cultivars from Lanzhou, Zhonglan-1 and Gannong-2 were located in the same subgroup. This result suggested that different geographical and environmental conditions may help to differentiate populations.
Figure 2. Dendrogram of seven Alfalfa populations generated by UPGMA cluster analysis of similarity values. Note: ZL, Zhonglan-1; GN2, Gannong-2; TS, Tianshui; LZ, Longzhong; LD, Longdong; HX, Hexi; GN1, Gannong-1.
Analysis of molecular variation showed that the majority of the genetic variation was within cultivars (60.4%) (p <0.001), with a relatively smaller proportion being due to the differences between cultivars (39.6%) (p <0.001).
Discussion
In this paper we report that the genetic diversity by use of RAPD markers helps to discriminate between alfalfa cultivars in Northwest China. Previous RAPD marker analysis suggested that the single-plant approach revealed the high polymorphism present in alfalfa populations and made it quite suitable for studying genetic relatedness among populations (Mengoni et al., Citation2000; Yang et al., Citation2008). We investigated the genetic diversity here using 280 individual plants in 7 alfalfa populations from Northwest China. To analyse genetic diversity in different alfalfa populations with RAPD markers, primers that provide sufficient and reliable information need to be selected. In this study, the primers selected amplified 117 polymorphic loci in the seven populations, which is comparable to the study with eight cultivated and natural populations of M. sativa and M. falcata using five RAPD primers (Ghérardi et al., Citation1998). However, this is slightly lower than the number of polymorphic bands using six RAPD markers to investigate 14 alfalfa cultivars (Tucak et al., Citation2008), and higher than the 50 polymorphic loci observed in 27 alfalfa cultivars using 7 RAPD primers (Li & Su, Citation1998). This difference may be due to the genetic materials themselves and the marker types used. The 117 polymorphic RAPD bands amplified in this study were also comparable with the study on RAPD reproducibility in other plant species (dos Santos et al., Citation1994; Skroch & Nienhuis, Citation1995). The degree of polymorphism displayed with RAPDs in alfalfa was as high as expected from its allogamous nature. An average of 13.4 bands was generated per primer. The value was higher than that of the 5–10 bands per primer reported in other alfalfa genotypes (Ghérardi et al., Citation1998; Hu et al., Citation2000; Wei, Citation2004). This suggested that there was high degree of genetic polymorphism of alfalfa cultivars in Northwest China, and RAPD could be useful for assessing genetic variation in tetraploid alfalfa (Yu & Pauls, Citation1993; Mengoni et al., Citation2000; Tucak et al., Citation2008).
Since only a wide genetic base gives the opportunity to select genotypes with a trait of interest, it is essential to understand the extent and distribution of genetic variation in plant populations. It has been stated that long lived, out-crossing, late successional species retain most of their genetic variability within populations (Nyborn, Citation2000). Data on the genetic diversity of alfalfa cultivars with different geographical origins and breeding histories were presented in this study. The relationships among alfalfa populations in Northwest China showed that overall within-cultivar variation in this study accounted for 60%, which is much lower than that reported by Mengoni et al. (Citation2000) in four ecotypes and two varieties of alfalfa from Italian and Egyptian germplasm, being 80–88% based on RAPD or 77% based on SSRs, respectively. Tucak et al. (Citation2008) reported that most of the genetic variability estimated by AMOVA was attributed to variation among individuals within 14 European, Australian, and South and North American M. sativa and M. media cultivars and one French wild population M. falcate cultivars (91.86%). These results indicated that the genetic structure of alfalfa cultivars was related to their geographic distribution and sources. The high level of intra- and inter-population variation detected in this study could be also related to both the out-crossing and the tetraploid nature of alfalfa (Brummer et al., Citation1991).
The estimates of genetic diversity here suggest that polymorphism is high in Chinese alfalfa cultivar populations; this agrees with other researchers’ experiments, which showed that there is a high heterozygosity within Chinese alfalfa genotypes (Hu et al., Citation2000; Wei, Citation2004). In this study, the highest genetic distance was between local cultivar Tianshui and commercial variety Gannong-1, while the lowest was between the two commercial cultivars Gannong-2 and Zhonglan-1 (). In the UPGMA cluster analysis (), genetic divergence of the seven cultivated alfalfa populations based on RAPD markers was also found to correspond well to their habitats and breeding backgrounds. For example, the clusters for cultivars originating from the same environment, e.g., Longdong and Longzhong, both originally from semi-arid conditions and loess soil, were clustered together. In contrast, Tianshui, which was originally from wet habitats (600-mm annual rainfall), was clearly distinguished from other cultivars originating from arid (e.g., Hexi, 120 mm/year) or semi-arid regions (e.g., Qinyang and Dingxi, 300–320 mm/year). Gannong-2 and Zhonglan-1 were both synthetic populations with the same number of parental plants included in their development (Chinese Herbage Cultivar Registration Board, Citation1999). The diversity data observed in these cultivars suggest that the information generated through molecular markers is valuable for planning future alfalfa breeding programs. For example, crosses between Zhonglan-1 and Longdong are a good combination to improve biotic and abiotic tolerance of alfalfa in arid and semi-arid regions for their larger genetic distance.
Molecular markers are valuable to enable us to monitor and detect reductions of genetic diversity associated with breeding activities. In addition, understanding the extent and distribution of genetic variation within a breeding program is essential to the better utilization of the germplasm available and to help us devise breeding strategies able to develop new materials as well as keeping appropriate levels of variability to support further genetic advances.
Acknowledgements
This study was supported by The National Basic Research Program of China (2007CB108904), Natural Scientific Foundation of Gansu Province (No. 3ZS051-A25-066), and Natural Science and Technology Program of Lanzhou University (582402, 582403).
References
- Ariss , J.J. and Vandemark , G.J. 2007 . Assessment of genetic diversity among nondormant and semi-dormant alfalfa populations using sequence-related amplified polymorphisms . Crop Science , 47 : 2274 – 2284 .
- Barnes , D.K. 1993 . Alfalfa . In : OECD Traditional crop breeding practices: An historical review to serve as a baseline for assessing the role of modern biotechnology (pp. 135 146 ). Paris , , France .
- Bolaric , S. , Barth , S. , Melchinger , A.E. and Posselt , U.K. 2005 . Genetic diversity in European perennial ryegrass cultivars investigated with RAPD markers . Plant Breeding , 124 : 161 – 166 .
- Brummer , E.C. , Kochert , G. and Bouton , J.H. 1991 . RFLP variation in diploid and tetraploid alfalfa . Theoretical and Applied Genetics , 83 : 89 – 96 .
- Chinese Herbage Cultivar Registration Board 1999 . Licensed cultivars of herbage crops in China . Beijing , , China : China Agricultural University Publishing (in Chinese).
- Crochemore , M.L. , Huyghe , C. , Kerlan , M.C. and Julier , B. 1996 . Partitioning and distribution of RAPD variation in a set of populations of Medicago sativa complex . Agronomie , 16 : 421 – 432 .
- dos Santos , J.B. , Nienhuis , J. , Skroch , P. , Tivang , J. and Slocum , M.K. 1994 . Comparison of RAPD and RFLP genetic markers in determining genetic similarity among Brassica oleracea L. genotypes . Theoretical and Applied Genetics , 87 : 909 – 915 .
- Excoffier , L. , Smouse , P.E. and Quattro , M. 1992 . Analysis of molecular variance inferred from metric distances among DNA haplotypes: application to human mitochondrial DNA restriction data . Genetics , 131 : 479 – 491 .
- Flajoulot , S. , Ronfort , J. , Baudouin , P. , Barre , P. , Huguet , T. , Huyghe , C. and Julier , B. 2005 . Genetic diversity among alfalfa (Medicago sativa) cultivars coming from a breeding program, using SSR markers . Theoretical and Applied Genetics , 111 : 1420 – 1429 .
- Gen , H.Z. , Wu , Y.F. , Cao , Z.Z. 1995 . Chinese alfalfa (pp. 1 6 ). Beijing , , China : China Agriculture Press (In Chinese) .
- Ghérardi , M. , Mangin , B. , Goffinet , B. , Bonnet , D. and Huguet , T. 1998 . A method to measure genetic distance between allogamous populations of alfalfa (Medicago sativa) using RAPD molecular markers . Theoretical and Applied Genetics , 96 : 406 – 412 .
- Gulhan , A.E. , Melih , T. and Kenan , T. 2004 . Analysis of genetic diversity in Turkish sesame (Sesamum indicum L.) populations using RAPD markers . Genetic Resources and Crop Evolution , 51 : 599 – 607 .
- Hu , B.Z. , Liu , D. , Hu , F.G. , Zhang , A.Y. , Jiang , S.J. 2000 . Random amplified polymorphic DNA study of local breeds in Chinese alfalfa . Chinese Journal of Plant Ecology , 24 , 697 701 ( In Chinese with English abstract ).
- Iqbal , A. , Khan , A.S. , Khan , I.A. , Awan , F.S. , Ahmad , A. and Khan , A.A. 2007 . Study of genetic divergence among wheat genotypes through random amplified polymorphic DNA . Genetics and Molecular Research , 6 : 476 – 481 .
- Julier , B. 2009 . A program to test linkage disequilibrium between loci in autotetraploid species . Molecular Ecology Resources , 9 : 746 – 748 .
- Kidwell , K.K. , Austin , D.F. and Osborn , T.C. 1994 . RFLP evaluation of nine Medicago populations representing the original germplasm sources for the North American alfalfa cultivars . Crop Science , 34 : 230 – 236 .
- Li , X. and Brummer , E.C. 2009 . Inbreeding depression for fertility and biomass in advanced generations of inter- and intrasubspecific hybrids of tetraploid alfalfa . Crop Science , 49 : 13 – 19 .
- Li , Y.J. , Su , J.K. 1998 . Study of the genetic diversity of the alfalfa local varieties (Medicago sativa L.) based on RAPD markers . Acta Agresia Sinica , 6 , 105 114 ( In Chinese with English abstract ).
- Maureira-Butler , I.J. , Udall , J.A. and Osborn , T.C. 2007 . Analyses of a multi-parent population derived from two diverse alfalfa germplasms: testcross evaluations and phenotype-DNA associations . Theoretical and Applied Genetics , 115 : 859 – 867 .
- Mengoni , A. , Gori , A. and Bazzicalupo , M. 2000 . Use of RAPD and microsatellite (SSR) to assess genetic relationships among populations of tetraploid alfalfa, Medicago sativa . Plant Breeding , 119 : 311 – 317 .
- Mertens , D.R. 2002 . Nutritional implications of fiber and carbohydrate characteristic of corn silage and alfalfa hay (pp. 94 107 ). Fresno, CA , , USA : California Animal Nutrition Conference .
- Moreno-Gonzalez , J. , Cubero , J.I. 1993 . Selection strategies and choice of breeding materials . In : M.D. Hayward , N.O. Bosemark , & I. Romagosa Plant breeding: principles and prospects (pp. 281 313 ). London , , UK : Chapman and Hall .
- Musial , J.M. , Basford , K.E. and Irwin , J.A.C. 2002 . Analysis of genetic diversity within Australian cultivars and implications for future genetic improvement . Australian Journal of Agricultural Research , 53 : 620 – 633 .
- Nei , M. and Li , W. 1979 . Mathematical model for studying genetic variation in terms of restriction endonucleases . Proceedings of the National Academy of Sciences of the USA , 76 : 5269 – 5273 .
- Nyborn , H. 2000 . Effects on life history traits and sampling strategies on genetic diversity estimates obtained with RAPD markers in plants . Perspectives in Plant Ecology, Evolution and Systematics , 3 : 93 – 114 .
- Osborn , T.C. , Brouwer , D.J. , Kidwell , K.K. , Tavoletti , S. , Bingham , E.T. 1998 . Molecular marker application to genetics and breeding of alfalfa . In : B.C. Brummer , & C.A. Roberts Molecular and cellular technologies for forage improvement (pp. 25 31 ). Madison, WI , , USA : CSSA special publication .
- Pupilli , F. , Labombarda , P. , Scotti , C. and Arcioni , S. 2000 . RFLP analysis allows for the identification of alfalfa ecotypes . Plant Breeding , 119 : 271 – 276 .
- Riday , H. and Brummer , E.C. 2002 . Forage yield heterosis in alfalfa . Crop Science , 42 : 716 – 723 .
- Rocco , L. , Ferrito , V. , Costagliola , D. , Marsilio , A. , Pappalardo , A.M. , Stingo , V. and Tigano , C. 2007 . Genetic divergence among and within four Italian populations of Aphanius fasciatus (Teleostei, Cyprinodontiformes) . Italian Journal of Zoology , 74 : 371 – 379 .
- Rohlf , F.J. 1990 . NTSYS-Pc: Numerical taxonomy and multivariate analysis system, Version 2.02 . New York , , USA : Exeter Publishing .
- Skroch , P. and Nienhuis , J. 1995 . Impact of scoring error and reproducibility RAPD data on RAPD based estimates of genetic distance . Theoretical and Applied Genetics , 91 : 1086 – 1091 .
- Saghai-Maroof , M.A. , Soliman , K.M. and Jorgensen , R.A. 1984 . Ribosomal DNA spacer length polymorphism in barley . Proceedings of the National Academy of Sciences of the USA , 81 : 8104 – 8108 .
- Sandoval , M.A. , Stolpe , N.B. , Zagal , E.M. and Mardones , M. 2007 . The effect of crop-pasture rotations on the C, N and S contents of soil aggregates and structural stability in a volcanic soil of South-central Chile . Acta Agriculturae Scandinavica, Section B — Soil & Plant Science , 57 : 255 – 262 .
- Tucak , M. , Popovic , S. , Cupic , T. , Grljusic , S. , Bolaric , S. and Kozumplik , V. 2008 . Genetic diversity of alfalfa (Medicago spp.) estimated by molecular markers and morphological characters . Periodicum Biologorum , 110 : 243 – 249 .
- Wei , Z.W. 2004 . DNA fingerprint of Medicago sativa variety genomes using SSR, ISSR and RAPD . Acta Prataculturae Sinica , 13 , 62 67 ( In Chinese with English abstract ).
- Yang , X.L. , Chen , L. , Ban , T. , Han , P. , Wang , X.J. 2008 . RAPD analysis of genetic diversity of alfalfa germplasms in Gansu Province . Acta Agresia Sinica , 16 , 129 134 ( In Chinese with English abstract ).
- Yu , P. and Pauls , K.P. 1993 . Rapid estimation of genetic relatedness among heterogeneous populations of alfalfa by random amplification of bulked genomic DNA samples . Theoretical and Applied Genetics , 86 : 788 – 794 .
- Zaccardelli , M. , Gnocchi , S. , Carelli , M. , Scotti , C. 2003 . Variation among and within Italian alfalfa ecotypes by means of bio-agronomic characters and amplified fragment length polymorphism analyses . Plant Breeding , 122 , 61 65 .