Abstract
After thermal incubation at 48 °C for 10 min, single variants of Moloney murine leukemia virus reverse transcriptase, V433R and V433K in which a surface hydrophobic residue, Val433, was mutated, retained 55% of initial reverse transcription activity, while the wild-type enzyme retained 17%. After thermal incubation at 50 °C for 10 min, multiple variants D108R/E286R/V433R and D108R/E286R/V433R/D524A, in which Val433→Arg was combined with stabilizing mutations we identified previously, Asp108→Arg and Glu286→Arg, and RNase H activity-eliminating mutation Asp524→Ala, retained 70% of initial activity, exhibiting higher stability than V433R or V433K.
Reverse transcriptase (RT) [EC 2.7.7.49] from Moloney murine leukemia virus (MMLV) is widely used in cDNA synthesis.Citation1) For cDNA synthesis, a higher reaction temperature is desirable, because this reduces RNA secondary structures and nonspecific primer binding. However, MMLV RT is not thermostable: the temperature reducing the initial reverse transcription activity by 50% during 10 min incubation (T50) is 44 °C.Citation2) Hence to increase the thermostability of MMLV RT is an important aim.
MMLV RT is a 75-kDa monomer comprising the fingers, palm, thumb, and connection subdomains and the RNase H domain. Like other RTs, it shows RNA- and DNA-dependent DNA polymerase and RNase H activities. The active site of the DNA polymerase reaction resides in the fingers/palm/thumb domain, while that of the RNase H reaction lies in the RNase H domain. The thermostability of MMLV RT was first improved by eliminating RNase H activity.Citation3-Citation5) Recently, Arezi and Hogrefé increased thermostability by random mutagenesis in combination with activity screening at high temperature and generated a highly stable multiple variant, E69K/E302R/W313F/L435G/N454KCitation6) (In this report, mutation of a residue, e.g. Glu69 to Lys, is designated Glu69→Lys, and the MMLV RT variant, e.g. bearing Glu69→Lys, is designated E69K). The optimum temperature for reverse transcription activity was 50−55 °C, higher than that of wild-type MMLV RT (WT) (45 °C).Citation6) Baranauskas et al. increased thermostability by random mutagenesis in combination with compartmentalized ribosome display evolution, and generated a highly stable multiple variant, L139P/D200N/T330P/L603W/E607K.Citation7) Its half-life (t1/2) for reverse transcription activity during incubation at 50 °C was 492 min, higher than that of WT (40 min).Citation7) We increased thermostability by introducing positive charges by site-directed mutagenesis at positions that have been implicated in interaction with the template–primer (T/P), and generated highly stable multiple variants, E286R/E302K/L435R (MM3) and E286R/E302K/L435R/D524A (MM4).Citation8) Their T50 values, of MM3 and MM4, were 54 and 56 °C, respectively, higher than that of WT (44 °C).Citation8) We increased the thermostability of avian myeloblastosis virus (AMV) RT by the same strategy.Citation9,Citation10)
It is generally thought that surface hydrophobic residues decrease protein stability, but according to a summary of characterization of about 700 variants of the phage T4 lysozyme,Citation11) most surface residues contribute little to stability and the number of stabilizing mutations of surface residues is small, suggesting that the chances that one increases the thermostability of MMLV RT by replacing a surface hydrophobic residue with a charged one are low. Sequence analysis has revealed that MMLV RT has five consecutive hydrophobic residues, Leu432-Val433-Ile434-Leu435-Ala436, in the connection domain.Citation12) Das and Georgiadis increased the solubility of MMLV RT by a mutation of Leu435 into Lys.Citation12) They determined the three-dimensional structure of variant L435K and found that MMLV RT has consecutive surface hydrophobic residues, Phe303-Leu304 and Leu432-Val433-Ile434 (Fig. ).Citation13)
Fig. 1. Structure of MMLV RT Variant L435K.
Note: The structure is based on Protein Data Bank No. 1RW3. (A) Overall structure. (B) Close-up view of the surface region, in which Phe303 and Leu304 are located. (C) Close-up view of the surface region, in which Leu432, Val433, and Ile434 are located.
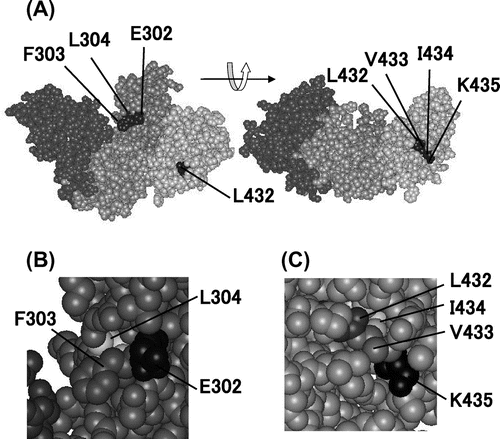
On the assumption that MMLV RT stability increases upon introduction of the charged residue into one of these consecutive surface hydrophobic residues, 10 variants (F303R, F303K, L304R, L304K, L432R, L432K, V433R, V433K, I434R, and I434K) were constructed in which one of these five residues was replaced with Arg or Lys. C-terminal (His)6-tagged variants were expressed in Escherichia coli and purified from the cells by a method described previously.Citation14) Following SDS-PAGE under reducing conditions, the purified enzyme preparations yielded a single band with a molecular mass of 75 kDa (Fig. (A)).
Fig. 2. Analysis of Single MMLV RT Variants.
Note: (A) SDS-PAGE under reducing conditions. Coomassie Brilliant Blue-stained 10% SDS-polyacrlamide gel is shown. The arrow indicates the band corresponding to MMLV RT. (B) Specific activity. One unit was defined as the amount that incorporates 1 nmol of dTTP into poly(rA)-p(dT)15 in 10 min. Relative specific activity was defined as the ratio of the specific activity of RT to that of WT (34,000 units/mg). (C) Thermal stability. RT at 50 nM was incubated in 10 mM phosphate buffer (pH 7.6), 2 mM DTT, 0.2% v/v Triton X-100, 10% v/v glycerol, and 28 μM poly(rA)-p(dT)15 at 48 or 50 °C for 10 min. Then a dTTP incorporation reaction was carried out at 37 °C. Relative activity was defined as the ratio of the initial reaction rate of RT with 10 min incubation at 48 or 50 °C to that without the incubation.
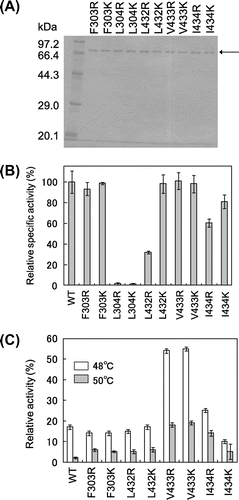
Fig. (B) shows the specific activities of the reverse transcription reaction for WT and all the single variants. The reaction was carried out in 10 mM Tris-HCl buffer (pH 8.3) containing 50 mM KCl, 2 mM dithiothreitol (DTT), 5 mM MgCl2, 12.5 μM poly(rA)-p(dT)15 (this concentration is expressed based on p(dT)15), 0.2 mM [3H]dTTP (1.85 Bq/pmol), and 5 nM MMLV RT at 37 °C. The reaction rate was determined by the time-course of the amounts of [3H]dTTP incorporated into poly(rA)-p(dT)15. The RT concentration was determined with Protein Assay CBB Solution (Nacalai Tesque, Kyoto, Japan) with bovine serum albumin (Nacalai Tesque) as standard. All the variants were classifiable into three groups: (i) The specific activities of L304R and L304K were close to zero; (ii) the specific activities of L432R and I434R were 40−60% of that of WT; and (iii) the specific activities of the other six variants were 80−100% of that of WT.
Fig. (C) shows the relative activities of WT and all single variants except for L304R and L304K, the activities of which were markedly reduced. Relative activity was defined as the ratio of the reaction rate for 10 min incubation at 48 or 50 °C in the presence of T/P to the rate without incubation. All variants were classifiable into two groups: (i) The relative activities of V433R and V433K were 3−5-fold higher than that of WT and (ii) the relative activities of other six variants were similar to that of WT.
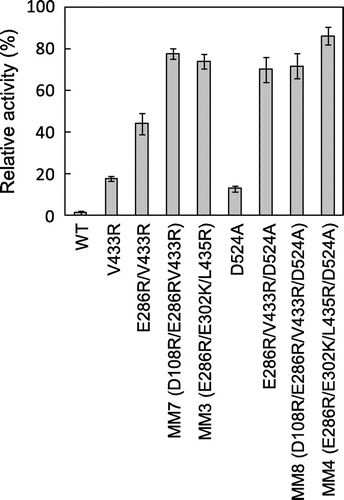
It is generally thought that if mutated sites are not in contact, the chances that mutational effects will be additive are high.Citation11) Indeed, we have generated highly stable MMLV RT variants MM3 and MM4: MM3 has stabilizing mutations Glu286→Arg, Glu302→Lys, and Leu435→Arg, and MM4 has these three mutations and RNase H activity-eliminating and stabilizing mutation Asp524→Ala.Citation8) In this study, we selected Val433→Arg as the mutation to be combined. Fig. shows the relative activities of WT and variants for incubation at 50 °C for 10 min in the presence of T/P. Relative activities increased with increasing numbers of mutations combined to Val433→Arg, and reached 70% for D108R/E286R/V433R (which we termed MM7) and D108R/E286R/V433R/D524A (MM8), indicating that the effects of the stabilizing mutations were additive. As Fig. also indicates, the stabilities of MM7 and MM8 were similar to those of MM3 and MM4, which we generated previously.Citation8) The specific activities of the enzyme preparations were 31,000 units/mg for MM3, 35,000 units/mg for MM4, 33,000 units/mg for MM7, and 25,000 units/mg for MM8, almost the same.
Fig. 3. Thermal Stability of Multiple MMLV RT Variants.
Note: RT at 50 nM was incubated with 28 μM poly(rA)-p(dT)15 at 50ºC for 10 min. Then a dTTP incorporation reaction was carried out at 37 °C. Relative activity was defined as the ratio of the initial reaction rate of RT with 10 min incubation at 50 °C to that without incubation.
MMLV RT is an unstable enzyme. It aggregates easily. In this study, all purification procedures were conducted at 4 °C in the presence of 2.0 mM DTT and 10% v/v glycerol, and storage was done at −80 °C in the presence of 2.0 mM DTT and 50% glycerol. It is generally thought that two steps, the formation of intermolecular disulfide bonds and intermolecular interaction of hydrophobic surfaces, are important in protein aggregation. We think that the stabilizing effects of Val433→Arg and Val433→Lys result from a decrease in the interaction of hydrophobic surfaces. Thus, V433R and V433 K aggregate less easily than WT. Regarding this, we have found that glycerol inhibited the inactivation of MMLV RT during incubation at 43 °C.Citation15) Glycerol is an osmolyte that reduces the water activity of the solution. It stabilizes proteins and folds denatured proteins correctly.Citation16) Such a high glycerol concentration does not cause any problem in use of MMLV RT in cDNA synthesis, but does for physico-chemical analysis of MMLV RT. We speculate that the mechanisms of the effects of glycerol and mutation Val433→Arg on MMLV RT stability are similar. If this is true, the glycerol concentration required to minimize aggregation can be reduced for variants with mutation Val433→Arg. As for the fidelity of reverse transcription, we think that Val433→Arg and Val433→Lys have no effect on it, because the active site of DNA polymerase reaction resides in the fingers/palm/thumb domain, not in the connection subdomain in which Val433 is located.
We think that the thermostable multiple MMLV RT variants that weCitation8) and othersCitation6,Citation7) have generated and which we generated in this study are more valuable for various research projects, including transcriptome analysis and clinical diagnosis, than wild-type MMLV RT. However, without a precise comparison of fidelity and processivity, it is difficult to determine which variant is the most suitable. To address this, further characterization of the thermostable variants generated previously (MM3 and MM4)Citation8) and in this study (MM7 and MM8) by misincoporation, mispair extension, and primer extension assayCitation17,Citation18) is currently in progress.
Acknowledgments
This study was supported in part by Grants-in-Aid for Scientific Research (Nos. 19580104 and 21580110, to K. Y.) and for JSPS Fellows (No. 25-1955, to A. K.) from the Japan Society for the Promotion of Science.
References
- Kimmel AR, Berger SL. Preparation of cDNA and the generation of cDNA libraries: overview. Methods Enzymol. 1982;152:307–316.
- Yasukawa K, Nemoto D, Inouye K. Comparison of the thermal stabilities of reverse transcriptases from avian myeloblastosis virus and Moloney murine leukaemia virus. J. Biochem. 2008;143:261–268.
- Kotewicz ML, D’Alessio JM, Driftmier KM, Blodgett KP, Gerard GF. Cloning and overexpression of Moloney murine leukemia virus reverse transcriptase in Escherichia coli. Gene. 1985;35:249–258.
- Gerard GF, Potter RJ, Smith MD, Rosenthal K, Dhariwal G, Lee J, Chatterjee DK. The role of template-primer in protection of reverse transcriptase from thermal inactivation. Nucleic Acids Res. 2002;30:3118–3129.
- Mizuno M, Yasukawa K, Inouye K. Insight into the mechanism of the stabilization of Moloney murine leukaemia virus reverse transcriptase by eliminating RNase H activity. Biosci. Biotechnol. Biochem. 2010;74:440–442.
- Arezi B, Hogrefé H. Novel mutations in Moloney murine leukemia virus reverse transcriptase increase thermostability through tighter binding to template-primer. Nucleic Acids Res. 2009;37:473–481.
- Baranauskas A, Paliksa S, Alzbutas G, Vaitkevicius M, Lubiene J, Letukiene V, Burinskas S, Sasnauskas G, Skirgaila R. Generation and characterization of new highly thermostable and processive M-MuLV reverse transcriptase variants. Protein Eng. Des. Sel. 2012;25:657–668.
- Yasukawa K, Mizuno M, Konishi A, Inouye K. Increase in thermal stability of Moloney murine leukaemia virus reverse transcriptase by site-directed mutagenesis. J. Biotechnol. 2010;150:299–306.
- Konishi A, Nemoto D, Yasukawa K, Inouye K. Comparison of the thermal stabilities of the αβ heterodimer and the α subunit of avian myeloblastosis virus reverse transcriptase. Biosci. Biotechnol. Biochem. 2011;75:1618–1620.
- Konishi A, Yasukawa K, Inouye K. Improving the thermal stability of avian myeloblastosis virus reverse transcriptase α subunit by site-directed mutagenesis. Biotechnol. Lett. 2012;34:1209–1215.
- Baase WA, Liu L, Tronrud DE, Matthews BW. Lessons from the lysozyme of phage T4. Protein Sci. 2010;19:631–641.
- Das D, Georgiadis MM. A directed approach to improving the solubility of Moloney murine leukemia virus reverse transcriptase. Protein Sci. 2001;10:1936–1941.
- Das D, Georgiadis MM. The crystal structure of the monomeric reverse transcriptase from Moloney murine leukemia virus. Structure. 2004;12:819–829.
- Konishi A, Shinomura M, Yasukawa K. Enzymatic characterization of human immunodeficiency virus type 1 reverse transcriptase for use in cDNA synthesis. Appl. Biochem. Biotechnol. 2013;169:77–87.
- Yasukawa K, Konishi A, Inouye K. Effects of organic solvents on the reverse transcription reaction catalyzed by reverse transcriptases from avian myeloblastosis virus and Moloney murine leukaemia virus. Biosci. Biotechnol. Biochem. 2010;74:1925–1930.
- Rariy RV, Klibanov AM. Correct protein folding in glycerol. Proc. Nat. Acad. Sci. USA. 1997;94:13520–13523.
- Barrioluengo V, Álvarez M, Barbieri D, Menéndez‑Arias L. Thermostable HIV-1 group O reverse transcriptase variants with the same fidelity as murine leukaemia virus reverse transcritpase. Biochem. J. 2011;436:599–607.
- Álvarez M, Barrioluengo V, Afonso-Lehmann RN, Menéndez-Arias L. Altered error specificity of RNase H-deficient HIV-1 reverse transcriptases during DNA-dependent DNA synthesis. Nucleic Acids Res. 2013;41:4601–4612.
