Abstract
Biofilms are communities of surface-attached microbial cells that resist environmental stresses. In this study, we found that low concentrations of ethanol increase biofilm formation in Pseudomonas aeruginosa PAO1 but not in a mutant of it lacking both Psl and Pel exopolysaccharides. Low concentrations of ethanol also increased pellicle formation at the air–liquid interface.
Pseudomonas aeruginosa is an opportunistic human pathogen that causes infections in burn wounds and in the lungs of patients suffering from cystic fibrosis.Citation1,2) This bacterium forms biofilms, which are surface-associated bacterial communities encased in a polymeric matrix, and it is used as a model organism in studying biofilm formation in Gram-negative bacteria. Bacterial cells in biofilms are more resistant to immune systems and antibiotics than planktonic cells.Citation3,4)
Ethanol is the most popular antibactericidal agent. It is used in the disinfection of skin, medical apparatus, and cooking equipment in a variety of situations because it is volatile, leaves minimal residue, and is harmless, even if intraoral intake occurs. Although ethanol is used in many situations, the behavior of bacteria exposed to small amounts of ethanol is unknown. Thus far, several studies have found that environmental stresses trigger biofilm formation. For example, antibiotics, such as protein synthesis inhibitors, including aminoglycosides, tetracycline, and chloramphenicol, increase biofilm formation by P. aeruginosa and Escherichia coli.Citation5−8) In this study, we investigated the impact of ethanol on biofilm formation using P. aeruginosa as a model Gram-negative bacterium.
P. aeruginosa PAO1Citation9) was grown in Luria–Bertani (LB) medium containing several concentrations of ethanol in 96-well microtiter plates. The cell density of planktonic cells was measured at an optical density of 660 nm (OD660), and biofilm formation was quantified with 0.1% crystal violet, as described previously.Citation10,11) Under these experimental conditions, biofilm formation peaked at 6 h, and the attached cells were dispersed at 9 h. While 1 and 2% ethanol repressed cell growth, biofilm formation was increased by the presence of ethanol, particularly at 1%, at 6 h of culture (Fig. (A)). At 9 h of culture, biofilm formation was significantly enhanced in the presence of 1 and 2% of ethanol despite low cell densities (Fig. (B)). It was also increased, 2.7-fold, by 1% isopropanol at 6 h of culture. These data indicate that alcohol promotes microtitre plate-based biofilm formation.
Fig. 1. Low Concentrations of Ethanol Increased Biofilm Formation by P. aeruginosa PAO1.
Note: (A) Biofilm formation in LB with a 1% or 2% concentration of ethanol in microtiter plates at 37°C. Biofilm formation was quantified at 6 h and 9 h of incubation. Bar charts and dots indicate biofilm formation and cell densities of planktonic cells, respectively. Representative images of biofilm formation after crystal violet staining are shown at the bottom. Data are averages of eight replicates. (B) Confocal microscope images of P. aeruginosa biofilms. The images were obtained 12 h after inoculation in a flat-bottom flask. Projections show fields of 140 by 140 μm.
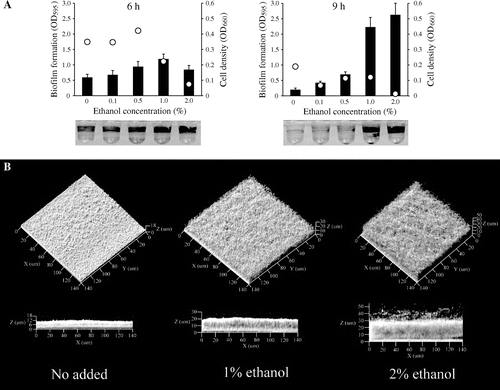
To corroborate the increase in P. aeruginosa biofilm formation due to small amounts of ethanol, the three-dimensional structure of biofilms was observed by microscopy. P. aeruginosa PAO1 was grown in LB medium containing 1 or 2% ethanol in a Nunc flat-bottom flask (Thermo Fisher Scientific, Waltham, MA) under static conditions at 37 °C, and the biofilm formed at the air–liquid interface on the glass was observed under a Carl Zeiss Pascal laser scanning microscope equipped with a numerical aperture water immersion objective (Carl Zeiss, Jena, Germany). Biofilm formation reached a peak at 12 h in this flask. When the P. aeruginosa biofilm incubated for 12 h was observed by a confocal reflection microscopy technique, as described previously,Citation12) the presence of 1 and 2% ethanol resulted in increased biofilm formation at a thickness greater than 20 μm, though it was less than 10 μm in the absence of ethanol (Fig. (B)). When the biomass was quantified with the COMSTAT computer program,Citation13) which functions within MATLAB software (Mathworks, Natick, MA), the biovolume increased 2.1-fold in the presence of 2% ethanol as compared to the absence of ethanol. Visualization of biofilms using a BacLight Live/Dead bacterial viability staining kit (Molecular Probes Inc., Eugene, OR) indicated that 1 and 2% ethanol did not kill the cells in the biofilm (Supplemental Fig. 1; see Biosci. Biotechnol. Biochem. Web site), suggesting that increased biofilm formation due to ethanol was not due to an accumulation of dead cells.
To date, it has been confirmed that the synthesis of extracellular polysaccharides is necessary for the development of a mature biofilm.Citation14) At least three exopolysaccharides are produced by P. aeruginosa: alginate, Psl, and Pel.Citation14) Although it has been reported that ethanol enhances alginate synthesis in P. aeruginosa,Citation15) PAO1 produces little or no detectable alginate in vitro.Citation16) Psl, a mannose and galactose-rich polysaccharide, is essential for mature biofilm formation, whereas Pel, a glucose-rich matrix polysaccharide polymer, is essential for pellicle formation at the air–liquid interface in P. aeruginosa PAO1.Citation17−21) To determine the impact of ethanol on exopolysaccharide synthesis, we constructed in-frame deletion mutants, including ΔpelA, ΔpslA, and ΔalgD, which do not synthesize Pel, Psl, and alginate, respectively, by a previously described protocol,Citation22) and biofilm formation in these mutants was examined in LB and M63 medium (0.4% glucose and 0.2% casamino acid as carbon sources). Deletion of pslA decreased biofilm formation in the microtiter plate assay (Fig. (A)). This result is consistent with previous reports that the biofilm-forming ability of PAO1 was attenuated in a mutant lacking Psl.Citation19,20) Low concentrations of ethanol repressed cell growth but stimulated biofilm formation in all strains except for the ΔpelAΔpslA mutant (Fig. (A)), suggesting that enhancement of biofilm formation by low concentrations of ethanol requires either Pel or Psl exopolysaccharide. Biofilm formation was remarkably enhanced by ethanol in M63 medium in all strains except for the ΔpelAΔpslA mutant (Fig. (B)). The mechanism for enhanced biofilm formation by ethanol in M63 remains unknown. The amount of biofilm formation by the ΔalgD mutant was similar to WT in both media (Fig. (A) and (B)). In summary, ethanol-induced biofilm formation depends on Pel and Psl exopolysaccharides.
Fig. 2. Effects of Ethanol on Biofilm Formation in WT and Exopolysaccharide Defective Mutants.
Note: P. aeruginosa PAO1 WT, ΔpelA, ΔpslA, ΔpelAΔpslA, and ΔalgD mutants were incubated statically in LB (A) or M63 (B) medium with and without 1–2% of ethanol at 37°C for 6 h. Bar charts and dots indicate biofilm formation and cell densities of planktonic cells, respectively. Data are averages of four independent cultures, and standard deviations are shown.
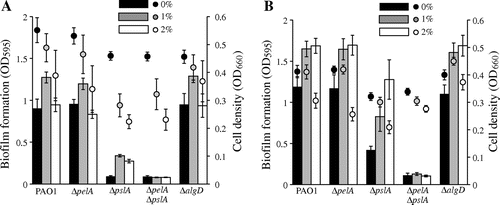
Fig. 3. Effects of Ethanol on Pellicle Formation in WT and Exopolysaccharide Defective Mutants.
Note: P. aeruginosa WT, ΔpelA, ΔpslA, ΔpelAΔpslA, and ΔalgD mutants were incubated statically in PI medium with and without 1 or 2% ethanol at 37°C for 4 ds.
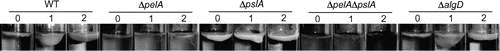
To corroborate the effects of polysaccharides, we examined pellicle formation in the presence and the absence of ethanol by the protocol described by Ghafoor et al.Citation19). The results indicated that low concentrations of ethanol significantly increased pellicle formation in WT (Fig. ). No pellicle was observed in the ΔpelA and ΔpelAΔpslA mutants, and this is consistent with a previous report that pellicle formation depends on Pel polysaccharides.Citation21) The ΔpslA mutant showed increased pellicle formation in both the absence and the presence of ethanol, perhaps because Pel production was enhanced in the ΔpslA mutant.Citation19) These results suggest that low concentrations of ethanol increase pellicle formation and that this effect is strongly associated with Pel polysaccharide.
To determine how ethanol affects Psl and Pel, the transcription of pslA and pelA was examined by real-time reverse transcription PCR. The results showed that expression was not altered by the presence of ethanol (0.8 ± 0.3-fold for pslA and 1.0 ± 0.2-fold for pelA as compared to the absence of ethanol) at 6 h incubation in M63 medium, suggesting that ethanol does not affect pslA and pelA expression at the transcriptional level. Hence, we concluded that ethanol increases Psl and Pel at the translational level, or enhances these functions to form biofilm and pellicle without affecting these processes of synthesis.
It has been reported that ethanol promotes extracellular polymeric substance synthesis: alginate synthesis and mucoid phenotype in P. aeruginosa,Citation15) polysaccharide intercellular adhesion synthesis and biofilm formation in Staphylococcus epidermidis,Citation23) and amyloid fibers curli synthesis and biofilm formation in E. coli.Citation24) In this study, we found that low concentrations of ethanol increased biofilm and pellicle formation in P. aeruginosa PAO1, and that these effects depended on Psl and Pel synthesis. The low concentrations of ethanol (1 and 2%) used in this study might occur as residuals of actual disinfection. These observations provide useful information on aspects of disinfection in the field of medicine and the food industry. In addition, when observing ecological points, they provide insight into interspecies communication by ethanol-synthesizing organisms.
Supplemental material
The supplemental material for this paper is available at http://dx.doi.org/10.1080/09168451.2014.877828.
Supplemental Fig. 1. Confocal Microscope Images of P. aeruginosa Biofilms with BacLight Live/Dead Bacterial Viability Staining Kit.
Download PDF (3.2 MB)Acknowledgments
We thank Dr Arne Heydorn for kindly providing us the COMSTAT program. This research was supported financially in part by the Japan Science and Technology Agency, CREST, and ALCA, and by a Grant-in-Aid for Scientific Research (60292520, to N. N.) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan. Y. Tashiro was supported by a Scientific Research Fellowship from the Japan Society for the Promotion of Science (JSPS).
References
- Tashiro Y, Yawata Y, Toyofuku M, Uchiyama H, Nomura N. Interspecies interaction between Pseudomonas aeruginosa and other microorganisms. Microbes Environ. 2013;28:13–24.
- Tashiro Y, Uchiyama H, Nomura N. Multifunctional membrane vesicles in Pseudomonas aeruginosa. Environ. Microbiol. 2012;14:1349–1362.
- Mah T-FC, Pitts B, Pellock B, Walker GC, Stewart PS, O’Toole GA. A genetic basis for Pseudomonas aeruginosa biofilm antibiotic resistance. Nature. 2003;426:306–310.
- Costerton J, Stewart P, Greenberg E. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284:1318–1322.
- Hoffman LR, D’Argenio DA, Maccoss MJ, Zhang Z, Jones RA, Miller SI. Aminoglycoside antibiotics induce bacterial biofilm formation. Nature. 2005;436:1171–1175.
- Linares JF, Gustafsson I, Baquero F, Martinez JL. Antibiotics as intermicrobial signaling agents instead of weapons. Proc. Nat. Acad. Sci. 2006;103:19484–19489.
- May T, Ito A, Okabe S. Induction of multidrug resistance mechanism in Escherichia coli biofilms by interplay between tetracycline and ampicillin resistance genes. Antimicrob. Agents Chemother. 2009;53:4628–4639.
- Boehm A, Steiner S, Zaehringer F, Casanova A, Hamburger F, Ritz D, Keck W, Ackermann M, Schirmer T, Jenal U. Second messenger-mediated adjustment of bacterial swimming velocity. Mol. Microbiol. 2009;72:1500–1516.
- Holloway B, Krishnapillai V, Morgan A. Chromosomal genetics of Pseudomonas. Microbiol. Rev. 1979;43:73–102.
- O’Toole GA, Kolter R. Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol. Microbiol. 1998;30:295–304.
- Tashiro Y, Nomura N, Nakao R, Senpuku H, Kariyama R, Kumon H, Kosono S, Watanabe H, Nakajima T, Uchiyama H. Opr86 is essential for viability and is a potential candidate for a protective antigen against biofilm formation by Pseudomonas aeruginosa. J. Bacteriol. 2008;190:3969–3978.
- Yawata Y, Nomura N, Uchiyama H. Development of a novel biofilm continuous culture method for simultaneous assessment of architecture and gaseous metabolite production. Appl. Environ. Microbiol. 2008;74:5429–5435.
- Heydorn A, Nielsen AT, Hentzer M, Sternberg C, Givskov M, Ersboll BK, Molin S. Quantification of biofilm structures by the novel computer program COMSTAT. Microbiology. 2000;146:2395–2407.
- Ryder C, Byrd M, Wozniak D. Role of polysaccharides in Pseudomonas aeruginosa biofilm development. Curr. Opin. Microbiol. 2007;10:644–648.
- DeVault JD, Kimbara K, Chakrabarty AM. Pulmonary dehydration and infection in cystic fibrosis: evidence that ethanol activates alginate gene expression and induction of mucoidy in Pseudomonas aeruginosa. Mol. Microbiol. 1990;4:737–745.
- Hentzer M, Teitzel GM, Balzer GJ, Heydorn A, Molin S, Givskov M, Parsek MR. Alginate overproduction affects Pseudomonas aeruginosa biofilm structure and function. J. Bacteriol. 2001;183:5395–5401.
- Jackson KD, Starkey M, Kremer S, Parsek MR, Wozniak DJ. Identification of psl, a locus encoding a potential exopolysaccharide that is essential for Pseudomonas aeruginosa PAO1 biofilm formation. J. Bacteriol. 2004;186:4466–4475.
- Colvin KM, Gordon VD, Murakami K, Borlee BR, Wozniak DJ, Wong GCL, Parsek MR. The Pel polysaccharide can serve a structural and protective role in the biofilm matrix of Pseudomonas aeruginosa. PLoS Pathog. 2011;7:e1001264.
- Ghafoor A, Hay ID, Rehm BHA. Role of exopolysaccharides in Pseudomonas aeruginosa biofilm formation and architecture. Appl. Environ. Microbiol. 2011;77:5238–5246.
- Yang L, Hu Y, Liu Y, Zhang J, Ulstrup J, Molin S. Distinct roles of extracellular polymeric substances in Pseudomonas aeruginosa biofilm development. Environ. Microbiol. 2011;13:1705–1717.
- Friedman L, Kolter R. Genes involved in matrix formation in Pseudomonas aeruginosa PA14 biofilms. Mol. Microbiol. 2004;51:675–690.
- Tashiro Y, Sakai R, Toyofuku M, Sawada I, Nakajima-Kambe T, Uchiyama H, Nomura N. Outer membrane machinery and alginate synthesis regulators control membrane vesicle production in Pseudomonas aeruginosa. J. Bacteriol. 2009;191:7509–7519.
- Knobloch JK-M, Bartscht K, Sabottke A, Rohde H, Feucht H-H, Mack D. Biofilm formation by Staphylococcus epidermidis depends on functional RsbU, an activator of the sigB operon: differential activation mechanisms due to ethanol and salt stress. J. Bacteriol. 2001;183:2624–2633.
- Lim JY, May JM, Cegelski L. Dimethyl sulfoxide and ethanol elicit increased amyloid biogenesis and amyloid-integrated biofilm formation in Escherichia coli. Appl. Environ. Microbiol. 2012;78:3369–3378.
