Abstract
Acute rheumatic fever (ARF), caused by group A β-hemolytic streptococcus infection, is characterized by inflammation affecting several organs. There are few reports on magnetic resonance imaging (MRI) findings in patients with ARF. An 8-year-old Japanese boy presented with a prolonged fever of unknown cause and swelling of his right hand. MRI of his hand revealed tenosynovitis. Migratory arthritis and erythema marginatum appeared following the hand swelling. We diagnosed him as having ARF based on the clinical course and serological testing for group A β-hemolytic streptococcus. His serum interleukin-18 levels were lower than those typically seen in cases of systemic juvenile idiopathic arthritis (sJIA). After treatment with naproxen, his symptoms improved immediately. In conclusion, MRI findings of tenosynovitis may be useful for the diagnosis of not only sJIA but also ARF in patients presenting with a fever of unknown origin. Subsequently, the diagnosis of ARF can be confirmed with specific serological tests. Serum interleukin-18 levels may be helpful in the differential diagnosis of ARF and sJIA. Although ARF is rare in developed countries, including Japan, early diagnosis and appropriate treatment are important to prevent rheumatic heart disease.
Acute rheumatic fever (ARF) is characterized by an inflammatory process affecting several organs after infection with group A β-hemolytic streptococcus (GAS) [Citation1]. Recently, the incidence of ARF has decreased in developed countries such as Japan due to improvements in public hygiene [Citation1]. To the best of our knowledge, there have been no reports on magnetic resonance imaging (MRI) finding of the hands in patients with ARF. Here, we present the case of an 8-year-old Japanese boy with ARF who exhibited tenosynovitis that was detected via MRI.
A previously healthy 8-year-old Japanese boy presented to our hospital due to fever with painful swelling of the right hand. He had an 8-d history of remittent fever at admission. The antibiotic treatment administered at a hospital he had previously visited was not effective. The fever persisted and the symptoms affecting his right hand underwent repeated cycles of exacerbations and remissions.
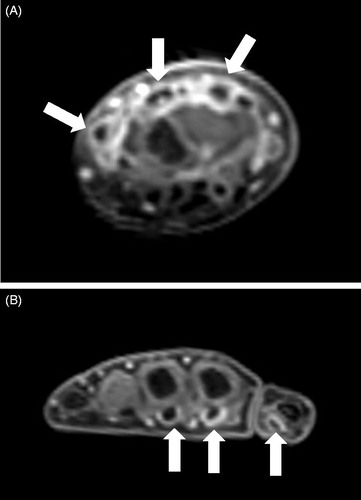
The results of the hematological examinations conducted at our hospital were as follows: leukocytes, 13.85 × 109/L; hemoglobin, 10.1 g/dL; platelet count, 659 × 109/L; erythrocyte sedimentation rate, 86 mm/h; C-reactive protein (CRP), 9.37 mg/dL (reference range [rr]: <0.14 mg/dL); procalcitonin, 0.08 ng/mL (rr: <0.05 ng/mL); lactate dehydrogenase, 214 U/L (rr: 124–222 U/L); ferritin, 89 ng/mL (rr: 15–250 ng/mL); β2-microglobulin, 1.7 mg/L (rr: <2.0 mg/L); matrix metalloproteinase-3, 43.7 ng/mL (rr: <20.0 ng/mL); immunoglobulin (Ig) G, 1873 mg/dL; IgA, 430 mg/dL; and IgM, 100 mg/dL (rrs: 861–1747 mg/dL, 93–393 mg/dL, and 33–183 mg/dL, respectively); complement component-3, 165 mg/dL; complement component-4, 31 mg/dL; and 50% hemolytic unit of complement, >60 U/mL (rrs: 73–138 mg/dL, 11–31 mg/dL, and 30–60 U/mL, respectively). Routine laboratory tests, including hepatic and renal function tests, urinalysis, and a fecal occult blood test, were normal. Among peripheral blood mononuclear cells, the numbers of T cells, B cells, and natural killer cells were normal, as analyzed by flow cytometry. Bacterial and fungal blood cultures yielded no growth. All tests of infectious agents, including adenovirus, cytomegalovirus, Epstein–Barr virus, human metapneumovirus, influenza virus, respiratory syncytial virus, Chlamydophila pneumoniae, Mycobacterium tuberculosis, and Mycoplasma pneumoniae, were negative. Rheumatoid factor, anti-nuclear, anti-Ro/SS-A, and anti-La/SS-B antibodies were not detected. Bone marrow aspiration, ophthalmologic, otorhinolaryngologic examinations, and echocardiography showed no abnormalities. To evaluate for the presence of Takayasu arteritis, a tumor, or an abscess, we carried out whole-body contrast-enhanced computed tomography, which showed no abnormalities. In contrast, MRI findings of the right hand revealed tenosynovitis of the wrist and finger tendons ().
Figure 1. Magnetic resonance imaging of the hand. Coronal contrast-enhanced T1-weighted images of the right hand. Tenosynovitis was seen in the wrist extensor (A) and finger flexor tendons (B) (arrows).
Bacterial infection was ruled out based on almost normal serum procalcitonin levels and the lack of response to antibiotic treatment. Although the patient’s fever persisted, CRP levels stayed relatively consistent. However, migratory arthritis of the right ankle and both knees and erythema marginatum appeared within 2 weeks after admission to our hospital. Based on his clinical course, a diagnosis of ARF was made. This diagnosis was confirmed using an anti-streptolysin O antibody (584 IU/mL; rr: < 240 U/mL) and anti-streptokinase antibody titer test (1:5120; rr: < 1:5120) that indicated a recent GAS infection. Furthermore, the patient fulfilled both the 1992 and 2015 revised Jones Criteria for ARF diagnosis [Citation2,Citation3]. At the time of diagnosis, he still had high CRP levels (10.03 mg/dL), while an echocardiography showed no abnormalities. Therefore, treatment with oral naproxen (20 mg/kg/d) was initiated; his fever and arthritis improved within 48 h after the start of treatment. Two weeks after treatment initiation, the patient’s CRP levels decreased to 0.74 mg/dL. Four weeks after treatment initiation, CRP levels returned to normal.
We studied serum cytokine profiles in this patient during the acute (before treatment with naproxen) and convalescent (4 weeks after treatment with naproxen) phases of ARF. Multi-target streaming protein quantitative technology (BD-Pharmingen Cytometric Bead Array; BD Biosciences, Franklin Lakes, NJ) was used to analyze the serum levels of interleukin (IL)-2, -4, -6, -10, tumor necrosis factor-α (TNF-α), and interferon-γ (IFN-γ), following the manufacturer's instructions. The lower detection limits for IL-2, IL-4, IL-6, IL-10, TNF-α, and IFN-γ are 2.6, 2.6, 2.5, 2.8, 2.8, and 7.1 pg/mL, respectively. Serum levels of IL-18 were determined using enzyme-linked immunosorbent assay kits (Medical & Biological Laboratories, Co., Ltd., Nagoya, Aichi, Japan) according to the manufacturers’ protocols. The lower detection limit for IL-18 is 12.5 pg/mL. Elevated serum IL-6 and IL-18 levels were found during the acute phase in the present case (45.3 pg/mL and 2259.8 pg/mL, respectively; rr: <9.5 pg/mL and <215.0 pg/mL, respectively). Serum IL-2, IL-4, IL-10, TNF-α, and IFN-γ levels were not elevated. Four weeks after treatment initiation, the serum IL-6 level returned to normal, whereas the IL-18 level was still high during the convalescent phase (2411.0 pg/mL).
In our case, the patient’s early clinical feature was tenosynovitis with fever. Only one case of tenosynovitis in a child with ARF has been reported, and no MRI was performed in this case [Citation4]. It has been shown that peripheral proinflammatory cytokine (e.g., IL-6) levels are elevated in ARF [Citation5]. However, to the best of our knowledge, there have been no reports on serum IL-18 levels in patients with ARF.
Tenosynovitis in febrile children is known to occur not only with ARF, but also with systemic juvenile idiopathic arthritis (sJIA) [Citation6]. Our patient’s serum IL-18 levels were lower than those typically seen in sJIA (>10000 pg/mL) [Citation7]. Therefore, serum IL-18 levels may be helpful for the differential diagnosis between ARF and sJIA.
As for using MRI for assessing ARF associated arthritis/tenosynovitis, only one case has been reported, which revealed knee osteomyelitis [Citation8]. Our case suggests that tenosynovitis may be a key feature in the early diagnosis of not only sJIA but also ARF, particularly before migratory arthritis or erythema marginatum appears. MRI findings may be important to diagnose patients with suspected ARF.
We administered naproxen, but not aspirin, because naproxen has been found to be as effective as aspirin for the treatment of ARF, and it has fewer side effects than aspirin [Citation9]. ARF patients have a high risk for recurrence, and rheumatic heart disease becomes more severe with each episode [Citation10]. Although our patient did not have carditis, it has been shown that all patients will experience carditis if they have a second ARF episode [Citation10]. Thus, ARF patients need to prevent recurrent GAS infection. The American Heart Association recommends continuous antimicrobial prophylaxis with intramuscular benzathine penicillin G (BPG), oral penicillin V, or oral sulfadiazine [Citation11]. BPG is available, while penicillin V and sulfadiazine are not available in Japan. Moreover, intramuscular BPG is not covered by insurance in Japan. We treated our patient using oral trimethoprim/sulfamethoxazole rather than BPG for GAS infection prophylaxis, since BPG-resistant bacteria are more prevalent in Japan (data not shown), and we used to administer trimethoprim/sulfamethoxazole for continuous antimicrobial prophylaxis, but not BPG. He has had no recurrence of GAS infection for 1 year since the start of prophylaxis.
In conclusion, MRI findings of tenosynovitis may be useful for diagnosing not only sJIA but also ARF in patients presenting with a fever of unknown origin. Serum IL-18 levels may be helpful for the differential diagnosis of ARF and sJIA. Although the incidence of ARF is low in developed countries such as Japan, early diagnosis and appropriate treatment are important to prevent cardiac complications.
Informed consent
Informed consent was obtained from the patient’s guardian for the publication of this case report.
Acknowledgements
The authors thank the patient, his family, and his friends for their cooperation.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Alsaeid K, Uziel Y. Acute rheumatic fever and poststreptococcal reactive arthritis. In: Petty RE, Laxer RM, Lindsley CB, Wedderburn LR, editors. Textbook of pediatric rheumatology. 7th ed. Philadelphia: Saunders Elsevier; 2016. p. 571–585.
- Guidelines for the diagnosis of rheumatic fever. Jones Criteria, 1992 update. Special Writing Group of the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease of the Council on Cardiovascular Disease in the Young of the American Heart Association. JAMA. 1992;268:2069–2073.
- Gewitz MH, Baltimore RS, Tani LY, et al. Revision of the Jones Criteria for the diagnosis of acute rheumatic fever in the era of Doppler echocardiography a scientific statement from the American Heart Association. Circulation. 2015;131:1806–1818.
- Boon RL, Baildam E. Rheumatic fever – a vignette. Rheumatology (Oxford). 2005;44:1198.
- Guilherme L, Kalil J. Rheumatic fever and rheumatic heart disease: cellular mechanisms leading autoimmune reactivity and disease. J Clin Immunol. 2010;30:17–23.
- De Benedetti F, Schneider R. Systemic juvenile idiopathic arthritis. In: Petty RE, Laxer RM, Lindsley CB, Wedderburn LR, editors. Textbook of pediatric rheumatology. 7th ed. Philadelphia: Saunders Elsevier; 2016. p. 205–216.
- Inoue N, Shimizu M, Tsunoda S, et al. Cytokine profile in adult-onset Still's disease: comparison with systemic juvenile idiopathic arthritis. Clin Immunol. 2016;169:8–13.
- Sato S, Chiyotanda M, Hijikata T, et al. Acute suppurative oligoarthritis and osteomyelitis: a differential diagnosis that overlaps with acute rheumatic fever. J Infect Chemother. 2015;21:610–612.
- Hashkes PJ, Tauber T, Somekh E, et al. Naproxen as an alternative to aspirin for the treatment of arthritis of rheumatic fever: a randomized trial. J Pediatr. 2003;143:399–401.
- Sheikh AM, Sadiq M, Rehman AU. Changing clinical profile of acute rheumatic fever and rheumatic recurrence. J Ayub Med Coll Abbottabad. 2016;28:141–145.
- Gerber MA, Baltimore RS, Eaton CB, et al. Prevention of Rheumatic Fever and Diagnosis and Treatment of Acute Streptococcal Pharyngitis: a scientific statement from the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee of the Council on Cardiovascular Disease in the Young, the Interdisciplinary Council on Functional Genomics and Translational Biology, and the Interdisciplinary Council on Quality of Care and Outcomes Research: endorsed by the American Academy of Pediatrics. Circulation. 2009;119:1541–1551.
