Abstract
Highly active mutant of NADPH-dependent acetoacetyl-CoA reductase (PhaB) was expressed in Nicotiana tabacum cv. Bright Yellow-2 cultured cells to produce poly(3-hydroxybutyrate) [P(3HB)]. The mutated PhaB increased P(3HB) content by three-fold over the control, indicating that the mutant was a versatile tool for P(3HB) production. Additionally, the PhaB-catalyzed reaction was suggested to be a rate-limiting step of P(3HB) biosynthesis in tobacco BY-2 cells.
Poly(3-hydroxybutyrate) [P(3HB)] is a bacterial polyester, which can be used as a biobased alternative to petroleum-derived plastics. P(3HB) has been produced using native producers such as Ralstonia eutropha (currently designated as Cupriavidus necator) and engineered bacteria using a variety of carbon source. In attempts to produce P(3HB) and related polymers at high concentrations, the feedstock has been a major factor contributing to the production cost of the polymer.Citation1) Therefore, transgenic plants expressing bacterial P(3HB) biosynthetic genes have attracted research interest,Citation2) because they can produce P(3HB) directly from CO2. To date, various plant speciesCitation3–5) including tobaccoCitation6,7) have been successfully transformed to produce P(3HB), but a further improvement in polymer productivity is required.
P(3HB) is synthesized from acetyl-CoA via three successive reactions. First, two molecules of acetyl-CoA are condensed into acetoacetyl-CoA by a β-ketothiolase (PhaA). Subsequently, NADPH-dependent acetoacetyl-CoA reductase (PhaB) converts acetoacetyl-CoA into (R)-3-hydroxybutyryl-CoA (3HB-CoA), which is finally polymerized into P(3HB) by polyhydroxyalkanoate synthase (PhaC). A set of P(3HB) biosynthetic genes (phaA, phaB, phaC) encoding these enzymes have been found in a variety of bacteria.Citation8) In particular, the genes from R. eutropha are widely used and well-characterized.Citation9) As mentioned, the expression of P(3HB) biosynthetic genes led to an accumulation of P(3HB) in non P(3HB)-producing organisms, including eukaryotes such as plants. For example, the transgenic tobacco expressing PhaB and PhaC from R. eutropha in cytosol produced P(3HB),Citation7) indicating the presence of intrinsic acetoacetyl-CoA supply in tobacco cytosol.
In our previous study, we developed the transgenic tobacco using the codon-optimized phaB and phaC genes for enhancing P(3HB) production. As a result, the expression of a codon-optimized phaB gene increased both the PhaB expression level and P(3HB) content.Citation10) This beneficial effect was not seen for the phaC gene. The result indicated that the PhaB-catalyzed reaction was a rate-determining step in P(3HB) biosynthesis in tobacco. Accordingly, we previously created an evolved PhaB (Thr173Ser) mutant through in vitro evolution technique based on the random mutagenesis and high-throughput screening.Citation11) The mutated PhaB(TS) exhibited 3.6-fold higher kcat value, and a 2.0-fold increased P(3HB) content in recombinant Corynebacterium glutamicum compared to the wild-type enzyme [PhaB(WT)]. Combining these results, it was expected that PhaB(TS) might improve P(3HB) production in tobacco. Therefore, the aim of this study was to express PhaB(TS) in tobacco and to examine the effect of the PhaB mutant on P(3HB) production. To meet this goal, we used BY-2 cultured tobacco cells, which are known to grow very rapidly. In general, development of transgenic plants and evaluation of the function of transgenes take long time, and need large laboratory space. Therefore, it is worth to pre-evaluate the function of the gene of interest using rapid and compact system. In this regard, BY-2 cells were favorable host because the culture was scalable and stable for long-term cultivation.Citation12)
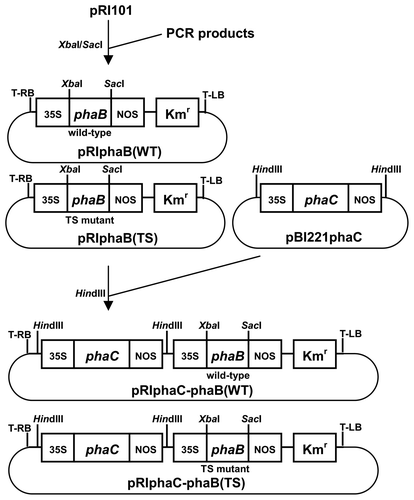
The plasmids used in this study were constructed as follows. The phaB(WT) and phaB(TS) genes were amplified by PCR using a pair of primers: phaB_f (5′-CAATCTAGAATAAAGAATGACTCAGC-3′) and phaB_r (5′-AACCAGAGCTCGAGGTCAGCCCAT-3′), as well as pGEM″CAB and pGEM″phaCAB(T173S) as templates, respectively.Citation11) The amplified fragments were digested using XbaI and SacI, and ligated with pRI101 (Takara, Japan) for the expression of the genes under control of 35S cauliflower mosaic virus promoter and NOS terminator. The plasmids were designated as pRIphaB(WT) and pRIphaB(TS), respectively. The HindIII fragment, which contained 35S promoter, the phaC gene from R. eutropha, and NOS terminator,Citation10) was digested from pBI221phaC and inserted into pRIphaB(WT) and pRIphaB(TS) to yield pRIphaC-phaB(WT) and pRIphaC-phaB(TS), respectively (Fig. ).
Fig. 1. The plasmids used in this study.
Notes: phaC: PHA synthase gene, phaB: acetoacetyl-CoA reductase gene (wild type and TS mutant), 35S: 35S cauliflower mosaic virus promoter, NOS: nopaline synthase terminator, Kmr: kanamycin resistance gene, T-LB and T-RB: left and right borders of T-DNA, respectively.
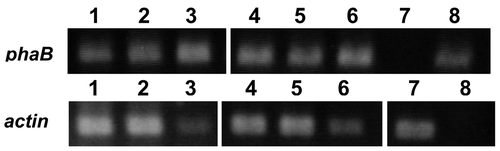
In this study, Nicotiana tabacum cv. Bright Yellow-2 (tobacco BY-2) cultured cells were chosen as a host because of its high transformation efficiency and ease of manipulation.Citation12) pRIphaC-phaB(WT) and pRIphaC-phaB(TS) were introduced into BY-2 cells using the Agrobacterium-mediated method as described.Citation13) The candidates of transformants, designated as WT and TS, respectively, were selected and maintained at 28 °C in the dark on modified Murashige and Skoog mediumCitation14) containing 30 g l−1 sucrose, 100 μg l−1 kanamycin, and 500 mg l−1 cefotaxime. The transformants expressing the phaB genes were selected using reverse transcription-PCR (Toyobo, Japan) using phaB_f and phaB_r primers (Fig. ). The actin gene was amplified as a positive control using a pair of primers; 5′-CTTGACGGAAAGAGGTTATT-3′ and 5′-GATCCTCCAATCCAGACACT-3′. Thirteen and ten lines of WT and TS expressing the phaB gene were obtained, respectively. For polymer production, the cells were cultured for ten days in 50 ml medium with rotary shaking of 130 rpm, and harvested. The lyophilized cells were ground with stainless hammer using multi-bead-shocker (YASUI KIKAI, Japan). The obtained powders were rinsed with n-hexane, and subsequently suspended in methanol, at 50 °C for 24 h, as described.Citation15) The polymer was extracted with chloroform at 60 °C for 48 h, and subjected to gas chromatography/mass spectrum analysis as described previously.Citation15)
Fig. 2. RT-PCR of transgenic tobacco harboring phaB and phaC genes.
Notes: 1-3: transformants of pRIphaC-phaB(WT), 4-6: transformants of pRIphaC-phaB(TS), 7: wild-type BY2, and 8: positive control (plasmid).
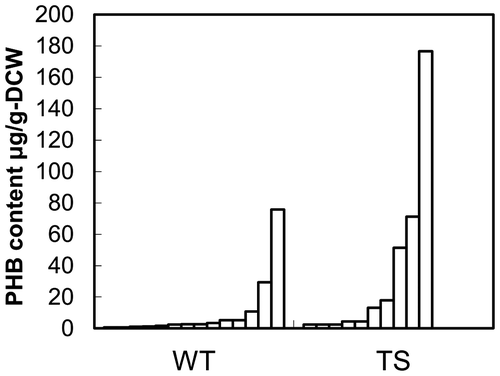
The P(3HB) contents in WT and TS lines are shown in Fig. . The polymer content varied from 0.61 μg g−1-cell dry weight to 177 μg g−1. The large distribution of the polymer content in P(3HB)-producing transgenic plants has been typically observed in various plant species.Citation15,16) This phenomenon could be partly due to the position effect of transgene insertion in the chromosomal DNA of the host plants and/or epigenetic gene silencing.Citation17) Despite the heterogeneity of the P(3HB) productivity, the average P(3HB) content of the TS lines (35 μg g−1) was approximately three-fold higher than the line harboring wild-type phaB (10 μg g−1) (Fig. ), indicating that there was a tendency that TS lines accumulated greater amount of P(3HB). In general, in order to obtain a highly polymer-producing line, a number of transformants have to be developed and screened because of the broad distribution in the polymer content in transgenic plants. Thus, considering the number of sample, the lines expressing phaB(TS) gene expectedly achieves a higher production than the wild-type phaB gene. Therefore, the mutated phaB gene could be a versatile tool for improving P(3HB) production in plants.
Fig. 3. P(3HB) content in transgenic tobacco BY-2 cells.
Notes: WT: transformants of pRIphaC-phaB(WT), TS: transformants of pRIphaC-phaB(TS).
The increase in P(3HB) content in tobacco expressing the TS mutant suggests that the PhaB activity was an important factor for the polymer production. However, it should be noted that the flux toward 3HB-CoA was determined by not only the kinetic parameters of PhaB, but also the concentration of the substrates, acetoacetyl-CoA and NADPH. Therefore, the result suggested that the conditions in BY-2 cells, namely the levels of acetoacetyl-CoA and NADPH, were suitable for exercising the capacity of PhaB(TS) as well as the activity of PhaB.
The increase in P(3HB) content by expression of the PhaB TS mutant suggested that the PhaB-catalyzed reaction is a rate-limiting step in P(3HB) synthesis in BY-2 cells. In fact, this hypothesis was consistent with the previous result that the polymer content was increased by using the codon-optimized phaB gene.Citation10) Therefore, PhaB should be an effective target to increase P(3HB) content in plants. As a simple example, the combination of the mutant and codon-optimization will be a potent approach to achieve this goal.
In conclusion, P(3HB) production in transgenic tobacco was successfully increased by using an evolved PhaB with enhanced activity. This result demonstrated that the evolved PhaB should be a powerful tool to improve the production of P(3HB) and 3HB-based polyesters in various platforms.
Acknowledgments
We thank Professor Junichi Yamaguchi in Hokkaido University for technical assistance for BY-2 manipulation. BY-2 cells were provided by the RIKEN BRC through the National Bio-Resource Project of the MEXT, Japan.
Additional information
Funding
References
- Sudesh K, Bhubalan K, Chuah JA, Kek YK, Kamilah H, Sridewi N, Lee YF. Synthesis of polyhydroxyalkanoate from palm oil and some new applications. Appl. Microbiol. Biotechnol. 2011;89:1373–1386.10.1007/s00253-011-3098-5
- Poirier Y, Dennis D, Klomparens K, Somerville C. Polyhydroxybutyrate, a biodegradable thermoplastic, produced in transgenic plants. Science. 1992;256:520–523.10.1126/science.256.5056.520
- Tilbrook K, Gebbie L, Schenk PM, Poirier Y, Brumbley SM. Peroxisomal polyhydroxyalkanoate biosynthesis is a promising strategy for bioplastic production in high biomass crops. Plant Biotechnol. J. 2011;9:958–969.10.1111/pbi.2011.9.issue-9
- Snell KD, Peoples OP. Polyhydroxyalkanoate polymers and their production in transgenic plants. Metab. Eng. 2002;4:29–40.
- Matsumoto K, Murata T, Nagao R, Nomura CT, Arai S, Arai Y, Takase K, Nakashita H, Taguchi S, Shimada H. Production of short-chain-length/medium-chain-length polyhydroxyalkanoate (PHA) copolymer in the plastid of Arabidopsis thaliana using an engineered 3-ketoacyl-acyl carrier protein synthase III. Biomacromolecules 2009;10:686–690.10.1021/bm8013878
- Bohmert-Tatarev K, McAvoy S, Daughtry S, Peoples OP, Snell KD. High levels of bioplastic are produced in fertile transplastomic tobacco plants engineered with a synthetic operon for the production of polyhydroxybutyrate. Plant Physiol. 2011;155:1690–1708.10.1104/pp.110.169581
- Nakashita H, Arai Y, Yoshioka K, Fukui T, Doi Y, Usami R, Horikoshi K, Yamaguchi I. Production of biodegradable polyester by a transgenic tobacco. Biosci. Biotechnol. Biochem. 1999;63:870–874.10.1271/bbb.63.870
- Doi Y. Microbial polyesters. New York (NY): VHC Publishers; 1990.
- Riedel SL, Lu J, Stahl U, Brigham CJ. Lipid and fatty acid metabolism in Ralstonia eutropha: relevance for the biotechnological production of value-added products. Appl. Microbiol. Biotechnol. 2014;98:1469–1483. doi:10.1007/s00253-013-5430-8.
- Matsumoto K, Morimoto K, Gohda A, Shimada H, Taguchi S. Improved polyhydroxybutyrate (PHB) production in transgenic tobacco by enhancing translation efficiency of bacterial PHB biosynthetic genes. J. Biosci. Bioeng. 2011;111:485–488.10.1016/j.jbiosc.2010.11.020
- Matsumoto K, Tanaka Y, Watanabe T, Motohashi R, Ikeda K, Tobitani K, Yao M, Tanaka I, Taguchi S. Directed evolution and structural analysis of NADPH-dependent acetoacetyl coenzyme A (Acetoacetyl-CoA) reductase from Ralstonia eutropha reveals two mutations responsible for enhanced kinetics. Appl. Environ. Microbiol. 2013;79:6134–6139.10.1128/AEM.01768-13
- Nagata T, Sakamoto K, Shimizu T. Tobacco BY-2 cells: the present and beyond. In Vitro Cell Dev. Biol. 2004;40:163–166.10.1079/IVP2003526
- Mayo KJ, Gonzales BJ, Mason HS. Genetic transformation of tobacco NT1 cells with Agrobacterium tumefaciens. Nat. Protoc. 2006;1:1105–1111.10.1038/nprot.2006.176
- Nagata T, Nemoto Y, Hasezawa S. Tobacco BY-2 cell-line as the Hela-cell in the cell biology of higher-plants. Int Rev Cytol. 1992;132:1–30.10.1016/S0074-7696(08)62452-3
- Matsumoto K, Nagao R, Murata T, Arai Y, Kichise T, Nakashita H, Taguchi S. Shimada H.,Doi Y, Enhancement of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) production in the transgenic Arabidopsis thaliana by the in vitro evolved highly active mutants of polyhydroxyalkanoate (PHA) synthase from Aeromonas caviae. Biomacromolecules;2005;6:2126–2130.10.1021/bm050113g
- Petrasovits LA, Purnell MP, Nielsen LK, Brumbley SM. Production of polyhydroxybutyrate in sugarcane. Plant Biotech. J. 2007;5:162–172.10.1111/pbi.2007.5.issue-1
- Matzke AJM, Matzke MA. Position effects and epigenetic silencing of plant transgenes. Curr. Opin. Plant Biol. 1998;1:142–148.10.1016/S1369-5266(98)80016-2
