Abstract
To better understand the ischemic-hypoxia-induced fracture healing impairment, we determined in this study the microRNA-210 expression in broken bone specimens and in osteoblasts under hypoxia and then determined the influence of microRNA-210 overexpression on the osteoblast cell proliferation and apoptosis. Results demonstrated that microRNA-210 expression was upregulated with an association with HIF-1α overexpression in clinical human catagmatic tissues and was upregulated HIF-1α-dependently in response to hypoxia in osteoblast MG-63 cells. CCK-8 assay indicated that microRNA-210 upregulation by microRNA-210 mimics reduced the chemotherapeutic 5-FU-induced osteoblast cell death, and colony formation assay demonstrated that microRNA-210 mimics promoted osteoblast cells growth. Moreover, the microRNA-210 mimics transfection inhibited the hypoxia-induced MG-63 cell apoptosis via inhibiting the activation of caspase 3 and caspase 9. Therefore, our research indicated a protective role of microRNA-210 in response to hypoxia. And microRNA-210 might serve as a protective role in bone fracture healing.
microRNA-210 ameliorates the hypoxia induced apoptosis in MG-63 cells.
Inadequate blood supply is a significant contributing factor for delayed fracture healing or nonunion.Citation1,2) The disrupted blood flow post-fracture usually leads to a hypoxic environment at the site of fracture,Citation3) as follows by cell death, delayed chondrocyte and osteoblast differentiation, and impaired fracture healing.Citation4) Thus, better understanding of the mechanisms that impair fracture healing under ischemic-hypoxic condition facilitates exploring novel therapies to stimulate fracture healing. Oxygen is involved in multiple basic cellular processes which are implicated in fracture healing, for example aerobic metabolism such as hydroxylation, oxidation, and cyclodehydration.Citation5,6) And an adequate oxygen supply is also important for the collagen synthesis process Citation7) and for the expression of several angiogenic genes through the hypoxia-inducible factor (HIF) pathway.Citation8) In addition, tissue oxygen levels may modulate stem cell maintenance, mobilization, and recruitment to sites of fracture,Citation9–13) and regulate the proliferation, mineralizationCitation14,15), and differentiation of alveolar osteoblasts. Therefore, the deep exploring of the hypoxic injury to osteoblasts and other cells implicated in fracture healing, and responded adaptation of these cells to the hypoxia lay light on strategies to stimulate fracture healing.
In recent years, accumulated factors have been reported to regulate a wide array of genes responsible for the metabolic changes under hypoxia, a pivotal component of which is hypoxia-inducible factor 1 (HIF-1).Citation16,17) HIF-1, existing as a heterodimer, is composed of a constitutively expressed HIF-1β subunit and an oxygen sensitive HIF-1α subunit. The HIF-1α/HIF-1β dimmer binds to a conserved DNA consensus on the promoters of its target genes and induces a vast array of gene products controlling essential cellular processes crucial for hypoxic adaptation.Citation18) HIF-1α plays a key role in hypoxia response, including glucose metabolism, vascular remodeling, and erythropoiesis.Citation19) Furthermore, when cells are exposed hypoxia, HIF-1α will initiate the protective and adaptive mechanism, if which is not sufficient to rescue cells from the severe hypoxia, cells die via apoptosis and even necrosis.Citation20)
microRNAs (miRNAs) are approximately 22-nt noncoding RNAs that regulate gene expressionCitation21) in a wide variety of organisms, including humans, and in a broad array of cell processes in mammals.Citation22) Recently, accumulating microRNAs have been recognized to be regulated in response to hypoxia, and to pose protective or aggravating effect in the hypoxia-induced injury. microRNA-199a has been shown to affect the HIF-1α-dependent lung structure maintenance and contribute to the pathogenesis of chronic obstructive pulmonary disease (COPD).Citation23), while microRNA-21 protects against ischemia-reperfusion and hypoxia-reperfusion-induced cardiocyte apoptosis via the phosphatase and tensin homolog/Akt-dependent mechanism.Citation24) And overexpression of microRNA-494 upregulates HIF-1α expression via PI3 K/Akt pathway and protects against hypoxia-induced apoptosis.Citation25) microRNA-210 is widely induced by hypoxia in various types of cells, such as cardiomyocytes,Citation26) keratinocyteCitation27), and other cells. microRNA-210 has been indicated to exert neuroprotective effects against oxygen-glucose deprivation through apoptosis inhibition in PC12 cells,Citation28) and to induce angiogenesis and neurogenesis in the normal adult mouse brain.Citation29) The protective role of microRNA-210 has also been recognized in hypoxic cardiomyocytes through Akt- and p53-dependent pathways.Citation26) And what is more in a rat model indicates that injection of ds microRNA-210 is effective in promoting the healing of partially torn anterior cruciate ligaments through enhancement of angiogenesis.Citation30) Taken together, microRNA-210 exerts protection in various types of cells against hypoxia, and this study will be focused on the protective effect of microRNA-210 in hypoxia accompanied with bone fracture.
In this study, we determined the microRNA-210 expression in bone specimens with fracture and in osteoblasts under a hypoxia condition, and then determined in detail the influence of microRNA-210 overexpression on the osteoblast cell proliferation and apoptosis, and the possible mechanism into the microRNA-210 regulation on the osteoblast cell proliferation.
Materials and methods
Human tissue specimens
Utilization of all catagmatic tissues and the normal bone tissues was approved by our hospital Internal Review Board (IRB) in Renmin Hospital of Wuhan University. Thirty four catagmatic tissues were obtained from femoral comminuted fractures, with size less than 0.5 cm in diameter, and not suitable for reposition, in emergency from 24 to 36 h post-fracture. And 18 normal bone tissues were from remaining femoral bone graft. All tissue specimens were stored at −80°C before utilizing.
Cell culture and treatment with reagents
The human osteoblast MG-63 cells were provided by the cell resource center of Wuhan University and were grown in Eagle’s Minimum Essential Medium (EMEM) (Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS; Gibco, Rockville, MD, USA) at 37 °C, with 5% CO2. For hypoxia treatment, cells were placed in a hypoxia incubator with 5% CO2 and 2 % oxygen. Oxygen concentration was monitored continuously (Forma 3130; Thermo Scientific, Rockford, IL, USA). MG-63 cells post-hypoxia or normoxia treatment for 0, 12, 24, 48, or 72 h, were collected for miRNA or mRNA isolation, for cellular protein extraction, or for apoptosis analysis. RNAi technology was used to knockdown HIF-1α expression, with siHIF-1α 1 (CUC AAG CAA CUG UCA UAUA), siHIF-1α 2 (UGC CAC CAC UGA UGA AUUA) and siRNA control (UAA GGC CAG ACG CGA AUUA), which were synthesized by GenePharma Technology (Shanghai, China). In brief, MG-63 cells were transfected siRNA oligos with 50 nM by lipofectamine 2000, 6 hours later, the supernatant was updated and cells were subject to followed treatment. microRNA-210 mimics (CUG UGC GUG UGA CAG CGG CUGA) or mimics control (UCA CAA CCU CCU AGA AAGA) (QIAGEN, Valencia, CA, USA) and were utilized to manipulate the microRNA-210 level. 25 or 50 nM microRNA-210 mimics/mimics control was transfected into MG-63 cells with lipofectamine 2000. 5-FU (Sigma–Aldrich, St. Louis, MO, USA), which is a thymidylate synthase inhibitor, was utilized to inhibit the MG-63 cell proliferation with a concentration of 5 μg/mL and to induce MG-63 apoptosis with a concentration of 5 or 40 μg/mL.
RNA isolation, reverse transcription, quantitative real-time PCR
Total cellular RNA was isolated with PureLink® RNA Mini Kit (Invitrogen, Carlsbad, CA, USA) according to the manual, and reverse transcription (RT) was performed with M-MLV Reverse Transcriptase (Promega, Madison, WI, USA). RT-qPCR analysis of the HIF-1α in mRNA level was performed with Takara One Step RT-PCT kit (Takara, Dalian, China). All mRNA expression levels were normalized to β-actin. miRNA specimens was isolated with the mirVana miRNA Isolation Kit (Ambion, Austin, TX, USA) and quantitatively examined for microRNA-210 expression with the mirVana qRT-PCR miRNA Detection Kit (Ambion, Austin, TX, USA), with the U6 small nuclear RNA as internal control. ∆∆Ct method was used for relative quantification.Citation31)
Preparation and Western blot analysis of protein samples
Whole cellular proteins were extracted with a cell lysis reagent (Sigma–Aldrich, St. Louis, MO, USA) according to the manual and added with protease inhibitor cocktail (Roche, Basel, Switzerland). Polyclonal rabbit antibodies against HIF-1α (Abcam, Cambridge, UK), caspase 3 (Santa Cruz Biotechnology, Santa Cruz, CA, USA) or caspase 9 (Santa Cruz Biotechnology, Santa Cruz, CA, USA) were utilized to detect the HIF-1α level, cleaved caspase 3 (CASP 3) or cleaved caspase 9 (CASP 9) level by Western blot analysis. β-actin was used as an internal control with polyclonal rabbit antibodies against β-actin (Sinobio, Beijing, China). Goat anti-rabbit IgG conjugated to horseradish peroxidase (Pierce, Rockford, IL, USA), and ECL detection systems (Super Signal West Femto; Pierce, Rockford, IL, USA) were used for detection.
Cell proliferation, cell colony formation, and cell apoptosis assay
Cell proliferation assay was performed by CCK-8 assay (DOJINDO, Kumamoto, Japan). In brief, MG-63 cells post-microRNA-210 mimics or mimics control transfection were inoculated for 24, 36, or 48 h, in the presence of 5 μg/mL 5-FU and then were incubated with CCK-8. The 450 nm absorbance of cells was detected after visual color occurrence. Cell colony formation assay was performed as usual. 103 Cells were incubated in 12-well plates at 37°C containing 5% CO2, and were transfected with 0 nM or 50 nM microRNA-210 mimics or mimics control and were inoculated for another 2–5 days. Then, cells were stained with crystal violet (0.005%) for 20 minutes and recorded the colony numbers. MG-63 cell apoptosis was examined with an annexin V-FITC apoptosis detection kit (Sigma–Aldrich, St. Louis, Missouri). Briefly, approximately 5 × 105 cells were stained with annexin V-FITC and propidium iodide and were detected by a FACScan flow cytometer (BD Biosciences). The results were calculated using the CellQuestTM Pro software (BD Biosciences) and evaluated as the percentage of apoptotic cells to total cells.
Statistical analysis
Statistical analyses were performed using SPSS17.0 software (IBM SPSS, Armonk, NY, USA). The correlation between microRNA-210 expression and HIF-1α level was analyzed using the Spearman rank correlation. The HIF-1α in mRNA level, the caspase 3 or caspase 9 expression in protein level, or microRNA-210 expression was analyzed by Student’s t test between two groups, and were analyzed by one-way ANOVA test. Statistical significance was considered when a p value <0.05 or less.
Results
microRNA-210 is upregulated in human bone specimens post-fracture, in association with HIF-1α
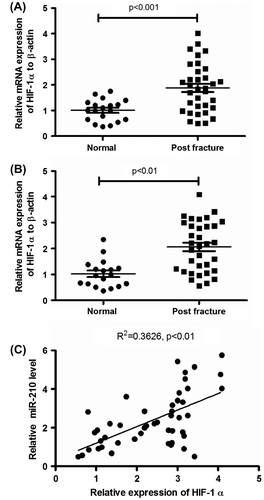
To compare the difference of microRNA-210 expression between human bone specimens with fracture and the normal bone tissues, we chose 34 aged patients with fractures in the study, who have a mean (±SD) diagnosis age of 47.2 ± 2.1 years, and we used bone specimens from 18 healthy age-matched volunteers as controls. To investigate the microRNA-210 preferentially expressed in human bone tissues to be involved in bone fracture healing, we assessed the expression of microRNA-210 in catagmatic tissues by real-time PCR. The mean (±SD) ∆∆CT value was 1.87 ± 1.23 in the 34 samples from patients with bone fracture and 1.00 ± 0.32 in healthy controls (p < 0.01; Fig. (A)). Therefore, we identified that the microRNA-210 in bone specimens significantly increased in patients with bone fracture. It has been well known that hypoxia-induced microRNA-210 via HIF-1α.Citation32) To investigate a possible upregulation of microRNA-210 by HIF-1α in catagmatic tissues, we firstly determined HIF-1α mRNA expression in the bone fracture group and normal specimens by RT-qPCR. Compared to controls, Fig. (C) showed a significant high level of HIF-1α mRNA expression in those catagmatic tissues (p < 0.01). We then investigated the correlation of microRNA-210 with HIF-1α mRNA expression. In bone fracture patients, microRNA-210 expression showed a positive correlation with HIF-1α mRNA (R2 = 0.3626, p < 0.01) (Fig. (D)).
Fig. 1. microRNA-210 is upregulated in bone tissues post-fracture and is positively correlated with HIF-1α levels.
Notes: (A) Relative microRNA-210 expression, examined by RT-qPCR, in normal bone tissues (n = 18) or bone tissues post-fracture (n = 34). (B) Relative HIF-1α mRNA level in bone tissues post-fracture (by RT-qPCR). (C) Correlation between the relative microRNA-210 expressions with the HIF-1α mRNA expression. And statistical significance was considered with a p value <0.05 or less.
microRNA-210 upregulation is regulated by HIF-1α
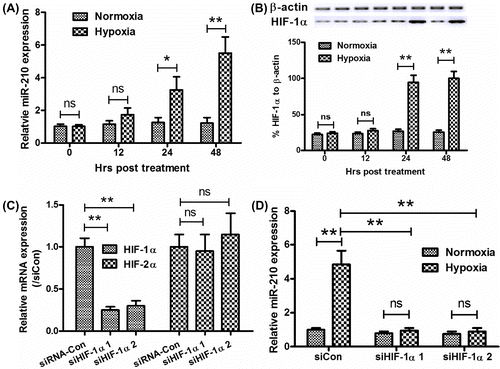
To further confirm the upregulation of microRNA-210 by hypoxia in catagmatic tissues or in osteoblasts, we determined the microRNA-210 level by real-time qPCR in MG-63 osteoblast cell line under normoxia or hypoxia. Coinciding with results of clinical specimens, the substantial and significant induction of microRNA-210 expression in the osteoblast cell line under hypoxia was confirmed in vitro with a time-dependence, from 24 h post-hypoxia, there was a significant upregulation of microRNA-210 (Fig. (A); p < 0.05 or p < 0.01) by the hypoxia. Moreover, HIF-1α was also upregulated by the hypoxia at both mRNA and protein levels (Fig. (B) and (C); either p < 0.05 or p < 0.01). To elucidate the correlation between microRNA-210 and HIF-1α, HIF-1α was knockdown by siRNAs targeting HIF-1α. Fig. (D) showed that the HIF-1α specific siRNA-1 or siRNA-2 significantly downregulated the mRNA level of HIF-1α (p < 0.01 for siHIF-1α-1 or siHIF-1α-2) rather HIF-2α. And the siHIF-1α-1 or siHIF-1α-2 could effectively abrogate the hypoxia-promoted miRNA-210 upregulation (Fig. (E)). Therefore, the microRNA-210 upregulation was associated with HIF-1α in catagmatic tissues, and its upregulation in MG-63 cells in response to hypoxia was HIF-1α-dependent.
Fig. 2. Hypoxia upregulates microRNA-210 expression in osteoblast cells in a HIF-1α-dependent way.
Notes: (A) microRNA-210 expression was upregulated by hypoxia (by RT-qPCR) in osteoblast MG-63 cells. (B) and (C) HIF-1α expression was upregulated by hypoxia at both mRNA (by RT-qPCR) and protein levels (by Western blot assay) in MG-63 cells. (D) Knockdown of HIF-1α by siRNAs transfection for 24 h in MG-63 cells. (E) Abrogation of hypoxia-induced microRNA-210 upregulation by the HIF-1α knockdown (transfection of siHIF-1α 1, siHIF-1α 2, or siRNA control for 24 h). siHIF-1α 1: siRNA-1 targeting siHIF-1α, siHIF-1α 2: siRNA-2 targeting siHIF-1α, siCon: Control siRNA. All experiments were performed in triplicate. Statistical significance was shown as *p < 0.05 and **p < 0.01, ns: no significance.
microRNA-210 mimics reduces the 5-FU-caused death and promotes the growth of osteoblast cells
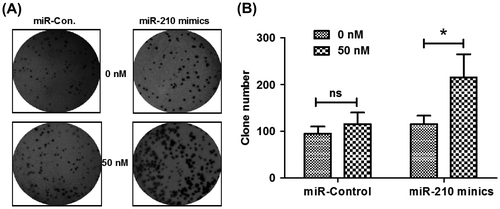
To investigate the effect of microRNA-210 to the osteoblast cell proliferation, microRNA-210 expression level in MG-63 cell lines was manipulated via transfection with microRNA-210 mimics. As Fig. (A) showed, microRNA-210 mimics induced a significant increasing in microRNA-210 level in MG-63 cells (p < 0.001 for 25 or 50 nM), compared to the control group. After that we used CCK-8 assay to detect the proliferation of MG-63 cells post-microRNA-210 mimics or miRNA control transfection and with a treatment with 5 μg/mL 5-FU. As expected, the microRNA-210 mimics, rather than miRNA control, reduced the 5-FU-caused cell death in time-dependent and dose-dependent manners in MG-63 cells (Fig. (B) and (C)). Furthermore, the difference in colony formation of MG-63 cells was investigated with microRNA-210 mimics or miRNA control transfection. Fig. showed that higher capability of colony formation for MG-63 cells post-transfecting with 50 nM microRNA-210 mimics than post-transfecting with 50 nM miRNA control (p < 0.05). In conclusion, these results demonstrated that upregulated microRNA-210 reduced the 5-FU-caused cell death and promoted the colony formation of MG-63 cells in vitro.
Fig. 3. Overexpression of microRNA-210 promotes the cellular proliferation of MG-63 cells in vitro.
Notes: (A) The upregulation of microRNA-210 level in MG-63 cells by microRNA-210 mimics transfection. (B) Dose-dependent promotion to the relative cellular proliferation of MG-63 cells in the presence of 5 μg/mL 5-FU by microRNA-210 mimics. (C) Time-dependent promotion to the relative cellular proliferation of MG-63 cells in the presence of 5 μg/mL 5-FU by microRNA-210 mimics. The experiments were performed respectively in triplicate. Statistical significance was shown as *p < 0.05, **p < 0.01, and ***p < 0.001, ns: no significance.
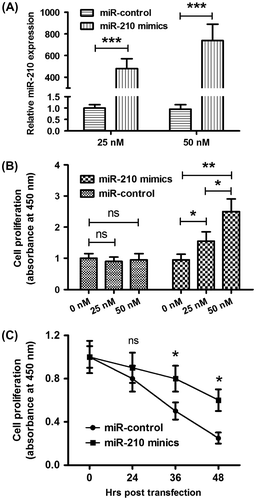
Fig. 4. microRNA-210 mimics stimulated the colony formation of MG-63 cells in vitro.
Notes: Colony formation by MG-63 cells was determined after cells’ transfection with 0 or 50 nM of microRNA-210 mimics or miR-control. The morphologic characteristics were shown of clones formated by MG-63 cells (A); the number of clones formed by MG-63 cells (B) was calculated as comparison. The experiments were performed respectively in triplicate. Statistical significance was shown as ns: no significance, *p < 0.05.
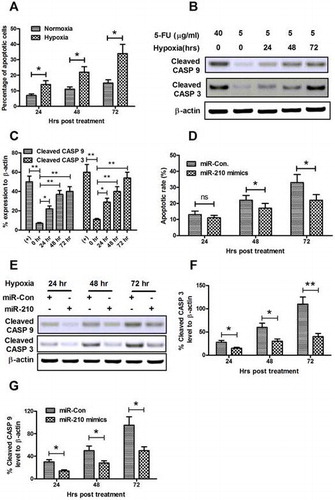
microRNA-210 mimics ameliorates the hypoxia-induced apoptosis in osteoblast cells in vitro
Recent reports showed that hypoxia induces apoptosis in various osteoblast cells.Citation33–35) It is the inhibition of the electron transport chain at the inner membrane of the mitochondria which is the most direct induction of hypoxia-induced apoptosis. Firstly, the sensitivity of MG-63 osteoblast cells to 5-FU was measured. At normoxia culture conditions, the 5-FU-induced low level of apoptosis with a slight time-dependent increasing, while hypoxia costimulates higher level of apoptosis with 5-FU in MG-63 cells (Fig. (A)). Next, the activation of caspase 3 (cleaved CASP 3) and of caspase 9 (cleaved CASP 9) was examined by Western blot assay, both of which are executive molecules in apoptosis. Results indicated that significant high levels of cleaved CASP 3 and cleaved CASP 9 were induced by a low dose of 5-FU under a hypoxia culture condition rather than under a normoxia condition (Fig. (B) and (C)).
Fig. 5. microRNA-210 mimics ameliorates the hypoxia-induced apoptosis in MG-63 cells.
Notes: (A) Hypoxia-induced apoptosis in MG-63 cells. Cells were treated with 5 μg/mL 5-FU under normoxia or hypoxia condition for 24, 48, or 72 h, then the apoptotic cells were counted by a FACScan flow cytometer. (B) Western blot assay of cleaved CASP 9 and cleaved CASP 3 expression induced by hypoxia. (C) Relative cleaved CASP 9 or cleaved CASP 3 expression as percentage to β-actin. (D) microRNA-210 mimics transfection reduced the hypoxia-induced apoptotic cells. (E–G) microRNA-210 mimics transfection inhibited activated CASP 3 and CASP 9 in MG-63 cells. Cells were transfected with 50 nM microRNA-210 mimics, and were treated with 5 μg/mL 5-FU, under hypoxia condition for 24, 48, or 72 h, then the apoptotic cells were counted by a FACScan flow cytometer; the levels of cleaved CASP 3 and cleaved CASP 9 were determined by Western blot assay. All results were the average of triple experiments. Statistical significance was shown as ns: no significance, *p < 0.05, **p < 0.01, ns: no significance.
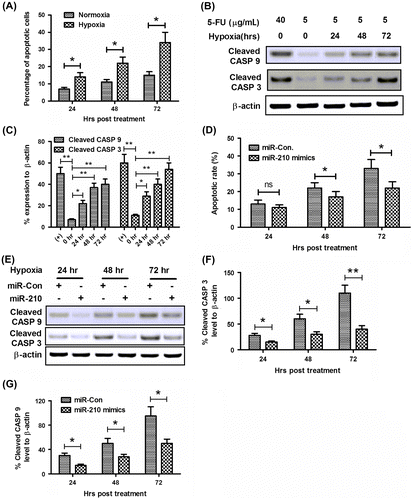
To recognize the role of microRNA-210 in the hypoxia-induced apoptosis in MG-63 cells, we transfected MG-63 cells with microRNA-210 mimics before subject to hypoxia. Fig. (C) demonstrated that from 48 hour post-hypoxia, less MG-63 cells post-microRNA-210 mimics transfection developed apoptosis (p < 0.05 for 48 or 72 hours post-treatment). And the Western blot assay for cleaved CASP 3 and cleaved CASP 9 reconfirmed the inhibition of microRNA-210 against hypoxia-induced apoptosis; less CASP 3 and CASP 9 were cleaved in the microRNA-210-transfected MG-63 cells (Fig. (E)–(G)). Therefore, the apoptosis promotion and the cleavage of both CASP 3 and CASP 9 were blocked by the microRNA-210 mimics transfection.
Discussion
In this study, we showed that microRNA-210 expression was upregulated with an association with HIF-1α overexpression in clinical human catagmatic tissues and was well upregulated HIF-1α-dependently in response to hypoxia in osteoblast cells. And the microRNA-210 upregulation by microRNA-210 mimics promoted osteoblast cells proliferation in vitro via inhibiting apoptosis. Therefore, our research indicated a protective role of microRNA-210 in response to hypoxia in vitro. microRNA-210 might serve as a protective role in bone fracture healing.
It has been well known that hypoxia-induced microRNA-210 via HIF-1α.Citation32) However, it is little known about the microRNA-210 regulation in the tissues of bones subject to fracture. Present study firstly revealed the upregulation of microRNA-210 in catagmatic tissues, with an association of HIF-1α upregulation; and there was a correlation between both molecules. To further confirm the upregulation of microRNA-210 by HIF-1α and hypoxia, we investigated the level of microRNA-210 and HIF-1α in osteoblast cell line, MG-63 cells, a significant induction of microRNA-210 and HIF-1α in the osteoblast cell line under hypoxia was also confirmed, and the microRNA-210 upregulation in MG-63 cells in response to hypoxia was HIF-1α-dependent. And what is more, present study, firstly confirmed that upregulated microRNA-210 promoted the osteoblast cell proliferation, revealing by both CCK-8 assay and colony formation assay.
Based on above-mentioned results, we speculated that the hypoxia-induced microRNA-210 upregulation might be an adaptative and protective response to hypoxia. And apoptosis in osteoblast cells have been widely accepted as a severe response to hypoxia.Citation33–35) We next investigated the role of microRNA-210 in the hypoxia-induced apoptosis in MG-63 cells. It was demonstrated that less MG-63 cells post-microRNA-210 mimics transfection developed apoptosis, and expressed the apoptosis-execution caspase, caspase 3 and caspase 9. Therefore, upregulated microRNA-210 protected MG-63 cells from hypoxia-induced apoptosis.
Oxygen is thought to be not only an indispensable metabolic substrate for a variety of in vivo enzymatic reactions, including mitochondrial respiration, but also a key regulatory signal in tissue development and homeostasis by controlling a specific genetic program. On the other side, hypoxia also plays an important role during development and cell differentiation.Citation36) Adaptation to hypoxia is a critical cellular event both in pathological settings, such as cancer, trauma healing and ischemia, and in biological processes, such as cell development and differentiation. It is known that disruption of the vascular supply and accompanying hypoxia are associated with modulated bone formation during development and fracture repair.Citation37) Multiple miroRNAs have been identified to regulate the complex process of osteogenic differentiation and osteoblastic bone formation.Citation38–44) Several miRNAs are involved in the regulation of osteoblast-specific gene expression and bone morphogenic protein 2 (BMP-2)-induced osteogenesis in vitro.Citation39,42) microRNA-210 in particular, has been well studied for its effects in rescuing cardiac function after myocardial infarcts via the upregulation of angiogenesis and inhibition of cardiomyocyte apoptosis.Citation45) Recent years, accumulated data show that it is significantly upregulated in various kinds of tumors, such as Head and Neck Paragangliomas,Citation46) lung cancer cells,Citation47) breast cancer cells.Citation48) Previous report revealed that overexpression of microRNA-210 could promote the survival of mesenchymal stem cells (MSCs) exposed to hypoxia.Citation49) However, the regulatory mechanism of microRNA-210 in the osteoblasts and other cells was not clear up to now, and it needs to be explored in future. Taken together, our finding lays light on the mechanism of osteoblastic bone formation followed by the hypoxic condition in bone fracture.
In summary, microRNA-210 is upregulated in a HIF-1α-dependent manner in both human catagmatic tissues and osteoblast cells post-hypoxia. And the microRNA-210 upregulation promotes osteoblast cells proliferation in vitro via inhibiting apoptosis.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgments
This study was supported by a grant from Renmin Hospital of Wuhan University.
Additional information
Funding
References
- Dickson KF, Katzman S, Paiement G. The importance of the blood supply in the healing of tibial fractures. Contemp. Orthop. 1995;30:489–493.
- Brinker MR, Bailey DJ. Fracture healing in tibia fractures with an associated vascular injury. J. Trauma. 1997;42:11–19.10.1097/00005373-199701000-00004
- Lu C, Rollins M, Hou H, Swartz HM, Hopf H, Miclau T, Marcucio RS. Tibial fracture decreases oxygen levels at the site of injury. Iowa Orthop J. 2008;28:14–21.
- Lu C, Miclau T, Hu D, Marcucio RS. Ischemia leads to delayed union during fracture healing: a mouse model. J. Orthop. Res. 2007;25:51–61.10.1002/(ISSN)1554-527X
- Zhang X, Schwarz EM, Young DA, Puzas JE, Rosier RN, O'Keefe RJ. Cyclooxygenase-2 regulates mesenchymal cell differentiation into the osteoblast lineage and is critically involved in bone repair. J. Clin. Invest. 2002;109:1405–1415.10.1172/JCI0215681
- Xie C, Liang B, Xue M, Lin AS, Loiselle A, Schwarz EM, Guldberg RE, O'Keefe RJ, Zhang X. Rescue of impaired fracture healing in COX-2−/− mice via activation of prostaglandin E2 receptor subtype 4. Am. J. Pathol. 2009;175:772–785.10.2353/ajpath.2009.081099
- Kivirikko KI, Prockop DJ. Enzymatic hydroxylation of proline and lysine in protocollagen. Proc. Nat. Acad. Sci. U.S.A. 1967;57:782–789.10.1073/pnas.57.3.782
- Fong GH. Regulation of angiogenesis by oxygen sensing mechanisms. J. Mol. Med. 2009;87:549–560.10.1007/s00109-009-0458-z
- Lin Q, Lee YJ, Yun Z. Differentiation arrest by hypoxia. J. Biol. Chem. 2006;281:30678–30683.10.1074/jbc.C600120200
- Thom SR, Bhopale VM, Velazquez OC, Goldstein LJ, Thom LH, Buerk DG. Stem cell mobilization by hyperbaric oxygen. Am. J. Physiol. Heart Circ. Physiol. 2006;290:H1378–H1386.
- Gallagher KA, Liu ZJ, Xiao M, Chen H, Goldstein LJ, Buerk DG, Nedeau A, Thom SR, Velazquez OC. Diabetic impairments in NO-mediated endothelial progenitor cell mobilization and homing are reversed by hyperoxia and SDF-1 alpha. J. Clin. Invest. 2007;117:1249–1259.10.1172/JCI29710
- Liu ZJ, Velazquez OC. Hyperoxia, endothelial progenitor cell mobilization, and diabetic wound healing. Antioxid. Redox Signal. 2008;10:1869–1882.10.1089/ars.2008.2121
- Ceradini DJ, Kulkarni AR, Callaghan MJ, Tepper OM, Bastidas N, Kleinman ME, Capla JM, Galiano RD, Levine JP, Gurtner GC. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat. Med. 2004;10:858–864.10.1038/nm1075
- Wu D, Malda J, Crawford R, Xiao Y. Effects of hyperbaric oxygen on proliferation and differentiation of osteoblasts from human alveolar bone. Connect. Tissue Res. 2007;48:206–213.10.1080/03008200701458749
- Nicolaije C, Koedam M, van Leeuwen JP. Decreased oxygen tension lowers reactive oxygen species and apoptosis and inhibits osteoblast matrix mineralization through changes in early osteoblast differentiation. J. Cell. Physiol. 2012;227:1309–1318.10.1002/jcp.22841
- Elks PM, van Eeden FJ, Dixon G, Wang X, Reyes-Aldasoro CC, Ingham PW, Whyte MK, Walmsley SR, Renshaw SA. Activation of hypoxia-inducible factor-1alpha (Hif-1alpha) delays inflammation resolution by reducing neutrophil apoptosis and reverse migration in a zebrafish inflammation model. Blood. 2011;118:712–722.10.1182/blood-2010-12-324186
- Eltzschig HK, Carmeliet P. Hypoxia and inflammation. N Engl. J. Med. 2011;364:656–665.
- Kaelin WJ, Ratcliffe PJ. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol. Cell. 2008;30:393–402.10.1016/j.molcel.2008.04.009
- Corn PG, Ricci MS, Scata KA, Arsham AM, Simon MC, Dicker DT, El-Deiry WS. Mxi1 is induced by hypoxia in a HIF-1-dependent manner and protects cells from c-Myc-induced apoptosis. Cancer Biol. Ther. 2005;4:1285–1294.10.4161/cbt.4.11.2299
- Piret JP, Mottet D, Raes M, Michiels C. Is HIF-1α a pro- or an anti-apoptotic protein? Biochem. Pharmacol. 2002;64:889–892.10.1016/S0006-2952(02)01155-3
- Ambros V. MicroRNA pathways in flies and worms: growth, death, fat, stress, and timing. Cell. 2003;113:673–676.10.1016/S0092-8674(03)00428-8
- Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233.10.1016/j.cell.2009.01.002
- Mizuno S, Bogaard HJ, Gomez-Arroyo J, Alhussaini A, Kraskauskas D, Cool CD, Voelkel NF. MicroRNA-199a-5p is associated with hypoxia-inducible factor-1alpha expression in lungs from patients with COPD. Chest. 2012;142:663–672.
- Yang Q, Yang K, Li A. microRNA-21 protects against ischemia-reperfusion and hypoxia-reperfusion-induced cardiocyte apoptosis via the phosphatase and tensin homolog/Akt-dependent mechanism. Mol. Med. Rep. 2014;9:2213–2220.
- Sun G, Zhou Y, Li H, Guo Y, Shan J, Xia M, Li Y, Li S, Long D, Feng L. Over-expression of microRNA-494 up-regulates hypoxia-inducible factor-1 alpha expression via PI3K/Akt pathway and protects against hypoxia-induced apoptosis. J. Biomed. Sci. 2013;20:100–108.10.1186/1423-0127-20-100
- Mutharasan RK, Nagpal V, Ichikawa Y, Ardehali H. microRNA-210 is upregulated in hypoxic cardiomyocytes through Akt- and p53-dependent pathways and exerts cytoprotective effects. Am. J. Physiol. Heart Circ. Physiol. 2011;301:H1519–H1530.10.1152/ajpheart.01080.2010
- Biswas S, Roy S, Banerjee J, Hussain SR, Khanna S, Meenakshisundaram G, Kuppusamy P, Friedman A, Sen CK. Hypoxia inducible microRNA 210 attenuates keratinocyte proliferation and impairs closure in a murine model of ischemic wounds. Proc. Nat. Acad. Sci. U.S.A. 2010;107:6976–6981.10.1073/pnas.1001653107
- Qiu J, Zhou XY, Zhou XG, Cheng R, Liu HY, Li Y. Neuroprotective effects of microRNA-210 against oxygen-glucose deprivation through inhibition of apoptosis in PC12 cells. Mol. Med. Rep. 2013;7:1955–1959.
- Zeng L, He X, Wang Y, Tang Y, Zheng C, Cai H, Liu J, Wang Y, Fu Y, Yang GY. MicroRNA-210 overexpression induces angiogenesis and neurogenesis in the normal adult mouse brain. Gene Ther. 2014;21:37–43.10.1038/gt.2013.55
- Shoji T, Nakasa T, Yamasaki K, Kodama A, Miyaki S, Niimoto T, Okuhara A, Kamei N, Adachi N, Ochi M. The effect of intra-articular injection of microRNA-210 on ligament healing in a rat model. Am. J. Sports Med. 2012;40:2470–2478.10.1177/0363546512458894
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408.10.1006/meth.2001.1262
- Kulshreshtha R, Ferracin M, Wojcik SE, Garzon R, Alder H, Agosto-Perez FJ, Davuluri R, Liu CG, Croce CM, Negrini M, Calin GA, Ivan M. A microRNA signature of hypoxia. Mol. Cell Biol. 2007;27:1859–1867.10.1128/MCB.01395-06
- Genetos DC, Wong A, Watari S, Yellowley CE. Hypoxia increases Annexin A2 expression in osteoblastic cells via VEGF and ERK. Bone. 2010;47:1013–1019.10.1016/j.bone.2010.08.024
- Utting JC, Robins SP, Brandao-Burch A, Orriss IR, Behar J, Arnett TR. Hypoxia inhibits the growth, differentiation and bone-forming capacity of rat osteoblasts. Exp. Cell. Res. 2006;312:1693–1702.10.1016/j.yexcr.2006.02.007
- Lechler P, Klein SM, Prantl L, Englert C, Renkawitz T, Grifka J. Hypoxic downregulation of cellular proliferation and loss of phenotype stability in human osteoblasts is mediated by HIF-1α. Clin. Hemorheol. Microcirc. 2011;49:279–286.
- Giaccia AJ, Simon MC, Johnson R. The biology of hypoxia: the role of oxygen sensing in development, normal function, and disease. Genes Dev. 2004;18:2183–2194.10.1101/gad.1243304
- Maes C, Carmeliet G, Schipani E. Hypoxia-driven pathways in bone development, regeneration and disease. Nat. Rev. Rheumatol. 2012;8:358–366.10.1038/nrrheum.2012.36
- Inose H, Ochi H, Kimura A, Fujita K, Xu R, Sato S, Iwasaki M, Sunamura S, Takeuchi Y, Fukumoto S, Saito K, Nakamura T, Siomi H, Ito H, Arai Y, Shinomiya K, Takeda S. A microRNA regulatory mechanism of osteoblast differentiation. Proc. Nat. Acad. Sci. U.S.A. 2009;106:20794–20799.10.1073/pnas.0909311106
- Li Z, Hassan MQ, Volinia S, van Wijnen AJ, Stein JL, Croce CM, Lian JB, Stein GS. A microRNA signature for a BMP2-induced osteoblast lineage commitment program. Proc. Nat. Acad. Sci. U.S.A. 2008;105:13906–13911.10.1073/pnas.0804438105
- Zhang J, Tu Q, Bonewald LF, He X, Stein G, Lian J, Chen J. Effects of miR-335-5p in modulating osteogenic differentiation by specifically downregulating Wnt antagonist DKK1. J. Bone Miner. Res. 2011;26:1953–1963.10.1002/jbmr.377
- Eskildsen T, Taipaleenmaki H, Stenvang J, Abdallah BM, Ditzel N, Nossent AY, Bak M, Kauppinen S, Kassem M. MicroRNA-138 regulates osteogenic differentiation of human stromal (mesenchymal) stem cells in vivo. Proc. Nat. Acad. Sci. U.S.A. 2011;108:6139–6144.10.1073/pnas.1016758108
- Itoh T, Nozawa Y, Akao Y. MicroRNA-141 and -200a are involved in bone morphogenetic protein-2-induced mouse pre-osteoblast differentiation by targeting distal-less homeobox 5. J. Biol. Chem. 2009;284:19272–19279.10.1074/jbc.M109.014001
- Huang J, Zhao L, Xing L, Chen D. MicroRNA-204 regulates Runx2 protein expression and mesenchymal progenitor cell differentiation. Stem Cells. 2010;28:357–364.
- Kapinas K, Kessler C, Ricks T, Gronowicz G, Delany AM. miR-29 modulates Wnt signaling in human osteoblasts through a positive feedback loop. J. Biol. Chem. 2010;285:25221–25231.10.1074/jbc.M110.116137
- Puisségur MP, Mazure NM, Bertero T, Pradelli L, Grosso S, Robbe-Sermesant K, Maurin T, Lebrigand K, Cardinaud B, Hofman V, Fourre S, Magnone V, Ricci JE, Pouyssegur J, Gounon P, Hofman P, Barbry P, Mari B. miR-210 is overexpressed in late stages of lung cancer and mediates mitochondrial alterations associated with modulation of HIF-1 activity. Cell Death Differ. 2011;18:465–478.10.1038/cdd.2010.119
- Merlo A, de Quiros SB, Secades P, Zambrano I, Balbin M, Astudillo A, Scola B, Aristegui M, Suarez C, Chiara MD. Identification of a signaling axis HIF-1α/microRNA-210/ISCU independent of SDH mutation that defines a subgroup of head and neck paragangliomas. J. Clin. Endocrinol. Metab. 2012;97:E2194–E2200.10.1210/jc.2012-2410
- Wang H, Bian S, Yang CS. Green tea polyphenol EGCG suppresses lung cancer cell growth through upregulating miR-210 expression caused by stabilizing HIF-1α. Carcinogenesis. 2011;32:1881–1889.10.1093/carcin/bgr218
- Camps C, Buffa FM, Colella S, Moore J, Sotiriou C, Sheldon H, Harris AL, Gleadle JM, Ragoussis J. hsa-miR-210 is induced by hypoxia and is an independent prognostic factor in breast cancer. Clin. Cancer Res. 2008;14:1340–1348.10.1158/1078-0432.CCR-07-1755
- Nie Y, Han BM, Liu XB, Yang JJ, Wang F, Cong XF, Chen X. Identification of MicroRNAs involved in hypoxia- and serum deprivation-induced apoptosis in mesenchymal stem cells. Int. J. Biol. Sci. 2011;7:762–768.10.7150/ijbs.7.762
