Abstract
The volatile oils were isolated from dried Schisandra chinensis Baill. seeds by Soxhlet extraction (SE), microwave-assisted extraction (MAE), and simultaneous distillation extraction (SDE), and fractions were identified by gas chromatography–mass spectrometry (GC–MS) and high-performance liquid chromatography (HPLC). The essential oils were assessed for their antioxidant and antibacterial activities. GC–MS results also revealed that the major ingredients in the oil extracted by SDE were terpenoids compounds such as ylangene (15.01%), α-phellandrene (8.23%), β-himachalene (6.95%), and cuparene (6.74), and the oil extracts of MAE and SE mainly contained aromatics such as schizandrins, wuweizisu C, and gomisin A. HPLC analysis results confirmed that more schizandrin was obtained through extraction by MAE (996.64 μg/g) and SE (722.13 μg/g). SDE oil extract showed more significant antioxidant activity than MAE or SE oil. Only volatile oil from SDE showed good antibacterial activity against all tested strains.
Schisandra chinensis Baill. (Family Magnoliaceae) was originally found in northern China and adjacent regions of Russia and Korea. Two species, S. chinensis and S. sphenanthera, are officially recognized as traditional Chinese herbal medicines for the treatment of coughs, asthma, night sweats, nocturnal emissions, and chronic diarrhea.Citation1) The dry fruit of S. chinensis contains approximately 20% essential oils, while its essential oil contains more monoterpene hydrocarbons than S. spenanthera fruit.Citation2) Song et al.Citation3) have reported that the S. chinensis essential oil is rich in sesquiterpene derivatives such as δ-cadinene (25.6%), β-himachalene, and santalol, and shows significant anti-inflammatory and antitumor activities.Citation4)
The essential oils and volatile fractions of natural plants are traditionally separated by hydrodistillation or Soxhlet extraction (SE) using organic solvents with low polarity (hexane, acetone, ether, and so on). SE is a commonly used technique to extract essential oils as it provides satisfactory yields with no necessity of filtration. However, the process has several drawbacks such as a long processing time and a substantial consumption of energy. Novel techniques such as microwave-assisted extraction (MAE), supercritical fluid extraction (SFE), ultrasonic-assisted extraction, and simultaneous distillation extraction (SDE) have been developed recently for essential oil extraction. Chen et al.Citation5) believe that MAE has great promise to be developed as a main technique in the future for industrial essential oil production, and advocate intensive researches on MAE for essential oils from aromatic plants. At the same time, a number of researchers have used SDE of volatile compounds from different spices and aromatic plants for analytic purposes, or as a means of obtaining essential oils as flavor and fragrance ingredients for food, flavoring, and pharmaceutical industries.Citation6,Citation7)
Essential oil from S. chinensis berries has been isolated by solvent-free MAE, SFE, as well as the conventional hydrodistillation method,Citation8–Citation10) but rarely can any literature be found on the extraction of volatile compounds from the seeds of S. chinensis or on evaluation of the biological activities of S. chinensis seed oil. The aim of this study was to extract volatile oils from S. chinensis seeds using Soxhlet, microwave-assisted methods, and SDE methods, to identify the main chemical compounds of S. chinensis seed extracts using gas chromatography-mass spectrometry (GC–MS) and high-performance liquid chromatography (HPLC), and to evaluate the antioxidant and antibacterial activities of the extracted S. chinensis seed oils.
Materials and methods
Plant material
S. chinensis Baill. seeds were purchased in Mungyeong in the Gyeongbuk area in Korea. They were properly dried (moisture 8 ± 0.19%), ground into powder, packed in a polyethylene zipper bag, and stored in a refrigerator at −18 °C.
MAE apparatus and procedure
A MAE device (Soxwave 100, Prolabo, Fontenay, France) with focused irradiation power under atmospheric pressure conditions was used. The emission frequency was 2450 MHz and the microwave power was linearly adjustable between 60 and 300 W. S. chinensis seed powder (25 g) was placed in a quartz tube, blended with 100 mL of pure hexane solvent, and extracted at a microwave power of 180 W for 10 min. After that, the extract was removed from the vessel and filtered. The organic solvent (hexane) was then removed with a vacuum evaporator operating at 40 °C for 10 min. The volatile oil was then dehydrated with anhydrous sodium sulfate, collected (6.3750 g), transferred into an amber-colored vial, and stored at 4 °C until further analysis.
SE apparatus and procedure
S. chinensis seed powder (25 g) was placed in a cellulose thimble (28 mm i.d. × 100 mm long, Advantec, Tokyo), mixed with 100 mL of hexane, and boiled for 1 h. The extract was evaporated and dehydrated with anhydrous sodium sulfate. The collected volatile oil (7.0014 g) was stored in an amber-colored vial at 4 °C until further analysis and testing.
SDE apparatus and procedure
A SDE apparatus (Chromapck, Middelburg, Netherlands) was used. An amount of 50 g of S. chinensis seed powder was mixed with 500 mL of distilled water and soaked for 1 h at room temperature (20 °C) before extraction. The solvent flask containing 50 mL of hexane and the sample flask containing S. chinensis seed powder and distilled water were boiled for 8 h under atmospheric conditions with an efficient cooling condenser (−5 °C). Then, the organic solvent (hexane) was evaporated with continuous nitrogen gas. The moisture of the essential oil was removed by adding sodium sulfate anhydrate, and the dried oil was preserved in an amber-colored vial at 4 °C, and essential oil (0.6100 g) was collected.
Color measurement
Color was recorded with a colorimeter manufactured by Minolta (Model CR-300, Konica Minolta Sensing, Osaka, Japan). The device was calibrated with white reference tile. Color values were expressed by L* (lightness/darkness), a* (redness/greenness), and b* (yellowness/blueness).
GC–MS analysis
GC–MS analysis of the volatile oils extracted from the seeds of S. chinensis was carried out on an Agilent 6890N-5973 insert gas chromatograph (Agilent Technologies, Palo Alto, CA) with a capillary column (HP-5, 30 mm × 0.25 mm, film thickness 0.25 μm) equipped with an Agilent 6890N-5973 mass selective detector in electron impact mode. GC was operated under the following conditions: manual injection 1 μL, injector temperature 270 °C, carrier gas (He) flow 1 mL/min, and oven temperature programmed at 40–165 °C increasing at 10 °C/min, from 165 to 200 °C at 5 °C/min, and 200–250 °C at 10 °C/min. The detector temperature was 280 °C. The MS was operated under 70 eV at a scan range of 15 to 500 amu. The chemical compositions of the volatile oils were identified by comparison of their mass spectral pattern with that in NIST02 mass spectral library, as well as by calculation of retention indices (RI) using n-alkane standards (C8–C20 and C21–C40, Sigma-Aldrich, Milan, Italy).
HPLC analysis for schizandrin content
The lignans (schizandrin) in the volatile oils of the S. chinensis seeds were quantified by the HPLC system equipped with a pump, a column temperature control, and an ultraviolet detector (Jasco, Tokyo). Chromatographic separation was performed using a C18 reversed phase column (4.6 mm × 250 mm, 5 μm). Analysis conditions were imposed following Ma et al.Citation11) with a mobile phase consisting of acetonitrile–water–glacial acetic acid (60:40:0.1) at a flow rate of 1.0 μL/min, injection volume of 60 μL, and column temperature of 30 °C. Absorbance was recorded at a wavelength of 220 nm, and retention time was 60 min. The external standard of schizandrin (Sigma, St. Louis, MO) was dissolved in methanol and made into a series concentrations ranging from 12.5 to 100 μg/mL. A linear calibration curve (Yschizandrin = 109,960X-96,110, R2 = 0.9999) was obtained.
DPPH assay
The DPPH free radical-scavenging activities of the volatile oils obtained by MAE, SE, and SDE were determined by the method of Orak.Citation12) A 0.2 mM DPPH solution in 125 μL of methanol was mixed with 500 μL of essential oil, to which 375 μL of 99% methanol was added. The mixture was incubated for 30 min in the dark at room temperature. Scavenging activity was measured in a spectrometer by monitoring the decrease of absorbance at 517 nm. A lower absorbance of the reaction mixture indicated higher free radical-scavenging activity. DPPH radical-scavenging activity was calculated as follows:
where As and Ac are the absorbances of the sample and the control (without sample), respectively. The volatile essential oil concentration at which 50% inhibitive ability (IC50) was achieved against the free radical DPPH solution was calculated by plotting inhibitive percentages against sample concentrations.
β-carotene-linoleic acid bleaching assay
The ability of S. chinensis seed oil to prevent bleaching of β-carotene was evaluated according to Chen et al.Citation8) A stock solution of β-carotene/linoleic acid was prepared by dissolving 0.5 mg of β-carotene, 25 μL of linoleic acid, and 200 μL of Tween 40 in 1 mL of chloroform. Then, the chloroform was removed under vacuum in a rotatory evaporator at 40 °C, after which 100 mL of distilled water was added and the mixture was vigorously stirred. The working solution was freshly prepared immediately before the experiment. A working solution of 2 mL was transferred to a test tube containing 100 μL of essential oil extracts, and the absorbance of each sample was read at 470 nm after incubation for 3 h at 50 °C. BHT was used as positive standard, and all tests were carried out in triplicate. Relative antioxidant activity was calculated according to the following equation:
where C0 is the absorbance of control (without essential oil) at the beginning of incubation. At and Ct are the absorbances after incubation of the sample and the control, respectively.
Bacterial strains
Escherichia coli and Bacillus cereus 882 were obtained from Microbiology Lab of School of Food Science and Biotechnology, Kyungpook National University, Korea. Three other strains, including Enterobacter aerogens, Serratia marceseces, and Micrococcus luteus, were isolated from raspberry fruits, and were identified by a microbial identification system in Apparatus Central at Kyungpook National University.
Antibacterial activity test
The different extracts of volatile oils (MAE, SE, and SDE) from the S. chinensis seeds were tested using disc diffusion test.Citation13) A suspension (100 μL) containing 1.0 × 106 cfu/mL of bacteria was spread on a nutrient agar medium. Sterilized disc papers (diameter 8 mm) were impregnated with 20 μL of essential oil and placed onto the medium. Control was prepared using sterilized water instead of essential oil. The inoculated plates were incubated at 30 °C for 24 h. The diameter of the clear zone around the disc was measured and expressed in millimeters as its antibacterial activity. Four discs per plate were used and each test was run in triplicate.
Results and discussion
Chemical composition of volatile oils
Volatile essential oils of S. chinensis seeds were extracted by the SDE, MAE, and SE methods and the colors of the extracts obtained were quite different. Color parameters are reported in Table . The value of a showed an insignificant difference, but the values of L and b showed significant differences among the oil extracts. The oil extracts obtained by SE and MAE were golden yellow in color with a significantly higher b value, while the color of the SDE oil extract was milk-white and there was a strong aroma. The oil yield obtained by the SE method was the highest (in yield up to 28%) compared with the yield of MAE method (25.5%) and the SDE method (1.22%), which is fairly low. When essential oils were extracted from S. chinensis by SFE,Citation14) the oil obtained was golden orange in color with an extraction yield of 0.43%. Li et al.Citation15) used six different methods for the extraction of volatile fractions from S. chinesis seeds, including the standard extraction method for the extraction of essential oils, and steam distillation, ultrasonic, and Soxhlet with petroleum ether or diethyl ether for the extraction of volatile oils, but the highest yield reached only 11.2%. Recently, Chen et al.Citation8) reported a novel solvent-free MAE of essential oils from S. chinensis, but the average yield of the yellowish S. chinensis essential oil was only 1.75%. Therefore, compared with other extraction methods, MAE and SE with hexane as extraction medium can give higher extraction yields.
Table 1. Color parameters, oil yields, and schizandrin concentrations of essential oil obtained by various extraction methods (Soxhlet, MAE, and SDE).
Lignans in the S. chinensis seed oil were analyzed with HPLC. The schizandrin content in the MAE extracted oil was as high as 996.64 μg/g, and the SDE extract contained a low quantity of schizandrin (18.86 μg/g). GC–MS analysis was employed for S. chinensis seed oils, and more than 50 chemical compounds were detected and identified. The GC–MS results showing the RI of the extract constituents and their relative percentages are listed in Table . The major organic compounds detected in the S. Schisandra seed oil were dibenzocyclooctene derivatives, and the GC–MS profiles of the oil extracts obtained by SE and MAE were similar, while the SDE profile had a distinct contrast. In general, the chemical compounds present in simultaneous distillation extracted oil (SDEO) were simple but the quantities were higher, while the constituents of the microwave-assisted extracted oil (MAEO) and the Soxhlet-extracted oil (SEO) were more complex, but most of them were similar.
Table 2. GC–MS analysis results for the chemical compositions of S. chinensis seed oils obtained by MAE, SE, and SDE.
The main components of SDEO were ylangene (15.01%), followed by dehydroaromadendrene (9.26%), α-phellandrene (8.23%), β-himachalene (6.95%), and cuparene (6.74). Similar constituents have been identified in steam distillation extracts of S. chinensis by GC–MS,Citation8) and according to Zhu et al.Citation10), the major lignan in S. chinensis was ylangene (up to 14.34%), in good agreement with the SDEO constituents. Only a small amount of schizandrin was traced in the oil extracts of SDE. In contrast to SDE, the MAE and SE methods are capable of extracting abundant lignans from S. chinensis seeds, including schizandrin, γ-schizandrin, wuweizisu C, and gomisin A, comprising 41.83% of MAEO and 42.97% of SEO. Depending on the extraction process (methods and solvent variation) as well as diverse analysis techniques, the species and quantities of chemical compounds differ in the literature. Tian et al.Citation16) employed micellar electrokinetic capillary chromatography for the separation and determination of lignans from seeds of S. chinensis and identified four lignans, including schizandrin, schisanderin, deoxyschizandrin, and γ-schizandrin, as major lignans. In contrast, a similar application of capillary eletrochromatography conducted by Kvasnic Kova et al.Citation17) identified schizandrin, wuweizisu C, and gomisin A and N as the major lignans in S. chinensis seeds. In the present study, relative concentrations of lignans in the S. chinensis seeds differed significantly when different extraction methods were used, although the major lignans in MAEO and SEO were similar. Bornyl acetic ether and Terpinyl acetate were detected only in MAEO and SEDO, while wuweizisu C and gomisin F were found exclusively in the SEO extract.
Antioxidant activity
Since various compounds are related to complex antioxidant mechanisms, it is more reliable to employ more than one test method, as argued by AruomaCitation18) and Koleva et al.Citation19) Hence, two types of common used antioxidant assays (DPPH assay and β-carotene-linoleic acid bleaching assay) were carried out in the present study.
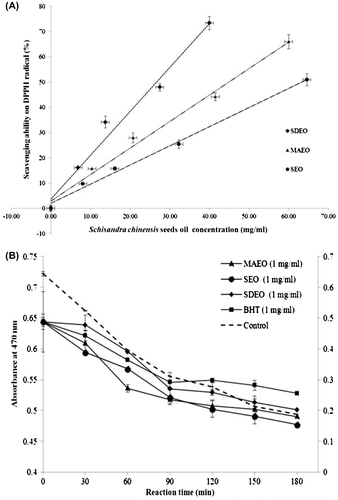
The free radical-scavenging capacities of the volatile essential oils from S. chinensis seeds as obtained by MAE, SE, and SDE were evaluated by DPPH assay. Fig. (A) shows positive correlations between DPPH radical-scavenging activity and the concentration of volatile oil of S. chinensis seeds, and lower IC50 value indicates higher antioxidant activity. The IC50 of SEO against the DPPH free radical solution was 63.32 mg/mL, much higher than that of MAEO (44.89 mg/mL) and SDEO (26.53 mg/mL). The inhibitive activity of S. chinensis seeds oil (1 mg/mL) in β-carotene-linoleic acid is represented in Fig. (B). A decrease in absorbance was observed and the inhibition ratios of the volatile essential oils from SE, MAE, and SDE were 63.46, 66.30, and 68.71% respectively, as compared with that of BHT (positive control) at 74.62%. SDEO had the strongest antioxidant effects, even higher than the synthetic antioxidant BHT within the initial 60 min. Slanina et al.Citation20) and Choi et al.Citation21) have reported that S. chinensis flesh extract showed good scavenging activity for DPPH free radicals, which may contributed by lignans such as schizandrin and gomisin. According to our research, S. chinensis seed oils also showed good antioxidant activities against DPPH free radical, and the β-carotene-linoleic acid system, but SDE showed higher antioxidant activity than that obtained by MAE or SE. HPLC and GC–MS results have revealed that only a small amount of schizandrin was presented in SDEO, and lignans such as gomisin A and F, schizandrin, and wuweizisu C were detected only in the MAE and SE oils, suggesting that lignan is not the primary source responsible for antioxidant activity. According to Yanishlieva et al.Citation22) and Foti and IngoldCitation23), terpenes have antioxidant and antiradical activity that may be comparable to the activity of phenols and α-tocopherol. S. chinensis seed oil extracted by SDE contained more monoterpenes such as α-thujene, α-pinene, β-pinene, and cymene than MAE and SE, which might yield enhanced scavenging activity.
Fig. 1. Antioxidant activities of volatile essential oils from seeds of S. chinensis: (A) free radical-scavenging activity assay and (B) β-carotene-linoleic acid bleaching assay. MAEO, microwave-assisted extracted oil; SDEO, simultaneous distillation extracted oil; SEO, Soxhlet-extracted oil; BHT, butylated hydroxytoluene.
Antibacterial activity
The antimicrobial activity of spices and essential oils is well recognized.Citation24) Many naturally occurring compounds found in edible and medicinal plants, herb, and spices have been found and identified, possessing antimicrobial functions that might serve as sources of antimicrobial agents against food pathogens, without side effects caused by synthetic antimicrobial agents. Since no relevant research had been done on the antibacterial effect of S. schisandra seed oils, five bacterial strains were tested, including food-borne pathogens of E. coli and B. cereus, as well as three kinds of bacteria that can cause small fruit (raspberry and mulberry) deterioration. The oil by SDE extraction showed good antibacterial activities against all tested bacterial strains, but the SE and MAE oil extracts showed no significant antibacterial activity. The disc diffusion method indicated that SDE oil was very effective against M. luteus and E. coli, with inhibition zones measured at 18.7 and 12.8 mm, respectively. In contrast, B. cereus, E. aerogens, and S. marcesecens showed modest sensibility to SDE oil extracts. The minimal inhibitive concentrations (MICs) of SDE oil from S. chinensis seeds against all the studied strains are summarized in Table . The essential oil showed the strongest activity against M. luteus (MIC 40 μg/mL), followed by E. coli with a MIC of 80 μg/mL. The MIC values of SDEO for B. cereus, E. aerogens, and S. marcesecens were 160 μg/mL.
Table 3. Inhibitive effects and MICs of essential oils from S. chinensis on E. coli, B. cereus, E. aerogens, S. marcesecens, and M. luteus.
The antibacterial activities of essential oils might be due to good hydrophilic ability, allowing to penetrate through outside membrane of bacterial cells and cause severe damage, but beside of that, particular chemical compounds (phenols, terpene hydrocarbons, and their derivatives) have direct inhibitive effects against bacteria.Citation25) In our research, S. chinensis seed oils extracted by different methods showed significant differences in antibacterial effects. Since there was no hydrophilic difference, the only explanation for this is the different chemical constitutions of S. chinensis seed oils as obtained by the various extraction methods. According to our previous GC–MS results, SDE extracted more terpenes, β-pinene and borneol were found exclusively in SDE oil, and α-pinene as well as limonene were much higher in abundance in the SDE oil than in the SE or MAE oil. These compounds were considered to have strong antibacterial activities according to Mourey and Canillac,Citation26) which explains why only the SDE oil exhibited strong antioxidant capacity.
Microwave-assisted and SE techniques can extract high-yield and high-quality volatile oils with schizandrin and its derivatives as major constituents, showing good antioxidant activity but no antibacterial effect. In contrast, SDE requires long processing times and intensive labor, while volatile fractions obtained possess strong aroma and higher antioxidant activity and showed significant antibacterial activities against all tested strains. The main compounds found in the SDE-extracted S. chinensis seeds were ylangene, dehydroaromadendrene, β-himachalene, and cuparene, and only a small amount of schizandrin compounds was detected.
Notes
Abbreviations: MAE, microwave-assisted extraction; SE, Soxhlet extraction; SDE, simultaneous distillation extraction.
References
- He XG, Lian LZ, Lin LZ. Separation and determination of active components in Schisandra chinensis Baill. and its medicinal preparations by non‐aqueous capillary electrophoresis. J. Chromatogr. A. 1997;757:81–87.
- Huyke C, Engel K, Simon-Haarhaus B, Quirin KW, Schempp CM. Composition and biological activity of different extracts from Schisandra sphenanthera and Schisandra chinensis. Planta Med. 2007;73:1116–1126.
- Song L, Ding JY, Tang C, Yin CH. Compositions and Biological Activities of Essential Oils of Kadsura longepedunculata and Schisandra sphenanthera. Am. J. Chin. Med. 2007;35:353–364.
- Menichini F, Conforti F, Rigano D, Formisano C, Piozzi F, Senatore F. Phytochemical composition, anti-inflammatory and antitumour activities of four Teucrium essential oils from Greece. Food Chem. 2009;115:679–686.
- Chen ZL, Chao JP, Cao HL, Bi WT, Cui HY, Li ML. Research on the extraction of plant volatile oils. Procedia Environ. Sci. 2011;8:426–432.
- Dı́az-Maroto MC, Pérez-Coello MS, Cabezudo MD. Headspace solid-phase microextraction analysis of volatile components of spices. J. Chromatogr. A. 2002;947:23–29.
- Cai JB, Liu BZ, Su QD. Comparison of simultaneous distillation extraction and solid-phase microextraction for the determination of volatile flavor components. J. Chromatogr. A. 2001;930:1–7.
- Chen XQ, Zhang Y, Zu YG, Fu YJ, Wang W. Comparison of simultaneous distillation extraction and solid-phase micro-extraction for the determination of volatile flavor components. LWT – Food Sci. Technol. 2011;44:2047–2052.
- Wang BL, Hu JP, Tan W, Sheng L, Chen H, Li Y. Simultaneous quantification of four active schisandra lignans from a traditional Chinese medicine Schisandra chinensis (Wuweizi) in rat plasma using liquid chromatography/mass spectrometry. J. Chromatogr. B. 2008;865:114–120.
- Zhu YF, Du B, Li J, Gao H, Liu C. Food Ferment. Ind. 2008;34:149–152.
- Ma CH, Liu TT, Yang L, Zu YG, Chen XQ, Zhang L, Zhang Y, Zhao CJ. Ionic liquid-based microwave-assisted extraction of essential oil and biphenyl cyclooctene lignans from Schisandra chinensis Baill fruits. J. Chromatogr. A. 2011;1218:8573–8580.
- Orak HH. Total antioxidant activities, phenolics, anthocyanins, polyphenoloxidase activities of selected red grape cultivars and their correlations. Sci. Hort. 2007;111:235–241.
- Malekzadeh F, Ehsanifar H, Shahamat M, Levin M, Colwell RR. Antimicrobial activity of black myrobalan (Terminalia chebula Retz) against Helicobacter pylori. Int. J. Antimicrob. Agents. 2001;18:85–88.
- Li B, Meng XJ, Li YS, Xue X. Use of Box-Behnken Design for the Optimization of Supercritical Carbon Dioxide Extraction of Oil from Schisandra Chinensis (Turcz.) Baill. Biomedical Engineering and Informatics (BMEI), 2010 3rd International Conference on. Yantai, China: IEEE, 2010, 2:670–674.
- Li XN, Cui H, Song YQ, Liang YZ, Chau FT. Analysis of Volatile Fractions of Schisandra chinensis (Turcz.) Baill. using GC-MS and Chemometric Resolution. Phytochem. Anal. 2003;14:23–33.
- Tian K, Qi SD, Cheng YQ, Chen XG, Hu ZD. Separation and determination of lignans from seeds of Schisandra species by micellar electrokinetic capillary chromatography using ionic liquid as modifier. J. Chromatogr. A. 2005;1078:181–187.
- Kvasničková L, Glatz Z, Štěrbová H, Kahle V, Slanina J, Musil P. Application of capillary electrochromatography using macroporous polyacrylamide columns for the analysis of lignans from seeds of Schisandra chinensis. J. Chromatogr. A. 2001;916:265–271.
- Aruoma OI. Methodological considerations for characterizing potential antioxidant actions of bioactive components in plant foods. Mutat. Res. Fund. Mol. Mech. Mutagen. 2003;523–524:9–20.
- Koleva II, van Beek TA, Linssen JPH, de Groot A, Evstatieva LN. Screening of plant extracts for antioxidant activity: A comparative study on three testing methods. Phytochem. Anal. 2002;13:8–17.
- Slanina J, Bøezinová L, Paulová H, Humpa O. Antioxidant lignans from petroleum ether extract of Schisandra chinensis. Planta Med. 2008;74:952–952.
- Choi YW, Takamatsu S, Khan SI, Srinivas PV, Ferreira D, Zhao JP, Khan IA. Schisandrene, a dibenzocyclooctadiene lignan from Schisandra chinensis: structure-antioxidant activity relationships of dibenzocyclooctadiene lignans. J. Nat. Prod. 2006;69:356–359.
- Yanishlieva NV, Marinova EM, Gordon MH, Raneva VG. Antioxidant activity and mechanism of action of thymol and carvacrol in two lipid systems. Food Chem. 1999;64:59–66.
- Foti MC, Ingold KU. Mechanism of inhibition of lipid peroxidation by γ-terpinene, an unusual and potentially useful hydrocarbon antioxidant. J. Agric. Food Chem. 2003;51:2758–2765.
- Kim J, Marshall MR, Wei C. Antibacterial activity of some essential oil components against five foodborne pathogens. J. Agric. Food Chem. 1995;43:2839–2845.
- Cristani M, D’Arrigo M, Mandalari G, Castelli F, Sarpietro MG, Micieli D, Venuti V, Bisignano G, Saija A, Trombetta D. Interaction of four monoterpenes contained in essential oils with model membranes: implications for their antibacterial activity. J. Agric. Food Chem. 2007;55:6300–6308.
- Mourey A, Canillac N. Anti-Listeria monocytogenes activity of essential oils components of conifers. Food Control. 2002;13:289–292.
