Abstract
Galacto-oligosaccharides (GOSs) are recognized as prebiotics beneficial to human health through their abilities to modulate gut microbiota. On the other hand, it has been reported that immediate allergic reactions are caused by a GOS product (Bc-GOS) produced by treating lactose with β-galactosidase derived from Bacillus circulans. The objective of this study was to create a safer GOS product that is less likely to cause GOS-induced allergy (GOS-AL). First, we identified two derivatives of tetrasaccharide sugar chains in Bc-GOS as the factors responsible for GOS-AL by histamine release test (HRT) using blood samples obtained from two GOS-AL patients. Through our search for non-allergic GOS, we developed a new GOS product, SK-GOS, which was produced by catalyzing lactose with β-galactosidase derived from Sporobolomyces singularis and Kluyveromyces lactis. We regard it as a hypoallergic and safe GOS product that does not cause GOS-AL.
Graphical Abstract
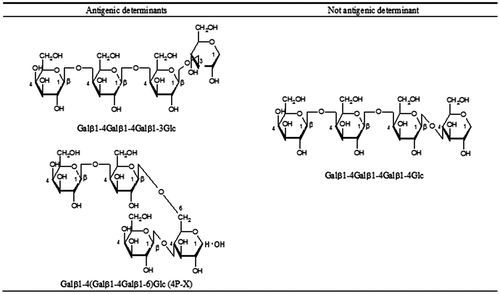
Galβ1-4(Galβ1-4Galβ1-6)Glc (4P-X) in GOS was an allergen. 4P-X exhibited strong histamine release activity. GOS having little 4P-X level was hypoallergenic.
Many studies have found that various indigestible carbohydrates can selectively promote the growth of beneficial micro-organisms colonizing the gut lumen, including bifidobacteria, and they have attracted considerable attention. Such substances were named prebiotics by Gibson and Roberfroid.Citation1) Galacto-oligosaccharide (GOS) is a generic term for di- to hexa-saccharides produced by β-galactosidase that transfers the galactose (Gal) to the lactose.Citation2,Citation3) Natural GOS is found in human breast milkCitation4,5) and bovine colostrum.Citation6) It has been found to promote the growth of bifidobacteria in the human intestine,Citation7−Citation12) thus improving constipation,Citation13,14) and to reduce the levels of intestinal putrefaction substances.Citation8,15) It has also been reported that it promotes mineral absorptionCitation16,17) and reduces the risk of cancer development.Citation18) Hence, GOS is considered a typical prebiotic beneficial to human health. It is used in various food products, including infant formula, as a low-calorie, non-digestible sugar with a moderate degree of sweetness.Citation1,3,19,20) The combination of a probiotic with a prebiotic is defined as a synbiotic, and these are expected to combine the advantages of both agents. Clinical trials in which patients with short bowel syndrome,Citation21) subjected to biliary cancer surgery,Citation22) or suffering from severe systemic inflammatory response syndrome (SIRS)Citation23) were given synbiotics revealed the benefits of these in medical treatment. Commercially available GOS can be divided into two types, 6′-GOS and 4′-GOS, in which sugar chains are bound predominantly via β1-6 bonds and β1-4 bonds, respectively.Citation3) This is dependent on the difference in the Gal-binding site selected by the transgalactosylation activity of the β-galactosidase used.Citation24)
With regard to the safety of GOS, general toxicity and genetic toxicity studies have raised no safety concerns.Citation25,26) GOS products supplied by three manufacturers were approved in the GRAS Notice Inventory of the US FDA (GRAS Notice Nos. GRN 236, 285 and 286, 334). These facts also confirm the safety of GOS products.
Recently, two reports on allergic cases attributable to GOS were published in successionCitation27,28) and we have encountered the occurrence of immediate allergic reactions caused by both a 6′-GOS product and a 4′-GOS product. A case of allergic reaction caused by 6′-GOS, referred to as 6′-GOS-AL, has been reported by an oyster-farming workshop in Hiroshima Prefecture, Japan, where an oyster-shucking worker developed allergic symptoms after tasting a lactic acid bacteria beverage containing a 6′-GOS product, AS-GOS.Citation29) That report points out an association of sea-squirt asthma, an occupational disease of oyster-shucking workers, with 6′-GOS-AL. It is assumed that there is an antigenic epitope common to 6′-GOS and sea-squirt antigen. Furthermore, HRT revealed high reactivity to 6′-GOS and low reactivity to 4′-GOS using blood from patients who had developed 6′-GOS-AL. Based on our investigative results, we produced 4′-GOS by treating lactose with β-galactosidase derived from Bacillus circulans and gave it the prototype name Bc-GOS as a substitute for 6′-GOS. However, the occurrence of an allergic reaction to a 4′-GOS termed 4′-GOS-AL was also reported in a man (PO-1, 14 years old) and a woman (PO-2, 46 years old) residing in Okinawa who developed allergic symptoms immediately after drinking a lactic acid bacteria beverage containing 4′-GOS. They experienced typical symptoms of immediate allergy, including urticaria, itchy eyes, lip numbness, swelling of face and lips, coughing, breathing difficulty, and respiratory distress. Several cases of allergic reaction to 4′-GOS-containing lactic acid bacteria beverages have been reported outside Okinawa, mostly in western Japan, and similar symptoms were observed in case studies done in Hiroshima. After that Okinawa cases were occurred, 12 people in total including four children (age range, 1.5–14 years), among whom eight patients resided outside Okinawa Prefecture, experienced allergic reactions after consuming the same beverage. Although most of the symptoms were minor and subsided quickly, three patients who experienced respiratory symptoms required hospitalization and treatment. All of these GOS-AL cases occurred in people who had an allergic predisposition to asthmatic or urticarial episodes.
The ultimate objective of this study was to create a safer GOS product that is less likely to cause GOS-AL, but it was necessary to identify the allergens involved in GOS-AL before achieving the ultimate goal. The study found that sugar chains constituting GOS were allergens responsible for GOS-AL. The difference in β-binding sites between Gal and Gal or between Gal and glucose (Glc) in sugar chain structures determines whether the sugar chain can be an allergen. This suggests that GOS containing no allergenic sugar chains should not cause any allergic reaction. Hence, we attempted to identify a safer type of GOS that is less likely to cause an allergic reaction by examining the allergenicity of various GOSs produced with β-galactosidases derived from various microbial species. We identified a safer type of GOS, the allergenicity of which was almost undetectable.
The finding that a low-molecular-weight sugar chain constituting GOS is the allergen responsible for GOS-AL, unlike ordinary allergic reaction caused by protein allergens, and is highly interesting considering the pathogenesis and mechanism of food allergies.
Materials and methods
Test samples
Six different GOS products were used as test samples (Table ). AS-GOS was produced by a combination of two β-galactosidases derived from Aspergillus oryzae and Streptococcus thermophilus from lactose. Two 4′-GOSs, Bc-GOS, and Ss-GOS, were produced by reacting lactose with β-galactosidases derived with B. circulansCitation30) and Sporobolomyces singularisCitation31), respectively. SK-GOS were produced by a combination of two β-galactosidases, derived from S. singularis and Kluyveromyces lactis. Two GOS products (Vi-GOS and Tw-GOS) were produced by non-Japanese manufacturers using a β-galactosidase derived from B. circulans. Vi-GOS is the same as the product related to the cases in Singapore and Vietnam.Citation27,28) Tw-GOS was the product of Taiwanese manufacturer. Sugar derivatives isolated from Bc-GOS and SK-GOS were used as samples for testing.
Table 1. Microbial sources of β-galactosidase for the production of the GOSs tested.
Subjects and blood sampling
The subjects in this study consisted of three patients (two women and one man) who developed immediate allergic reactions after ingesting a lactic acid bacteria-fermented beverage supplemented with GOS products. Two of them were 4′-GOS-AL patients (PO-1 and PO-2) who were diagnosed as suffering from anaphylaxis at Okinawa Prefectural Chubu Hospital. Another subject (PH-2, No. 10 patient in the article reported by Jyo et al.Citation29)), who experienced breathing difficulty as a typical symptom of immediate allergy after consuming an AS-GOS-containing beverage, was diagnosed as suffering from anaphyraxis to GOS at the Katsutani Clinic in Hatsukaichi, Hiroshima Prefecture, and also suffered from sea-squirt asthma. In order to identify the causative allergens, the study was conducted utilizing the histamine release test (HRT) with heparinized peripheral venous blood obtained from these three patients. Informed consent was obtained from the patients, with the cooperation of the treating physicians.
Blood histamine release test (HRT)
Procedure for HRT was previously drawn upon in two studies.Citation32,33) Briefly, 0.2 mL of heparinized blood was mixed in an equal amount of a GOS sample diluted with Hank’s Balanced Salt Solution (HBSS). After incubation at 37 °C for 30 min, the mixture was centrifuged at 1,400 rpm for 10 min to separate the supernatant. In order to remove protein, 10 μL of 60% HClO4 was added to the supernatant, and the mixture was centrifuged at 12,000 rpm for 30 min. To obtain 100% release control, the heparinized blood was mixed with HBSS containing 60% HClO4 at a ratio of 1:1:0.05 (blood:HBSS:60% HClO4), and the supernatant was prepared by the same procedure. In addition, the supernatant prepared by the same procedure from heparinized blood mixed with HBSS alone was used as negative control. Fluorescent detection of histamine was performed by post-column derivatization with o-phthaldehyde by the HPLC system (Tosoh, Tokyo) with a precolumn (TSK precolumn His, 4.0 mm ID × 6.0 cm L, Tosoh) and an analytical column (TSKgel Histamine Pak, 4.0 mm ID × 6.0 cm L, Tosoh). Histamine release (HR, %) was calculated by the following mathematical formula: HR (%) = (histamine content in test sample – histamine content in negative control)/histamine content in 100% release control – histamine content in negative control) × 100. In a series of HRT for Bc-GOS and its tetrasaccharide fraction, at least one reaction showed on HR of more than 15%. In contrast, AS-GOS or the trisaccharide fraction of Bc-GOS resulted in an HR of less than 15% at all concentrations of tested in the 4′-GOS-AL patients (PO-1 and PO-2). Based on this, we decided that results would be judged as positive in case the rate of a histamine release is more than 15% at 100 μg/mL or below in concentration, pseudo-positive in case the rate is between 10 and 15%, and negative in case the rate is less than 10%.
Fractionation and structural analysis of sugar chains
Each GOS sample was separated into five fractions, of disaccharides, trisaccharides, tetrasaccharides, pentasaccharides, and hexa- or higher-saccharides, by open-column chromatography with Bio-gel P-2 (80 mm × 100 cm; Bio-Rad Laboratories Inc, DE, USA) for subsequent analysis. In order to separate these fractions definitely and to avoid cross-contamination, GOS was converted into pyridylamino (PA) derivatives after gel filtration, as previously described.Citation34) Briefly, the trisaccharide and tetrasaccharide fractions were further converted into reductive amination derivatives with 2-amino-pyridine (Tokyo Chemical Industry, Tokyo) and subjected to high-performance liquid chromatography (HPLC) with a preparative column (Shimadzu ODS STR-H) to separate PA-sugar chains with different sugar-chain-structure sites. The structure of the GOS component was analyzed by NMR. Pentasaccharides and longer oligosaccharides were estimated to be a broad array of sugar chains structurally and to be small in quantity. Hence, we did not conduct pyridylamination and NMR analysis of these sugar chains.
Quantitation of 4P-X
A PA derivative of Galβ1-4Galβ1-4Galβ1-4Glc, the principal component of tetrasaccharide, was separated by HPLC (HPLC-1) with a Shimadzu STR-ODS-II (4.6φ × 250 mm) column with 0.1% trifluoroacetic acid as eluent. The fraction obtained was analyzed by HPLC (HPLC-2) with a Shimadzu ODS column with 0.2 M sodium citrate buffer (pH 6.0) as eluent. The level of Galβ1-4(Galβ1-4Galβ1-6)Glc, a branched sugar chain named 4P-X, in the separated peak was measured by the following procedure: A 1% aqueous solution of GOS was subjected to gel permeation chromatography (GPC)-HPLC on a Shodex KS-802 column (8.0φ × 300 mm; Showa Denko, Tokyo) to determine the proportion of tetrasaccharides in solid GOS from a chromatogram. The level of 4P-X in solid oligosaccharide was determined from the proportion of tetrasaccharide, the peak area ratio of Galβ1-4Galβ1-4Galβ1-4Glc in the tetrasaccharide fraction as determined by HPLC-1, and the peak area ratio of 4P-X in the Galβ1-4Galβ1-4Galβ1-4Glc fraction as determined by HPLC-2.
Results
Characterization of allergens responsible for 4′-GOS-AL
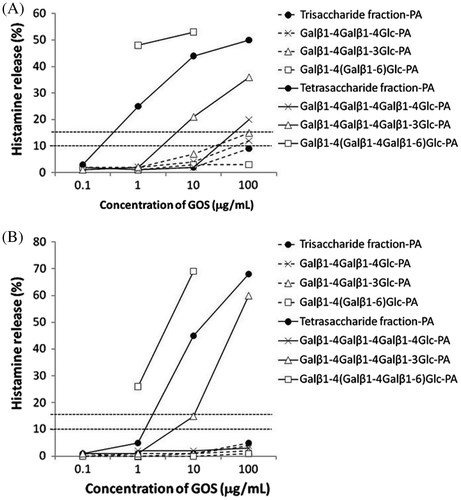
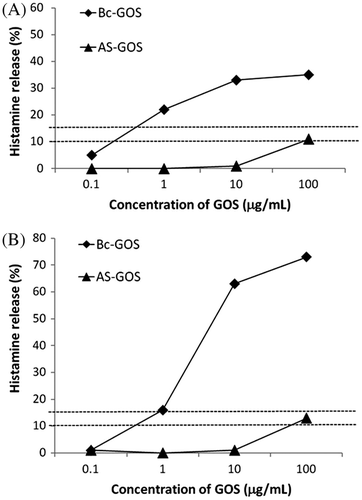
As shown in Fig. , HRT revealed high reactivity of 4′-GOS(Bc-GOS) and low reactivity of 6′-GOS (AS-GOS) to the blood of the patients (PO-1 and PO-2) who developed 4′-GOS-AL. The components of Bc-GOS were analyzed by HRT. As shown in Fig. , HRT with the trisaccharide fraction and trisaccharide PA-sugar chains revealed a pseudo-positive reaction to two PA-sugar chains (Galβ1-4Galβ1-4Glc-PA and Galβ1-4Galβ1-3Glc-PA) in one patient (PO-1), and negative results for all the trisaccharide fractions and the PA-sugar chains tested in the another patient (PO-2). Among the tetrasaccharide fractions, weak positive (PO-1) and negative (PO-2) reactions were observed for Galβ1-4Galβ1-4Galβ1-4Glc. In contrast, high HR-inducing activity was observed for Galβ1-4Galβ1-4Galβ1-3Glc and Galβ1-4(Galβ1-4Galβ1-6)Glc, a branched sugar chain named 4P-X, exhibited very strong HR-inducing activity. The chemical structures of these epitopes are shown in Fig. .
Fig. 1. Histamine release induced by 4′-GOS (Bc-GOS) and 6′-GOS (AS-GOS) in blood samples of 4′-GOS-AL patients. (A) Patient PO-1; (B) Patient PO-2. The horizontal dotted line indicates that a histamine release rate of more than 15% at all concentrations of samples was positive. Likewise, samples which showed histamine release rates below 10% were negative, from 10 to 15% was judged pseudo-positive.
Identification of hypoallergenic GOS
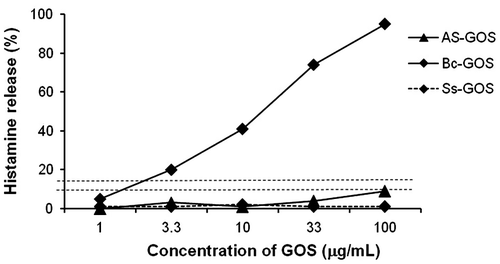
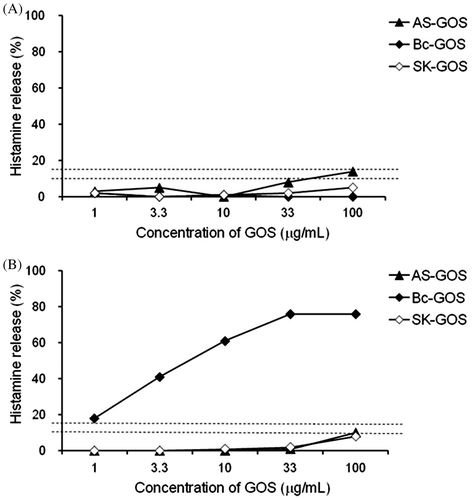
We attempted to identify another GOS that does not cause or is less likely to cause 6′-GOS-AL or 4′-GOS-AL. First we performed HRT for AS-GOS, Bc-GOS, and Ss-GOS using a blood sample obtained from the patient (PO-2) who had developed 4′-GOS-AL. The results indicated that AS-GOS and Ss-GOS did not induce HR in the blood sample obtained from this patient (Fig. ). Since the blood sample from the 6′-GOS-AL patient showed a positive response to AS-GOS, Ss-GOS was regarded as the only option available to these patients. On the other hand, one of the disadvantages to the use of Ss-GOS is that lactose crystals precipitate during storage due to a high residual lactose content. Hence, we tried to produce an improved form of Ss-GOS with reduced lactose content using not only a β-galactosidase derived from S. singularis in the primary reaction but also an enzyme derived from K. lactis in the secondary reaction. We named it SK-GOS. Since the β-galactosidase derived from K. lactis also produced 6′-GOS, SK-GOS was still associated with a risk-inducing 6′-GOS-AL. Despite this concern, SK-GOS demonstrated negative HR-inducing activity in HRT with blood samples collected from both the patient (PH-2) who developed 6′-GOS-AL and the one (PO-2) who developed 4′-GOS-AL (Fig. ).
Saccharide compositions, 4P-X level, and HRT results for commercially available GOS products
The saccharide composition of commercially available GOS products is presented in Table . There was no remarkable difference in saccharide composition among the GOS products analyzed. However, the quantity of Galβ1-4Galβ1-4Galβ1-3Glc, which had high HR-inducing activity, was lower in SK-GOS than in the other three products. In addition, no pentasaccharides or longer saccharides were detected in SK-GOS. In contrast, they were present at 4.7–6.0% in the remaining three GOS products produced with β-galactosidase derived from B. circulans.
Table 2. Average saccharide compositions of some commercially available GOS products.
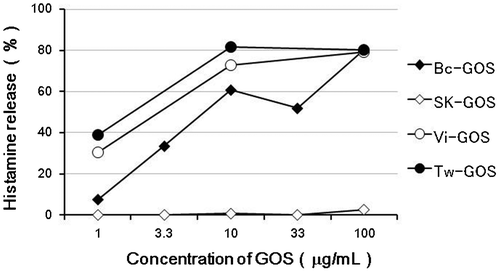
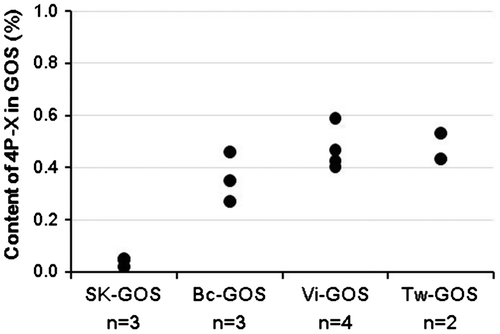
Next we measured the levels of 4P-X in commercially available GOS products, and found that the mean level of 4P-X was 0.039% in SK-GOS (n = 3) and 0.36% in Bc-GOS (n = 3), meaning that SK-GOS contained only one-ninth the amount of 4P-X in Bc-GOS. The 4P-X levels of two GOS products, Vi-GOS and Tw-GOS, were slightly higher than that of Bc-GOS (Fig. ). The results for HRT with these GOS products are shown in Fig. . SK-GOS showed the lowest HR-inducing activity, and Bc-GOS and the two GOS products supplied by other companies showed higher HR-inducing activity, indicating a positive correlation between 4P-X level and HR-inducing activity.
Fig. 6. Levels of 4P-X (Galβ1-4(Galβ1-4Galβ1-6)Glc) in several GOS products. Bc-GOS was produced by reacting lactose with β-galactosidases derived from circulans. SK-GOS was produced by a combination of two β-galactosidases, derived from S. singularis and K. lactis. Vi-GOS and Tw-GOS were commercial products produced by two different companies using a β-galactosidase derived from B. circulans.
Discussion
Proteins and glycoproteins account for a majority of allergens. In the case of GOS-AL, unlike the most usual allergies, particular sugar-chain structures constituting GOS were assumed to be allergens, because GOS does not contain proteins. Hence, we performed ASA and PCA on guinea pigs to examine the antigenicity of Bc-GOS (4′-GOS), but did not detect antigenicity (data not shown), but when we sensitized guinea pigs with Bc-GOS bound to carrier proteins, we found that the animals produced an antibody against GOS, demonstrating the immunogenicity of GOS. These results indicate that sugar-chain structures constituting GOS serve as haptens. Haptens are defined as molecules that can bind to antibodies but cannot induce an immune response by themselves. Thus a hapten must be bound to a protein or it does not induce an immune response. On the other hand, even though it is a low-molecular-weight, non-immunogenic molecule, GOS can induce an allergic reaction by itself, and this does not fit the concept of haptens. Moreover, our finding that particular tetrasaccharide sugar chains constituting GOS are the allergens responsible for GOS-AL is interesting. To date, this phenomenon has been hardly investigated and its clinical relevance is unknown.
Our conclusions are based on the observations obtained with blood samples from GOS-AL patients. Likewise, the recent reports from SingaporeCitation27) and VietnamCitation28) suggest that GOS itself can induce anaphylaxis as an allergen. Hence, we thought that it was necessary to identify the allergens involved in GOS-AL and to develop a safer GOS product to avoid this problem.
GOSs are present in human breast milk,Citation4,5) and 80% of GOSs available in the world are used as additives in infant formula. Nevertheless, no infant cases of GOS-AL have been detected. Polysaccharide pectinCitation35−Citation38) and sugar alcohol erythritolCitation39,40) have been reported to be causes of sugar-induced allergies. Many studies have also suggested the involvement of carbohydrates as epitopes in food allergy. A number of studies have examined the antigenicity of carbohydrates, and have provided experimental evidence of the production of anti-sugar-chain antibodies and the role of sugar chains as antigenicity enhancers.Citation41−Citation44) Studies of food allergies have shown that the sugar-chain structures of glycoproteins derived from taxonomically distant plants or animals play important roles as carbohydrate epitopes that confer cross-reactivity.Citation45−Citation49) Vieth et al.Citation50) have reported a case of food allergy in which galactose was an important epitope. Moreover, a recent study of an anaphylactic reaction caused by the molecular-targeted antitumor-agent cetuximab found that the IgE antibody against cetuximab specifically recognized galactose-α-1,3-galactose.Citation51−Citation53)
We attempted to identify specific anti-GOS IgE antibodies in sera obtained from the 6′-GOS-AL and 4′-GOS-AL patients by the enzyme immunoassay (EIA) method. Although the specific IgE antibodies were not detected (data not shown), we think that GOS-AL is not an IgE-independent allergy, because there was distinct specificity for responses on HRT. Rather, it is likely that the GOS-AL is IgE-dependent, because a specific skin-scratch reaction to Bc-GOS has been observed in a patient with 4′-GOS-AL.Citation54) Presumably, the fact of no detection of IgE was due to the low sensitivity of the EIA method or to the small amount of IgE in the serum antibody.
In HRT analysis to identify allergens in Bc-GOS, two tetrasaccharide sugar chains were identified as molecules responsible for inducing 4′-GOS-AL. HRT-negative or weak positive Galβ1-4Galβ1-4Galβ1-4Glc and HRT-positive Galβ1-4Galβ1-4Galβ1-3Glc differ only in the Gal–Glc bonds. This indicates that a slight difference in sugar binding sites can lead to a significant difference in the antigenicity of sugar chains. It is thought that the contribution of Galβ1-4Galβ1-4Galβ1-3Glc to the HR-inducing activity of Bc-GOS is small, judging by the HR-inducing activity and the levels of the Galβ1-4Galβ1-4Galβ1-3Glc and 4P-X in GOS products. Of these tetrasaccharide sugar chains, 4P-X exhibited very strong HR-inducing activity. The finding of a positive correlation between 4P-X levels and HR activity strongly indicates that the 4P-X levels in a GOS can be used as a risk marker for 4′-GOS-AL. We did not identify any antigenic structure common to AS-GOS and Bc-GOS that might be responsible for GOS-induced allergy (GOS-AL). On the other hand, our results indicate that tetrasaccharide or longer sugar chains are required for the induction of 4′-GOS-AL and of 6′-GOS-AL.Citation29) Chiang et al.Citation27) have reported that oligosaccharides containing three or more sugars induced positive reactions on a skin-prick test and a basophil activation test. To avoid cross-contamination of sugar chains different in length, a technique such as pyridylamination is needed to precisely separate sugar chains. According to the results of Chiang et al.Citation27), we supposed that cross-contamination by tetrasaccharide of the trisaccharide fraction can disturb the analysis. Through our search for non-allergic, safer types of GOS, we identified two 4′-GOS products, Bc-GOS and Ss-GOS, as hypoallergic GOS products that do not cause 6′-GOS-AL. We selected and are now marketing Bc-GOS as a substitute for AS-GOS, because the commercial availability of the enzyme used in producing Bc-GOS makes possible a stable supply of the product, but GOS-AL cases were also reported for this GOS product. One patient (PO-2) with allergy had a history of consuming a dairy product containing AS-GOS, but reported that she did not develop GOS-AL after consuming the product. Thus the HR level for AS-GOS can be defined as the target level at which the occurrence of GOS-AL can be avoided. The results of HRT with a blood sample collected from that patient indicated that the specific HR activity of Ss-GOS produced with a β-galactosidase derived from S. singularis was comparable to that of the non-allergic AS-GOS. Thus, the HR of Ss-GOS was below the target level and the product was considered a hypoallergic and safe product that does not cause 6′-GOS-AL or 4′-GOS-AL. One of the disadvantages associated with Ss-GOS is that lactose crystals precipitate during storage due to its high residual lactose content. For practical application of the product, we produced SK-GOS with reduced lactose levels by running a second reaction with a β-galactosidase derived from K. lactis. Since the β-galactosidase derived from K. lactis also produced 6′-GOS, SK-GOS was still associated with a risk of causing 6′-GOS-AL. Despite that concern, SK-GOS gave negative results on HRT with blood samples obtained from both a patient who developed 6′-GOS-AL and one who developed 4′-GOS-AL. The total level of 4P-X in SK-GOS was about one-ninth of that in Bc-GOS. Indeed, SK-GOS exhibited very low HR, and hence might prove a non-allergic and a safe GOS product.
It is an interesting question why Bc-GOS and Ss-GOS, both 4′-GOS products, exhibited different degrees of HR-inducing activity in the patient who developed 4′-GOS-AL. The most significant difference in the GOSs produced by β-galactosidases derived from B. circulans or S. singularis is the levels of the 4P-X molecule. Both enzymes exhibit high specificity in transferring Gal to a non-reducing terminal Gal, and both almost exclusively produce Galβ1-4 bonds. The enzymes function differently in transferring Gal to Glc. B. circulans-derived β-galactosidase produces Galβ1-6Glc at a certain rate.Citation55,56) Because the hydrolysis rate for the product was slower than those for other lactose isomers, Galβ1-2Glc and Galβ1-3Glc, a continuous long-term reaction with this enzyme is likely to result in an accumulation of Galβ1-6Glc or Galβ1-4(Galβ1-6)Glc in the reaction mixture. 4P-X is produced by the binding of one molecule of Gal to a non-reducing terminal Gal via a Galβ1-4 bond. In addition, there is analytical evidence that S. singularis-derived β-galactosidase very rarely produces sugar chains bound to the Glc-reducing terminus via Galβ1-6 bonds and long-chain oligosaccharides containing four or more monosaccharide units and branched oligosaccharides.Citation57)
SK-GOS has been carefully introduced to the market under the brand name Oligomate 55N. Although the lactic acid beverage-supplemented SK-GOS is consumed by about 1.5 million people daily, no occurrence of allergy has been reported over the past nine years.
Although GOS-AL is caused by sugar chains constituting a GOS and functioning as allergens, those who develop allergy to GOS are likely to have factors that facilitate sensitization. For 6′-GOS-AL, cross-reactivity between GOS and sea-squirt asthma antigens has been found,Citation29) but the background of sensitization to 4′-GOS-AL has not been identified.
Although detection of anti-GOS-specific IgE in the serum of the GOS-AL patient included in this study was not successful, the reactions were perhaps IgE-dependent on the basis of the following lines of evidence: (i) histamine release induced specifically by specific products or sugar chains was observed; (ii) no significant changes in HR activity were seen after chemical modification of the reducing terminal of the sugar chains by pyridylamination, indicating that sugar-chain moieties were specifically recognized; (iii) positive results on the skin-scratch test were obtained with a specificity similar to that for HR activity;Citation54) and (iv) positive results on the basophil activation test, which exhibits good accord with IgE-dependent clinical symptoms, were presented in the report of Singapore cases,Citation27) and this also probably represents GOS-AL.
In summary, although the number of samples used was small and only limited assays were done, we obtain evidence sufficient to conclude that GOS-AL represents IgE-dependent allergies. In addition, as for the ultimate goal of this study (the development of a hypoallergenic GOS product), we obtained consistent results by qualitative assessment as to whether the GOSs tested were positive or negative for the induction of HR regardless of HRT for a limited number of patients. Further investigation is needed to establish methods of detection of the anti-GOS-specific IgE antibody, the mechanism of cross-linking of the anti-GOS-specific IgE antibody as for low-molecular-weight sugar chains, the background of sensitization in GOS-AL, and the possibility of an IgE-independent manner.Citation58,59)
Acknowledgments
This study was conducted in the cooperation with Dr. Takashi Katutani (Katsutani Clinic, Hiroshima) and Dr. Eisaku Sakihara (Okinawa Prefectural Chubu Hospital). We would like to express our deepest gratitude to all of them. We also wish to thank Dr. Shuichi Kaminogawa (Professor Emeritus, University of Tokyo) for valuable suggestions. Here was no conflict of interest with any third party as to the contents of this study.
Notes
Abbreviations: GOS, galacto-oligosaccharide; GOS-AL, GOS-allergy; HRT, histamine release test; PA, pyridylamination; Gal, galactose; Glc, glucose.
References
- Gibson GR, Roberfroid MB. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J. Nutr. 1995;125:1401–1412.
- Matsumoto K, Kobayashi Y, Ueyama S, Watanabe T, Tanaka R, Kan T, Kuroda A, Sumihara Y. Japanese Technology Reviews, Section E: Biotechnology, Chapter 5, Oligosaccharides: production, properities, and applications. Nakakuki T, editor. New York, NY: Gordon and Breach Science; 1993. p. 90–106.
- Sako T, Matsumoto K, Tanaka R. Recent progress on research and application of non-digestible galcto-oligosaccharides. Int. Dairy J. 1999;9:69–80.
- Kobata A, Yamashita K, Tachibana Y. Oligosaccharides from human milk. Methods Enzymol. 1987;50:216–220.
- Boehm G, Stahl B. Oligosaccharaides from milk. J. Nutr. 2007;137:847S–849S.
- Saito T, Itoh T, Adachi S. Chemical structure of three neutral trisaccharides isolated in free form from bovine colostrum. Carbohydr. Res. 1987;165:43–51.
- Ito M, Deguchi Y, Miyamori A, Matsumoto K, Kikuchi K, Matsumoto K, Kobayashi Y, Yajima T, Kan T. Effects of administration of galacto-oligosaccharides on the human faecal microflora, stool weight and abdominal sensation. Microb. Ecol. Health Dis. 1990;3:285–292.
- Ito M, Deguchi Y, Matsumoto K, Kimura M, Onodera N, Yajima T. Influence of galacto-oligosaccharides on the human faecal microflora. J. Nutr. Sci. Vitaminol. 1993;39:635–640.
- Rowland IR, Tanaka R. The effects of transgalactosylated oligosaccharides on gut flora metabolism in rats associated with a human faecal microflora. J. Appl. Bacteriol. 1993;74:667–674.
- Ishikawa F, Takayama H, Matsumoto K, Ito M, Chonan O, Deguchi Y, Kikuchi-Hayakawa H, Watanuki M. Effects of b1-4 linked galactooligosaccharides on human fecal microflora. Bifidus. 1995;9:5–18. Japanese.
- Matsumoto K, Takada T, Yuki N, Kawakami K, Sakai T, Nomoto K, Kimura K, Matsumoto K, Iino H. Effects of transgalactosylated oligosaccharides mixture (N-GOS) on human intestinal microflora. J. Intest. Microbiol. 2004;18:25–35. Japanese.
- Davis LMG, Martínez I, Walter J, Hutkins R. A dose dependent impact of prebiotic galactooligosaccharides on the intestinal microbiota of healthy adults. Int. J. Food Microbiol. 2010;144:285–292.
- Deguchi Y, Matsumoto K, Ito M., Watanuki M. Effects of b1-4 galactooligosaccharides administration on defecation of healthy volunteers with constipation tendency. Eiyougaku Zassi. 1997;55:13–22. Japanese.
- Deguchi Y, Makino K, Iwadate E, Kimura M, Matsumoto K, Yamaoka Y, Komatsu H, Matsumoto K, Iino H. Influence of galacto-oligosaccharides on bowel habit in healthy young volunteers with constipation tendency. Nihon Shokuhin Shinsozai Kenkyu Kaishi. 2003;6:55–66. Japanese.
- Tohyama K, Tanaka R, Kobayashi Y, Mutai M. Relationship between the metabolic regulation of intestinal microflora by feeding Bifidobacterium and host hepatic function. Bifidobacteria Microflora. 1982;1:45–50.
- Chonan O, Watanuki M. Effect of galacto-oligosaccharides on calcium absorption in rats. J. Nutr. Sci. Vitaminol. 1995;41:95–104.
- Chonan O, Takahashi R, Watanuki M. Role of activity of gastrointestinal microflora in absorption of calcium and magnesium in rats fed b1-4 linked galactooligosaccharides. Biosci. Biotechnol. Biochem. 2001;65:1872–1875.
- Wijnands MV, Appel MJ, Hollanders VM, Woutersen RA. A comparison of the effects of dietary cellulose and fermentable galacto-oligosaccharide, in a rat model of colorectal carcinogenesis: fermentable fibre confers greater protection than non-fermentable fibre in both high and low fat backgrounds. Carcinogenesis. 1999;20:651–656.
- Fanaro S, Boehm G, Garssen J, Knol J, Mosca F, Stahl B, Vigi V. Galacto-oligosaccharides and long-chain fructo-oligosaccharides as prebiotics in infant formulas: a review. Acta Paediatr. Suppl. 2005;94:22–26.
- Veereman-Wauters G. Application of prebiotics in infant foods. Br. J. Nutr. 2005;93:S57–60.
- Kanamori Y, Hashizume K, Sugiyama M, Morotomi M, Yuki N. Combination therapy with Bifidobacterium breve, Lactobacillus casei, and galactooligosaccharides dramatically improved the intestinal function in a girl with short bowel syndrome: a novel synbiotics therapy for intestinal failure. Dig. Dis. Sci. 2001;46:2010–2016.
- Kanazawa H, Nagino M, Kamiya S, Komatsu S, Mayumi T, Takagi K, Asahara T, Nomoto K, Tanaka R, Nimura Y. Synbiotics reduce postoperative infections complications: a randomized controlled trial in biliary cancer patients undergoing hepatectomy. Langenbecks Arch. Surg. 2005;390:104–113.
- Shimizu K, Ogura H, Goto M, Asahara T, Nomoto K, Morotomi M, Matsushima A, Tasaki O, Fujita K, Hosotsubo H, Kuwagata Y, Tanaka H, Shimazu T, Sugimoto H. Synbiotics decrease the incidence of septic complications in patients with severe SIRS: a preliminary report. Dig. Dis. Sci. 2009;54:1071–1078.
- Torres DPM, Goncalves MDPF, Teixeire JA, Rodrigues LR. Galacto-oligosaccharides: production, properties, applications, and significance as prebiotics. Comp. Rev. Food Sci. Food Saf. 2010;9:438–454.
- Anthony JC, Merriman TN, Heimbach JT. 90-day oral (gavage) study in rats with galactooligosaccharides syrup. Food Chem. Toxicol. 2006;44:819–826.
- Kobayashi T, Yasutake N, Uchida K, Ohyama W, Kaneko K, Onoue M. Safety of a novel galacto-oligosaccharide: genotoxicity and repeated oral dose studies. Hum. Exp. Toxicol. 2009;28:619–630.
- Chiang WC, Huang CH, Llanora GV, Gerez I, Goh SH, Shek LP, Nauta AJ, Van Doorn WA, Bindels J, Ulfman LH, Knipping K, Delsing DJ, Knol EF, Lee BW. Anaphylaxis to cow’s milk formula containing short-chain galacto-oligosaccharide. J. Allergy Clin. Immunol. 2012;130:1361–1367.
- Vo TH, Le NH, Patel MS, Phan LT, Tran Minh NN. Acute allergic reactions in Vietnamese children after drinking a new milk product. Foodborne Pathog. Dis. 2012;9:156–159.
- Jyo T, Katsutani T, Otshuka T, Tsuboi S. Immediate type allergy in oyster-workers due to galactooligosaccharide. Occup. Environ. Allergy. 1996;3:12–20.
- Yanahira S, Kobayashi T, Suguri T, Nakakoshi M, Miura S, Ishikawa H, Nakajima I. Formation of oligosaccharides from lactose by Bacillus circulans beta-galactosidase. Biosci. Biotechnol. Biochem. 1995;59:1021–1026.
- Ishikawa E, Sakai T, Ikemura H, Matsumoto K, Abe H. Identification, cloning, and characterization of a Sporobolomyces singularis β-galactosidase-like enzyme involved in galacto-oligosaccharide production. J. Biosci. Bioeng. 2005;99:331–339.
- Daibo A, Kitazawa S, Jyo T, Katsutani T. The effect of blocking antibody on in vitro histamine release in the patients with sea-squirt asthma. J. Iwate Med. Assoc. 1988;40:511–520.
- Yamatodani A, Fukuda H, Wada H, Iwaeda T, Watanabe T. High-performance liquid chromatographic determination of plasma and brain histamine without previous purification of biological samples: cation-exchange chromatography coupled with post-column derivatization fluorometry. J. Chromatogr. 1985;344:115–123.
- Kimura K, Matsumoto K, Ishihara C, Harada K, Miyagi A. Structure determination of galacto-oligosaccharides by pyridylamination and NMR spectroscopy. Carbohydr. Res. 1995;270:33–42.
- Kraut A, Peng Z, Becker AB, Warren PW. Christmas candy maker’s asthma IgG4-mediated pectin allergy. Chest. 1992;102:1605–1607.
- Cohen AJ, Forse MS, Tarlo SM. Occupational asthma caused by pectin inhalation during the manufacture of jam. Chest. 1993;103:309–311.
- Baldwin JL, Shah AC. Pectin-induced occupational asthma. Chest. 1993;104:1936–1937.
- Jaakkola MS, Tammivaara R, Tuppurainen M, Lahdenne L, Tupasela O, Keskinen H. Asthma caused by occupational exposure to pectin. J. Allergy Clin. Immunol. 1997;100:575–576.
- Hino H, Kasai S, Hattori N, Kenjo K. A case of allergic urticaria caused by erythritol. J. Dermatol. 2000;27:163–165.
- Yunginger JW, Jones RT, Kita H, Saito K, Hefle SL, Taylor SL. Allergic reactions after ingestion of erythritol-containing foods and beverages. J. Allergy Clin. Immunol. 2001;108:650.
- Otani H, Tokita F. Contribution of the sugar moiety in the browning product between β-lactoglobulin and lactose as an antigenic determinant. Jpn. J. Zootech. Sci. 1982;53:344–350.
- Matsuda T, Nakashima I, Kato Y, Nakamura R. Antibody response to haptenic sugar antigen: immunodominancy of protein-bound lactose formed by amino-carbonyl reaction. Mol. Immunol. 1987;24:421–425.
- Matsuda T, Ishiguro H, Ohkubo I, Sasaki M, Nakamura R. Carbohydrate binding specificity of monoclonal antibodies raised against lactose-protein Maillard adducts. J. Biochem. 1992;111:383–387.
- Nakamura A, Watanabe K, Ojima T, Ahn DH, Saeki H. Effect of maillard reaction on allergenicity of scallop tropomyosin. J. Agric. Food Chem. 2005;53:7559–7564.
- Aalberse RC, Koshte V, Clemens JGJ. Cross-reactions between vegetable foods, pollen and bee venom due to IgE antibodies to a ubiquitous carbohydrate determinant. Int. Arch. Allergy Appl. Immunol. 1981;66:259–260.
- Tretter V, Altmann F, Kubelka V, Marz L, Becker WM. Fucose a1,3-linked to the core region of glycoprotein N-glycans creates an important epitope for IgE from honeybee venom allergic individuals. Int. Arch. Allergy Immunol. 1993;102:259–266.
- Fotisch K, Altmann F, Haustein D, Vieths S. Involvement of carbohydrate epitopes in the IgE response of celery-allergic patients. Int. Arch. Allergy Immunol. 1999;120:30–42.
- Mari A. IgE to cross-reactive carbohydrate determinants: analysis of the distribution and appraisal of the in vivo and in vitro reactivity. Int. Arch. Allergy Immunol. 2002;129:286–295.
- van Ree R. Carbohydrate epitopes and their relevance for the diagnosis and treatment of allergic diseases. Int. Arch. Allergy Immunol. 2002;129:189–197.
- Vieths S, Mayer M, Baumgart M. Food allergy: specific binding of IgE antibodies from plant food sensitized individuals to carbohydrate epitopes. Int. Arch. Allergy Immunol. 1994;6:453–463.
- Chung CH, Mirakhur B, Chan E, Le QT, Berlin J, Morse M, Murphy BA, Satinover SM, Hosen J, Mauro D, Slebos RJ, Zhou Q, Gold D, Hatley T, Hicklin DJ, Platts-Mills TA. Cetuximab-induced anaphylaxis and IgE specific for galactose-alpha-1,3-galactose. N. Engl. J. Med. 2008;358:1109–1117.
- Commins SP, Platts-Mills TA. Anaphylaxis syndromes related to a new mammalian cross-reactive carbohydrate determinant. J. Allergy Clin. Immunol. 2009;124:652–657.
- Commins SP, Platts-Mills TA. Allergenicity of carbohydrates and their role in anaphylactic events. Curr. Allergy Asthma Rep. 2010;10:29–33.
- Hamahata H, Maruno M, Uesato H. A case of anaphylaxis due to galacto-oligosaccharide (Abstract in Japanese). 13th Academic Convention in Jpn Soc Emerg Ped. 1999;429.
- Park AR, Oh DK. Galacto-oligosaccharides production using microbial β-galactosidase: current state and perspectives. Appl. Microbiol. Biotechnol. 2010;85:1279–1286.
- Yamamoto Y, Saito T, Ajisaka K. Study of the regioselectivity in the transglycosylation to D-galactose derivatives using β-galactosidase of various origins. J. Appl. Glycosci. 2004;51:335–339.
- Kimura K, Watanabe Y, Ishihara C, Miyagi A, Ikeda M, Matsumoto K. Analysis of regio-selectivity of transgalactosylation for several β-galactosidase by pyridylamination and NMR spectoroscopy. 21st International Carbohydrate Symposium in Carirne. Australia; 2001.
- Kojima T, Obata K, Mukai K, Sato S, Takai T, Minegishi Y, Karasuyama H. Mast cells and basophils are selectively activated in vitro and in vivo through CD200R3 in an IgE-independent manner. J. Immunol. 2007;179:7093–7100.
- Endo S, Hochman DJ, Midoro-Horiuti T, Goldblum RM, Brooks EG. Mountain cedar pollen induces IgE-independent mast cell degranulation, IL-4 production, and intracellular reactive oxygen species generation. Cell. Immunol. 2011;271:488–495.