Abstract
Previously we identified indole-3-acetic acid (IAA) biosynthesis inhibitors that act on the conversion of l-tryptophan to indole-3-pyruvic acid in the IAA biosynthesis of Arabidopsis. In the present study, we synthesized a new compound, indole-3-oxoethylphosphonic acid (IOEP), and found that IOEP had an inhibitory effect on IAA biosynthesis in Arabidopsis. The results suggest that IOEP is a novel inhibitor of auxin biosynthesis in Arabidopsis.
Indole-3-acetic acid (IAA), the most abundant naturally occurring auxin, regulates many aspects of plant growth and development, including embryogenesis, organogenesis, and tropism. IAA biosynthesis plays crucial regulatory roles in plant growth and in responses to environmental and developmental cues. l-Tryptophan (Trp)-dependent and Trp-independent routes have been proposed for the biosynthesis of IAA. The Trp-dependent indole-3-acetaldoxime (IAOx) and indole-3-pyruvic acid (IPyA) pathways have been studied intensively, and a series of enzymes and biosynthetic intermediates have been reported for Arabidopsis (Fig. (A)).Citation1–6) Indole-3-acetaldehyde (IAAld) has been also proposed as a biosynthetic intermediate in Arabidopsis (Fig. (A)),Citation7), but it is unclear whether IAAld is synthesized from IPyA or from other intermediates of Trp-dependent pathways. A flavin monooxygenase encoded by the YUCCA gene was reported to catalyze a rate-limiting step in the IPyA pathway in Arabidopsis.Citation5)
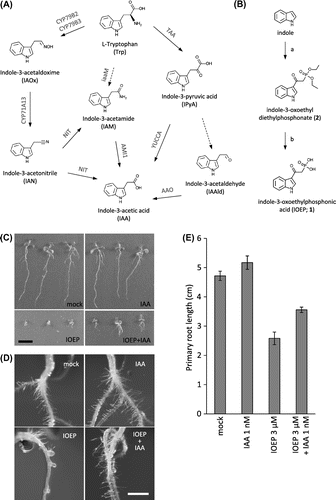
Fig. 1. Effect of IOEP on root growth in Arabidopsis seedlings.
Note: (A) IAA biosynthesis pathways and intermediates. (B) Synthesis of IOEP. a, diethyl phosphonoacetic acid, acetic anhydride in AcOH at 110 °C, 5 h, 37% yield; b, (CH3)3SiBr in MeCN at r.t., 40 h, 73% yield. Wild-type Arabidopsis thaliana (WT; ecotype Col-0) seeds were sterilized and grown for 10 d on half-strength MS agar medium containing 1% sucrose and 0.8% agar, pH 5.8 supplemented with 30 µm IOEP or 30 nm IAA. The seedlings were grown under continuous light at 22 °C. Scale bars: 1 cm (C), 2 mm (D). (E) WT seedlings were grown for 8 d on half-strength MS agar medium supplemented with 3 µm IOEP or 1 nm IAA under continuous light at 22 °C. Primary root length of the seedlings was analyzed with ImageJ software (NIH, MD). Data represent means ± SE (n = 13–15).
We have identified l-aminooxyphenylpropionic acid (AOPP) as an auxin biosynthesis inhibitor.Citation8) Presumably it targets tryptophan aminotransferase (TAA), because AOPP inhibited the conversion of Trp into IPyA in enzyme extracts from Arabidopsis and wheat. In addition, l-kynurenine was identified by chemical screening as another IAA biosynthesis inhibitor that targets TAA1, a TAA step enzyme of the biosynthesis pathway in Arabidopsis.Citation9) AOPP is an inhibitor of phenylalanine ammonia lyase.Citation10) The root growth inhibition caused by AOPP was almost completely eliminated by IAA when Arabidopsis seedlings were grown on 0.8% agar containing 1% sucrose.Citation8) However, because AOPP is a relatively unstable compound, it is not effective enough when added to half-strength Murashige–Skoog agar medium.Citation8) In an attempt to develop a novel auxin biosynthesis inhibitor, we designed a new compound, indole-3-oxoethylphosphonic acid (IOEP), based on an in silico analysis of the crystal structure of TAA1.Citation2) We synthesized IOEP (Fig. (B) and Supplemental data; see doi:10.1080/09168451.2014.877183) and characterized its inhibitory effect on auxin biosynthesis in Arabidopsis.
To investigate the effect of IOEP on the growth of Arabidopsis, seedlings were grown in the presence of 30 μm IOEP (Fig. (C)). They exhibited defects in both root growth and shoot growth (Fig. (C)). These growth defects were partially alleviated when the seedlings were treated with 30 nm IAA (Fig. (C)). Because root hair formation and elongation are promoted by auxin,Citation11) we observed the growth of root hairs in seedlings treated with IOEP (Fig. (D)). Root hair growth was inhibited by IOEP, and growth was restored by treatment with IAA (Fig. (D)). At a lower concentration (3 μm), IOEP inhibited growth of primary roots, which significantly recovered from growth inhibition in the presence of IAA (1 nm; Fig. (E)). These observations suggest that IOEP might inhibit auxin biosynthesis in Arabidopsis. On the other hand, we did not detect any significant changes in the numbers of lateral root when Arabidopsis seedlings were grown in the presence of IOEP (data not shown).
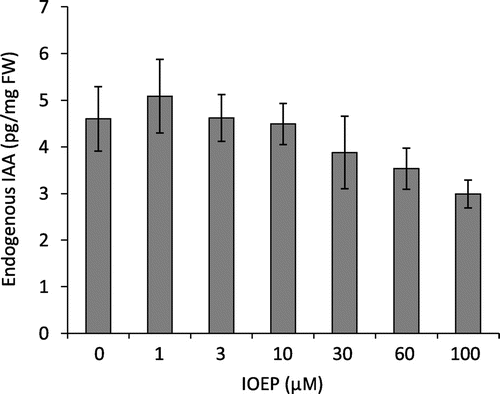
To assess the effect of IOEP on IAA biosynthesis, we treated Arabidopsis seedlings with various concentrations of IOEP and analyzed endogenous IAA levels by liquid chromatography-tandem mass spectroscopy (LC–MS/MS). IOEP treatment at concentrations of 30 μm and greater reduced endogenous IAA levels (Fig. ). These results suggest that IOEP plays a role in the inhibition of IAA biosynthesis in Arabidopsis.
Fig. 2. Effect of IOEP on endogenous IAA Levels in Arabidopsis seedlings.
Note: WT seeds were sterilized and grown on half-strength MS agar medium for 6 d. The seedlings were transferred to half-strength MS liquid medium containing 1% sucrose, pH 5.8, cultured for 1 d, and then treated with IOEP for 3 h. Endogenous IAA levels were analyzed as previously described.Citation8) Data represent means ± SE (n = 3).
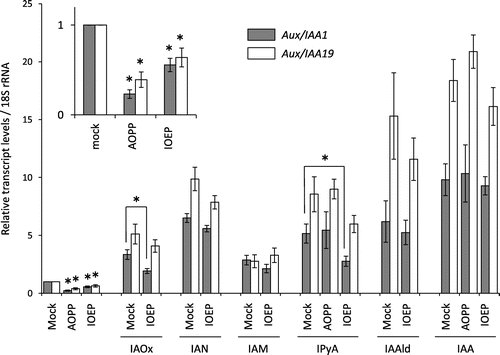
Next, we investigated the effect of IOEP on auxin activity by monitoring the expression of the early auxin-inducible genes Aux/IAA1 and Aux/IAA19 in Arabidopsis seedlings. Treatment of the seedlings with IOEP (3 h, 60 μm) repressed the expression of Aux/IAA1 and Aux/IAA19 similar to treatment with AOPP (3 h, 60 μm; Fig. , inset). Repression of Aux/IAA genes by AOPP or IOEP was eliminated by treatment with IAA (1 h, 10 μm; Fig. ). Together with the results for root growth recovery (10 d, IOEP 30 μm and IAA 30 nm; Fig. (C–E)) and the analysis of endogenous IAA levels (Fig. ), these results indicate that IOEP does not prevent auxin signaling, but inhibits auxin biosynthesis.
Fig. 3. qRT-PCR analysis of Aux/IAA1 and Aux/IAA19 gene expression in response to treatment with IOEP and IAA biosynthesis intermediates.
Note: WT seeds were sterilized and grown for 7 d in half-strength MS liquid medium. The seedlings were pretreated with 60 µm AOPP or 60 µm IOEP for 2 h and then treated with 10 µm IAA or its biosynthesis intermediate for 1 h. qRT-PCR analysis was done as described previously.Citation8) Data represent means ± SE (n = 3). Statistically significant differences relative to mock treatment are indicated by asterisks (student’s t-test, *p < 0.1).
We synthesized recombinant TAA1 protein in Escherichia coli and purified it to conduct an in vitro enzyme activity assay. TAA1 enzymatic activity was inhibited by AOPP (our unpublished results), but IOEP did not show inhibitory activity against the TAA1 enzyme in this in vitro system (data not shown). Previously, we identified AOPP and aminooxyacetic acid (AOA) as inhibitors of the conversion of Trp to IPyA in Arabidopsis enzyme extracts.Citation8,12) Both AOPP and AOA contain an anaminooxy residue and inhibit IAA biosynthesis, presumably at the TAA step.Citation8) IOEP has an inhibitory effect on IAA biosynthesis, but it lacks an aminooxy residue. Hence, we speculated that the target of IOEP in the IAA biosynthesis pathway is different from that of AOPP. An inhibitor that prevents an enzymatic step in IAA biosynthesis other than the TAA step has not been reported.
Analysis of the conversion of IAA intermediates to IAA is a conceptually straightforward approach to investigation of the target of IOEP in auxin biosynthesis, but this approach was difficult to employ for technical reasons. We took an alternative approach, as follows: the expression levels of auxin-inducible genes were determined in Arabidopsis seedlings pretreated with IOEP (2 h, 60 μm) and then treated with IAA biosynthesis intermediates (1 h, 10 μm) to determine the effects of IOEP on the conversion of these intermediates to IAA. Since Trp treatment did not induce Aux/IAA genes even in the absence of the biosynthesis inhibitor (data not shown), Trp was not included in this analysis. In our previous study,Citation13) we found that IAOx, indole-3-acetnitrile (IAN), indole-3-acetamide (IAM), IPyA, and IAAld efficiently induced the expression of the Aux/IAA genes. Hence, we assessed the effects of these intermediates (Fig. ). IAN, IAM, and IAAld induced the expression of both Aux/IAA1 and Aux/IAA19, but Aux/IAA gene expression was not significantly changed in the presence of IOEP (Fig. ). In contrast, IOEP treatment reduced IPyA-induced expression of the Aux/IAA1 and Aux/IAA19 genes (54 and 61%, respectively) with a statistically significant reduction in Aux/IAA1 expression relative to the mock treatment (Fig. ), while AOPP treatment did not reduce IPyA-induced expression of the Aux/IAA genes (Fig. ). IOEP treatment also reduced IAOx-induced expression of Aux/IAA1 and Aux/IAA19 (61 and 80%, respectively) (Fig. ). The results of these gene expression analyses suggest that IOEP inhibits the conversion of IPyA to IAA or of IAOx to IAA in Arabidopsis, while AOPP does not inhibit the conversion of IPyA to IAA.
In this study, we analyzed the inhibitory effect of IOEP on IAA biosynthesis in Arabidopsis. The results indicate that IOEP is a novel auxin biosynthesis inhibitor whose putative target site is different from that of AOPP. Previously reported auxin biosynthesis inhibitors, such as AOPP and l-kynurenine, contain aminooxy or amino residues. On the other hand, IOEP does not contain aminooxy or amino residues. Consequently, IOEP is a lead compound in the development of auxin biosynthesis inhibitor.
In our previous report, 3 h treatment of WT seedlings with 60 μm AOPP reduced the endogenous IAA level by more than 50%,Citation8) 3 h treatment with 60 μm IOEP reduced the endogenous IAA level by only 23% (Fig. ). Furthermore, qRT-PCR analysis indicated that AOPP inhibited the expression of auxin-inducible genes more effectively than IOEP (Fig. ). A possible explanation of the inhibitory activity of IOEP is that IOEP inhibits a minor IAOx-dependent pathway or a minor auxin biosynthesis pathway downstream of IPyA (Fig. (A), broken arrow), while AOPP inhibits a major auxin biosynthesis pathway. Another possible explanation is that IOEP targets the main pathway, YUCCA, but the inhibitory activity is weak. Further studies are required to investigate the target site of IOEP.
Since inhibition of Arabidopsis growth by IOEP was not fully alleviated in the presence of IAA (Fig. (C) and (E)), the effect of IOEP is not specific to auxin biosynthesis. Thus, further chemical screening or rational chemical design and development is required to produce a more specific IAA biosynthesis inhibitor.
Supplemental material
The supplemental material for this paper is available at http://dx.doi.org/10.1080/09168451.2014.877183.
Supplemental—Synthesis of IOEP
Download PDF (74.4 KB)Acknowledgments
We thank Mr Ko Kikuzato, Ms Akiko Stato, and Ms. Yuka Mitani for the technical assistance. The paper is contribution No. 1011 from Kihara Institute for Biological Research, Yokohama City University.
Funding
This work was supported by the Scientific Technique Research Promotion Program for Agriculture, Forestry, Fisheries, and Food industry (to Y.S. and K.S.). It was supported in part by Grants-in-Aid for Scientific Research [grant number 23580144] to M. S. and [grant number 23510285] to K. H.) from the Japan Society for the Promotion of Science.
Notes
Abbreviations: AOA, aminooxyacetic acid; AOPP, l-aminooxyphenylpropionic acid; IAA, indole-3-acetic acid; IAAld, indole-3-acetaldehyde; IAM, indole-3-acetamide; IAN, indole-3-acetnitrile; IAOx, indole-3-acetaldoxime; IOEP, indole-3-oxoethylphosphonic acid; IPyA, indole-3-pyruvic acid; qRT-PCR, quantitative real-time PCR; TAA, tryptophan aminotransferase of arabidospsis; Trp, l-tryptophan.
References
- Zhao Y, Hull AK, Gupta NR, Goss KA, Alonso J, Ecker JR, Normanly J, Chory J, Celenza JL. Trp-dependent auxin biosynthesis in Arabidopsis: involvement of cytochrome P450s CYP79B2 and CYP79B3. Genes Dev. 2002;16:3100–3112.
- Tao Y, Ferrer JL, Ljung K, Pojer F, Hong F, Long JA, Li L, Moreno JE, Bowman ME, Ivans LJ, Cheng Y, Lim J, Zhao Y, Ballaré CL, Sandberg G, Noel JP, Chory J. Rapid synthesis of auxin via a new tryptophan-dependent pathway is required for shade avoidance in plants. Cell. 2008;133:164–176.
- Stepanova AN, Robertson-Hoyt J, Yun J, Benavente LM, Xie DY, Doležal K, Schlereth A, Jürgens G, Alonso JM. TAA1-mediated auxin biosynthesis is essential for hormone crosstalk and plant development. Cell. 2008;133:177–191.
- Sugawara S, Hishiyama S, Jikumaru Y, Hanada A, Nishimura T, Koshiba T, Zhao Y, Kamiya Y, Kasahara H. Biochemical analyses of indole-3-acetaldoxime-dependent auxin biosynthesis in Arabidopsis. Proc. Nat. Acad. Sci. USA. 2009;106:5430–5435.
- Mashiguchi K, Tanaka K, Sakai T, Sugawara S, Kawaide H, et al. The main auxin biosynthesis pathway in Arabidopsis. Proc. Nat. Acad. Sci. USA. 2011;108:18512–18517.
- Won C, Shen X, Mashiguchi K, Zheng Z, Dai X, et al. Conversion of tryptophan to indole-3-acetic acid by TRYPTOPHAN AMINOTRANSFERASES OF ARABIDOPSIS and YUCCAs in Arabidopsis. Proc. Nat. Acad. Sci. USA. 2011;108:18518–18523.
- Sekimoto H, Seo M, Kawakami N, Komano T, Desloire S, et al. Molecular cloning and characterization of aldehyde oxidases in Arabidopsis thaliana. Plant Cell Physiol. 1998;39:433–442.
- Soeno K, Goda H, Ishii T, Ogura T, Tachikawa T, et al. Auxin biosynthesis inhibitors, identified by a genomics-based approach, provide insights into auxin biosynthesis. Plant Cell Physiol. 2010;51:524–536.
- He W, Brumos J, Li H, Ji Y, Ke M, et al. A small-molecule screen identifies L-kynurenine as a competitive inhibitor of TAA1/TAR activity in ethylene-directed auxin biosynthesis and root growth in Arabidopsis. Plant Cell. 2011;23:3944–3960.
- Amrhein N, Gerhardt J. Superinduction of phenylalanine ammonia-lyase in gherkin hypocotyls caused by the inhibitor, L-alpha-aminooxy-beta-phenylpropionic acid. Biochim. Biophys. Acta. 1979;583:434–442.
- Pitts RJ, Cernac A, Estelle M. Auxin and ethylene promote root hair elongation in Arabidopsis. Plant J. 1998;16:553–560.
- Ishii T, Soeno K, Asami T, Fujioka S, Shimada Y. Arabidopsis seedlings over-accumulated indole-3-acetic acid in response to aminooxyacetic acid. Biosci. Biotechnol. Biochem. 2010; 74:2345–2347.
- Ishida Y, Nakamura A, Mitani Y, Suzuki M, Soeno K, Asami T, Shimada Y. Comparison of indole derivatives as potential intermediates of auxin biosynthesis in Arabidopsis. Plant Biotechnol. 2013;30:185–190.
