Abstract
Two similarly sulfated triterpene saponins from Pearsonothuria graeffei were prepared to investigate the anti-obesity effects of echinoside A (EA) and holothurin A (HA). The in vitro inhibitory activities of EA and HA toward pancreatic lipase were investigated, and two in vivo studies were performed: (i) Male Wistar rats were orally administered the lipid emulsion with or without a saponin (HA or EA). The serum’s total triglyceride concentration was measured at various times. (ii) C57BL/6 mice were assigned to four groups, high fat (HF), EA (0.03%), HA (0.04%), and orlistat (0.01%), and the weight of adipose tissue and level of fatty acids excreted in the feces were determined. Both EA and HA repressed the pancreatic lipase activity and increased fatty acid excretion in the feces. Treatment with EA and HA significantly decreased the adipose tissue accumulation in mice. EA and HA manifested different inhibitory activities in vitro, but each of them dramatically inhibited lipid absorption in vivo and showed strong anti-obesity activity.
Obesity and overweight are associated with an increased risk of morbidity due to such diet-related chronic diseases as type 2 diabetes, cardiovascular disease, and several forms of cancer, all of which, in turn, are associated with high mortality.Citation1,2) Obesity is a growing pandemic affecting millions of people worldwide. There is thus increased interest in the development of anti-obesity medications that help prevent corpulence and mitigate the consequences of obesity.
Several studies have shown that dietary saponins may reduce obesity and hyperlipidemia.Citation3Citation−Citation5) Triterpene glycoside, a secondary metabolite and bioactive component of sea cucumber, has exhibited several biological activities;Citation6Citation−Citation8) however, its structure-activity relationship is unknown, and further study is required to elucidate the mechanism for its bioactivity.
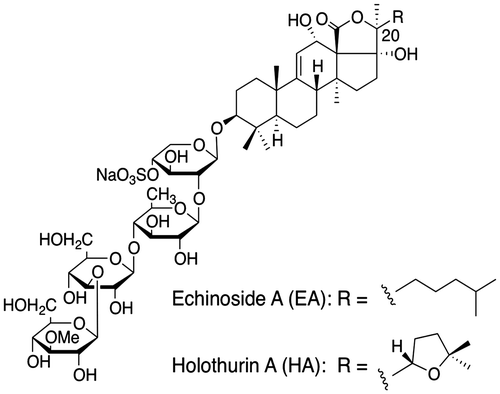
Pancreatic lipase is a key enzyme involved in the absorption of dietary triacylglycerol and the hydrolysis of triacylglycerols into 2-monoacylglycerol and fatty acids. Orlistat, a clinically approved drug for obesity treatment, has been shown to act via pancreatic lipase inhibition.Citation9) The success of orlistat has prompted studies for the development of novel pancreatic lipase inhibitors with fewer side effects. Previous studies have indicated that such plant-derived saponins as those found in green tea, ginseng, and Platycodi radix reduced fat absorption by inhibiting the pancreatic lipase activity.Citation10Citation−Citation12) The saponin from sea cucumber has a different structure from that of plant saponins. We have previously reported that crude saponins from sea cucumber could reduce the effects of aberrant lipid metabolism induced by a high-fat diet.Citation13) Little is currently known about the functions of the primary components of sea cucumber: echinoside A (EA) and holothurin A (HA). Both glycosides present the same degree of sulfation and only differ in the side chain at position 20 (Fig. ). An initial study was therefore undertaken to compare the effects of EA and HA on lipid metabolism.
Materials and methods
Preparation of the saponins from sea cucumber
HA and EA were isolated by the procedure described by Dong.Citation14) Briefly, an ethanolic extract of P. graeffei was sequentially submitted to macroporous resin, Si-gel, and reversed ODS Si-gel chromatography to achieve the final purification of HA and EA.
HPLC assay of EA and HA
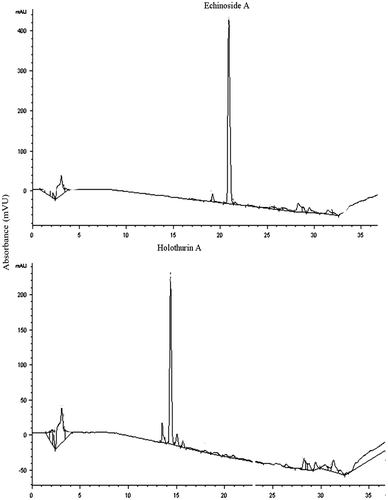
The HPLC system (Agilent) consisted of an injector (100 μL), a column oven (30 °C), a pump, and a diode-array detector (UV6000). A reversed-phase C-18 column (150 × 4.6 mm; Agilent) was used, with gradient elution by acetonitrile (A) and 0.1% NH4HCO3 (B). The initial concentration was 30% A, which was linearly changed to 60% A from 5 min to 30 min. Over the next 5 min, the percentage of mobile-phase A was decreased linearly to 30% (Fig. ). The flow rate was 1.0 mL/min, and UV absorption was measured at a 205 nm wavelength.
In vitro analysis of the inhibitory activity of the saponins on pancreatic lipase
The porcine pancreatic lipase (Sigma) activity was determined from the rate of oleic acid release from triolein, as previously described.Citation12,15) Briefly, a mixture of triolein (80 mg), phosphatidylcholine (10 mg), and taurocholic acid (5 mg) in 9 mL of a 0.1 M Tris–HCl buffer (pH 7.0) with 0.1 M of NaCl was sonicated for 5 min. This sonicated substrate suspension (0.1 mL) was incubated with 20 units of pancreatic lipase and 0.1 mL of various concentrations of the saponin or orlistat solutions for 30 min at 37 °C in a final volume of 0.25 mL. The assay was conducted with two replicates for each treatment.
Docking simulation
Protein preparation
The crystal structure of the porcine pancreatic lipase–colipase complex (PDB code 1ETH)Citation16) was obtained from the Protein Data Bank, and chain A was extracted for docking simulation.
Ligand preparation
The structure of EA was built by using Avogadro v. 1.0.3, an MMFF94s force field being used to minimize its structure. The partial atomic charge of EA was calculated with MOPAC2009 by using a PM3 Hamiltonian.
Docking simulation
The docking experiment was performed with AutoDock 4.2 and MGLTools 1.5.6 RC3. The Lamarckian genetic algorithm (GA) was employed for the docking simulation. A docking grid was set around the active site of the lipase.
Docking parameters
Number of GA runs, 100; population size, 150; maximum number of evaluations, 1,000,000; maximum number of generations, 27,000.
Animal maintenance
All experimental animals were individually housed in a room maintained with a 12-h light/dark cycle, a constant temperature of 24 ± 2 °C, and relatively humidity of 65 ± 15%. All aspects of the experiment were conducted according to guidelines provided by the ethical committee of experimental animal care at Ocean University of China (OUC, China).
Digestion and absorption of lipids in vivo
Male Wistar rats (6 weeks old; 180–220 g) were obtained from the Laboratory Animal Centre of Qingdao. After 1 week of adaptation, the rats were randomly assigned to 3 groups: the control group, EA-treated group, and HA-treated group. Each group comprised 6 rats. After 10 h of fasting, the rats were orally administered 2 mL of the lipid emulsion, which had been prepared by following the method of Han et al.Citation10) containing 6 mL of corn oil, 80 mg of cholic acid, 2 g of cholesteryl oleate, and 6 mL of physiological saline in the presence or absence of a saponin (0.27 g HA or 0.2 g EA). Blood samples were collected from the tail vein 0, 1, 2, 3, 4, and 5 h after administering the lipid emulsion with or without a saponin and centrifuged at 7500 rpm for 10 min to obtain the plasma. The triglycerol concentration was determined by using enzymatic reagent kits from Biosino (Beijing, China) according to the manufacturer’s instructions.
Weight-loss experiment on the high-fat diet-fed mice
A C57BL/6 male mice (18–22 g) were purchased from Vital River (Beijing, China). After a 5-d adaptation period, the mice were randomly assigned to the following four groups consisting of 7 mice each with similar mean body weight: (i) a model group (HF) fed with the AIN-93G dietCitation17) containing 25% fat; (ii) a group (EA) fed with the diet of the HF group supplemented with 0.03% EA; (iii) a group (HA) fed with the diet of the HF group supplemented with 0.04% HA; and (iv) a group (orlistat) fed with the diet of the HF group supplemented with 0.01% orlistat (Table ). All the mice had free access to water and food for 6 weeks. The mice were killed after overnight fasting at the end of the feeding period. Serum was separated from whole blood by centrifugation at 7500 rpm for 10 min at 4 °C. The liver, perirenal adipose tissue, and epidymal adipose tissue were quickly excised, weighed, frozen in liquid nitrogen, and stored at −80 °C until needed for analysis (Table ).
Table 1. Compositions of the experimental diets (g/kg of diet).
Analysis of fatty acid contents in the feces
Feces were collected for three consecutive days and freeze-dried. Total lipids were extracted by a chloroform/methanol mixture (2:1, v/v) as previously described.Citation18) The fatty acid analysis was conducted by hydrolyzing the total lipids with ethanolic KOH and simultaneously saponifying and methylating with methanol/HCl (5:1, v/v). The fatty acid methyl esters were analyzed by capillary gas chromatography (Agilent 6890) using an HP-INNO Wax capillary column (Agilent, 30 m × 0.32 mm × 0.25 μm). The temperature of the detector and injector were kept constant at 240 °C, and the oven temperature was increased from 170 to 240 °C at 3 °C/min, before being held at 240 °C for 15 min. Nitrogen was used as the carrier gas at a flow rate of 1.2 mL/min.
Statistical analyses
All the values in tables and figures are expressed as the mean ± standard error of the mean. Data were analyzed by one-way ANOVA, and then differences among means were analyzed by using the Duncan’s multiple range test. p < 0.05 was considered statistically significant.
Results and discussion
HA and EA were isolated with the procedure described by Dong.Citation14) Briefly, an ethanolic extract of P. graeffei was sequentially submitted to macroporous resin, Si-gel, and reversed ODS Si-gel chromatography to achieve the final purification of HA and EA.
The hydrolytic activity of porcine pancreatic lipase toward an emulsified triolein system was determined in the presence of an increasing concentration of each saponin or orlistat, as described previously.Citation12,15) EA inhibited the triolein-hydrolyzing activities of pancreatic lipase in a concentration-dependent manner, showing 50% inhibition at 9.6 μg/mL. In contrast, HA showed weak inhibitory activity. Orlistat exhibited significant inhibitory activity even at a concentration lower than 1 μg/mL (Fig. ).
Fig. 3. Inhibition of porcine pancreatic lipase activity by EA, HA, orlistat, and crude saponin.
Note: (A) inhibition of lipase by EA or HA. Values are the mean ± standard error, n = 3. (B) Inhibition of lipase by EA and orlistat. Values are the mean ± standard error, n = 3. (C) Double-reciprocal plot of the reaction rate vs. triolein concentration in the presence of the crude saponin.
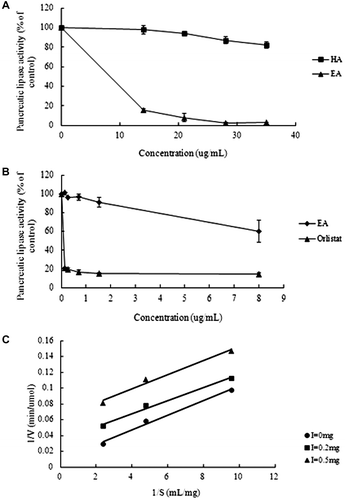
To elucidate the enzyme-inhibiting mechanism, the kinetics of the inhibitory effect of each crude saponin on pancreatic lipase were examined. The mode of inhibition was analyzed by changing the substrate concentration. A double-reciprocal plot of the reaction rate vs. triolein concentration was linear in the presence of each crude saponin, suggesting that the observed inhibition of lipase was anti-competitive.
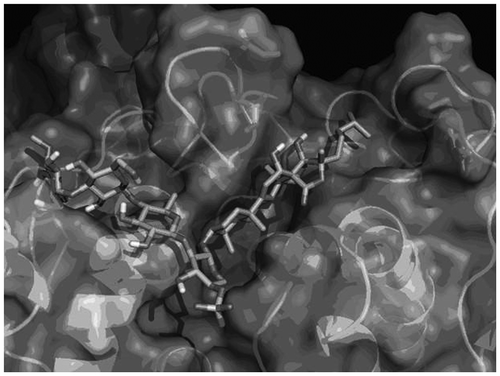
The distinct binding abilities of EA and HA to pancreatic lipase may have caused different inhibitory effects. A molecular docking simulation was therefore carried out to estimate the interaction between EA and porcine pancreatic lipase (PDB code: 1ETH)Citation16) by using AutoDock 4.2. The top-ranked docking result is shown in Fig. . The steroidal part of EA (thin chain) in this complex is located at the hydrophobic active site cleft of the lipase, and the isohexyl side chain of EA is close to the hydrophobic residues (stick), suggesting that the hydrophobicity of the steroidal part of EA might have been involved in the inhibition. The weak inhibitory activity of HA could be ascribed to the additional hydrophilic oxygen atom in the shortened side chain, a tetrahydrofuran ring consequently being formed.
Fig. 4. Predicted binding model of EA complexed with porcine pancreatic lipase.
Note: The protein structure is shown by a ribbon representation of the solvent-accessible surface. EA is shown by a stick representation.
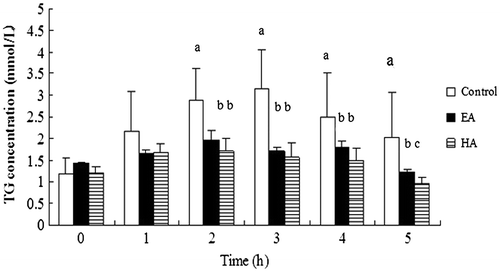
EA and HA exhibited different inhibitory activities toward pancreatic lipase in vitro, and more studies are needed to investigate their effects in vivo. Fig. shows that the plasma triacylglycerol concentrations of the rats had a different pattern in response to treatment with the lipid emulsion with or without a saponin, indicating that both EA and HA affected the dietary lipid absorption. The results show that the serum triglyceride levels of the control group increased in the period from 0 to 3 h after administering the lipid emulsion, following which the levels decreased. Two hours after administering the lipid emulsion, the plasma triacylglycerol concentration in the rats of the EA-treated and HA-treated groups were significantly lower than that of the control group (p < 0.05). In addition, the effect of HA was moderately better than that of EA. These results suggest that the saponins each had a lipid-lowering effect which could lead to the partial suppression of fat absorption.
Fig. 5. Effects of EA and HA on rat plasma triglyceride levels after the oral administration of a lipid emulsion.
Note: Each rat was given 2 mL of a lipid emulsion in the presence or absence of the saponins. Results are presented as the mean ± standard error of 6 rats. Those not sharing a letter differ, p < 0.05 (one-way ANOVA, followed by Duncan’s multiple range test).
Inhibiting the digestion and absorption of dietary fat is a key to controlling the body weight and adipose tissue weight.Citation19) We next examined the food intake, body weight, and adipose tissue weight of the mice. The initial body weight was similar among the mice groups, but after feeding for 6 weeks, the body weight gain of the EA-, HA-, and orlistat-treated mice was markedly lower than that of the mice in the HF group (p < 0.05). There was no significant difference in the average food intake and liver weight among these groups throughout the experiment (Table ).
Table 2. Effect of EA, HA, and orlistat on the body weight, food intake, and liver weight of mice.
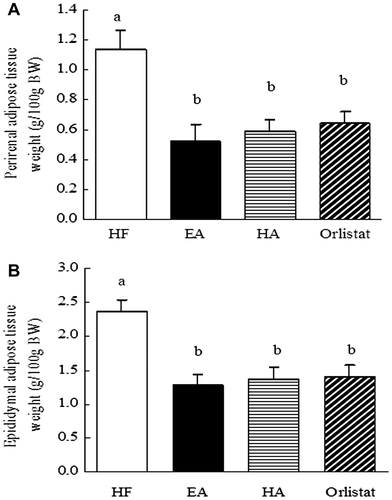
EA, HA, and orlistat all exhibited apparent suppressive effects on the weights of the perirenal adipose tissue and epididymal adipose tissue. These results confirmed that EA, HA, and orlistat had the potential to induce weight loss, and this anti-obesity effect did not depend on a decreased food or energy intake, because no significant difference was apparent among the different dietary groups (Fig. ).
Fig. 6. Effects of EA, HA, and orlistat on the adipose tissue weight in mice.
Note: (A) effects of EA, HA, and orlistat on the perirenal adipose tissue weight in mice. (B) effects of EA, HA, and orlistat on the epididymal adipose tissue weight in mice. Data are presented as the mean ± standard error of the mean of 7 rats. Those not sharing a letter differ, p < 0.05 (one-way ANOVA, Duncan’s multiple range test).
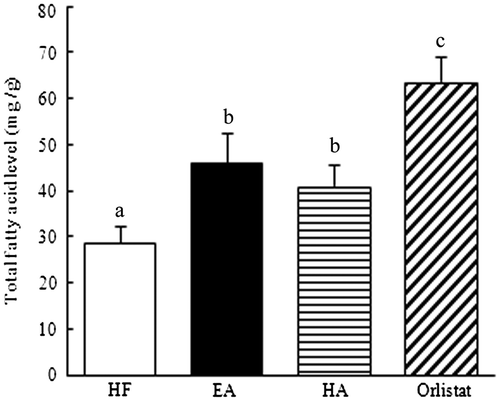
To clarify whether the saponins were associated with the absorption-inhibiting effect, the excretion of fatty acids in the feces was also assessed. Compared with the HF group, the EA- and HA-treated groups both showed significantly increased levels of total fatty acids in the feces (40.8 and 25.8%, respectively; p < 0.05). Furthermore, the orlistat group showed a markedly higher fatty acid level in the feces than that shown by the EA-treated and HA-treated groups (p < 0.05). These results suggest that a pathway that blocked lipid absorption may underlie the reduced accumulation of adipose tissue and increased excretion of fatty acids induced by the consumption of saponins (Fig. ).
Fig. 7. Effects of EA, HA, and orlistat on fatty acid excretion in the feces of mice.
Note: C57/BL6 mice were fed a high-fat diet with or without sea cucumber for 6 weeks. Feces were collected for three consecutive days and freeze-dried. Data are given as the mean ± standard error of the mean of 7 rats. Those not sharing a letter differ, p < 0.05 (one-way ANOVA, followed by Duncan’s multiple range test).
The sea cucumber has attracted considerable attention because of such beneficial health effects as antifungal, antineoplastic, hemolytic, cytostatic, and immunomodulatory activities.Citation6Citation−Citation8) Several studies have indicated that the whole body of the sea cucumber had beneficial effects on lipid metabolism.Citation20,21) According to Hu,Citation21) saponin was the most effective substance in sea cucumber owing to its hypolipidemic effect. We have additionally observed that crude saponins induced weight-loss and significantly decreased the adipose tissue weight.Citation13) Crude saponins, administered by using an emulsified triolein system, have inhibited the pancreatic lipase activity in vitro.Citation21) Furthermore, the rats administered with crude saponins displayed markedly reduced serum triglyceride and cholesterol concentrations, and saponin supplementation increased the excretion of fecal cholesterol. These results indicate that the anti-obesity effect of saponins might be related to their inhibitory effect on lipid absorption.
The inhibitory effect of saponins obtained from several plant sources on pancreatic lipase has been previously demonstrated. For example, despite their different structures, monomer saponins Rc, Rb1, and Rb2 obtained from Panax quinquefolium L., respectively, inhibited 100, 96, and 97% of pancreatic lipase activity.Citation22) However, platycodins A, C, and D, respectively, inhibited 3.3, 5.2, and 34.8% of pancreatic lipase activity.Citation23) EA and HA, as the two primary saponins in sea cucumber (P. graeffei), have respective contents of 0.84 and 0.56%. EA resembles HA in its molecular structure, with a sulfuric acid moiety linked to a steroid glycoside. The inhibitory activities of EA and HA toward pancreatic lipase were studied in vitro to elucidate the structure-activity relationship and the anti-obesity effects of EA and HA. It is interesting that the inhibitory activity of EA was significantly greater than that of HA. Unlike the inhibitory activity of saponins, the inhibitory activity of orlistat toward lipase was robust. The results of a computational docking study implied the possibility that EA bound directly to lipase. Structural modification of the EA side chain might therefore be a good strategy to develop powerful EA derivatives, although further experiments are required to elucidate the mechanism involved in EA-mediated lipase inhibition.
We have demonstrated in this study that EA and HA markedly reduced the serum triglyceride level. This finding is consistent with that of Hu,Citation21) indicating that EA and HA may inhibit lipid absorption. However, the hypolipidemic effect of EA and HA was similar, this being inconsistent with the findings of in vitro experiments.
Supplementing the diet of the C57BL/6 mice with EA, HA, or orlistat led to a remarkable reduction in the adipose tissue weight, no statistically significant difference being found in the effects of the three supplements. Saponins with complicated structures and from various species have been extensively studied for several years.Citation5,24−Citation30) The saponin-rich fraction of Platycodin grandiflorum radix has been screened for its anti-obesity effects, and these effects correlate with the inhibition of pancreatic lipase. Dioscin isolated from a methanol extract of Dioscorea nipponica powder has been shown to inhibit the pancreatic lipase activity and to suppress the time-dependent increase of plasma triglyceride level in mice injected with corn oil. Oolong tea has been reported to have anti-obesity and hypolipidemic effects, and tea saponins have dose-dependently inhibited the pancreatic lipase activity.Citation10,30) The saponins from sea cucumber have a structure different from that of plant saponins. Most of the sea cucumber saponins can be sulfated at the level of the sole xylosem, whereas plant saponins cannot be sulfated. Whether the sea cucumber saponins would have unique inhibitory effects was not known, so an initial study was undertaken to compare the effects of sea cucumber saponins with those of orlistat.
Supplementing the mouse diet with EA and HA notably increased the fecal excretion of fatty acids. It is notable that the effect of orlistat was markedly robust, unlike that of the saponins, indicating its powerful inhibitory activity toward pancreatic lipase that has been previously reported. Nevertheless, the effect of EA and HA was not statistically significant.
It is interesting that, unlike the powerful inhibitory action of EA, the inhibitory activity of HA was weak in vitro. In contrast, the anti-obesity effect of EA and HA in vivo was similar. The underlying inhibitory mechanisms are poorly understood. The data presented here also show that orlistat induced more fecal fatty acid excretion than the saponins did. However, the reduced adipose tissue weight induced by orlistat resembled that induced by the saponins, suggesting that another pathway may have contributed to the weight loss and that this pathway would be distinct from the one involved in inhibiting the pancreatic lipase activity. Indeed, we have observed that such hepatic lipogenic enzymes as fatty acid synthase, malic enzyme, and glucose-6-phosphate dehydrogenase were inhibited by a saponin treatment.Citation31) The saponin-fed rats also showed increased carnitine palmitoyl transferase activity in the liver. Consequently, after digestion, the influence of sea cucumber saponins on lipogenesis and lipodieresis possibly accounted for its anti-obesity effect.
Orlistat, a clinically approved drug for obesity treatment, has such unpleasant side effects as oily stools, oil spotting, and flatulence. The results presented here demonstrate that although an EA and HA treatment induced lower fatty acid excretion than that induced by an orlistat treatment, the anti-obesity effect of EA and HA was similar to that of orlistat.
In summary, EA and HA, two sea cucumber saponins with a similar molecular structure, showed distinct inhibitory activity toward pancreatic lipase in vitro and significantly inhibited lipid absorption in vivo, thereby exhibiting a powerful anti-obesity effect.
Funding
This work was supported by the National Key Technology Research and Development Program of the Ministry of Science and Technology of China [grant number 2012BAD33B07], and National Natural Science Foundation of China [grant number 31000795].
Notes
Abbreviations: EA, echinoside A; HA, holothurin A; HF, high fat.
References
- Hill JO, Peters JC. Environmental contributions to the obesity epidemic. Science. 1998;280:1371–1374.
- Gura T. Obesity drug pipeline not so fat. Science. 2003;299:849–852.
- Francis G, Kerem Z, Makkar HP, Becker K. The biological action of saponins in animal systems: a review. Br. J. Nutr. 2002;88:587–605.
- Lee SO, Simons AL, Murphy PA, Hendrich S. Soyasaponins lowered plasma cholesterol and increased fecal bile acids in female golden Syrian hamsters. Exp. Biol. Med. 2005;230:472–478.
- Yoshizumi K, Hirano K, Ando H, Hirai Y, Ida Y, Tsuji T, Tanaka T, Satouchi K, Terao J. Lupane-type saponins from leaves of Acanthopanax sessiliflorus and their inhibitory activity on pancreatic lipase. J. Agric. Food Chem. 2006;54:335–341.
- Kalinin VI. System-theoretical (holistic) approach to the modelling of structural-functional relationships of biomolecules and their evolution: an example of triterpene glycosides from sea cucumbers (Echinodermata, Holothurioidea). J. Theor. Biol. 2000;206:151–168.
- Zou ZR, Yi YH, Wu HM, Wu JH, Liaw CC, Lee KH. Intercedensides A-C, three new cytotoxic triterpene glycosides from the sea cucumber Mensamaria intercedens Lampert. J. Nat. Prod. 2003;66:1055–1060.
- Dong P, Xue CH, Yu LF, Xu J, Chen SG. Determination of triterpene glycosides in sea cucumber (Stichopus japonicus) and its related products by high-performance liquid chromatography. J. Agric. Food Chem. 2008;56:4937–4972.
- Birari RB, Bhutani KK. Pancreatic lipase inhibitors from natural sources: unexplored potential. Drug Discov. Today. 2007;12:879–889.
- Han LK, Kimura Y, Kawashima M, Takaku T, Taniyama T, Hayashi T, Zheng YN, Okuda H. Anti-obesity effects in rodents of dietary tea saponin, a lipase inhibitor. Int. J. Obesity. 2001;25:1459–1464.
- Karu N, Reifen R, Kerem Z. Weight gain reduction in mice fed Panax ginseng saponin, a pancreatic lipase inhibitor. J. Agric. Food Chem. 2007;55:2824–2828.
- Han LK, Zheng YN, Xu BJ, Okuda H, Kimura Y. Saponins from platycodi radix ameliorate high fat diet-induced obesity in mice. J. Nutr. 2002;132:2241–2245.
- Hu XQ, Li ZJ, Xue Y, Xu J, Xue CH, Wang JF, Wang YM. Dietary saponins of sea cucumber ameliorate obesity, hepatic steatosis, and glucose intolerance in high-fat diet–fed mice. J. Med. Food. 2012;15:1–8.
- Dong P. [PhD Thesis]. Studies on the isolation, identification, structure-modification and bioactivities of triterpene glycosides in Pearsonothuria graeffei. Ocean University of China, 2009.
- Zapf J, Schoenle E, Waldvogel M, Sand I, Froesch ER. Effect of trypsin treatment of rat adipocytes on biological effects and binding of insulin-like growth factors through the insulin receptor. Eur. J. Biochem. 1981;113:605–609.
- Hermoso J, Pignol D, Kerfelec B, Crenon I, Chapus C, Fontecilla-Camps JC. Lipase Activation by Nonionic Detergents. The crystal structure of the porcine lipase-colipase-tetraethylene glycol monooctyl ether complex. J. Biol. Chem. 1996;271:18007–18016.
- Reeves PG. Components of the AIN-93 diets as improvements in the AIN-76A diet. J. Nutr. 1997;127:838S–841S.
- Folch J, Lees M, Slane-Stanley GH. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957;226:497–506.
- Ros E. Intestinal absorption of triglyceride and cholesterol. Dietary and pharmacological inhibition to reduce cardiovascular risk. Atherosclerosis. 2000;151:357–379.
- Tanaka K, Nishizono S, Kase A, Ogura S, Kurita M, Murakami T, Kugino K, Matsumoto H, Ikeda I. Effects of dietary black sea cucumber on serum and liver lipid concentrations in rats. J. Jpn. Soc. Nutr. Food Sci. 2003;56:175–179.
- Hu XQ, Xu J, Xue Y, Li ZJ, Wang JF, Wang JH, Xue CH, Wang YM. Effects of bioactive components of sea cucumber on the serum, liver lipid profile and lipid absorption. Biosci. Biotechnol. Biochem. 2012;76:2214–2218.
- Zhang J, Zheng Y, Li X. Effects of saponins from Panax quinquefolium Linne on the metabolism of lipid. J. Jilin Agric. Univ. 2002;24:62–63.
- Zheng Y, Liu K, Xu B. Studies on effects of Platycodi Radix on lipid metabolism of mice with high fat diet-induced obesity. J. Jilin Agric. Univ. 2002;24:42–46.
- Han LK, Xu BJ, Kimura Y, Zheng YN, Okuda H. Platycodi Radix affects lipid metabolism in mice with high fat diet-induced obesity. J. Nutr. 2000;130:2760–2764.
- Zao HL, Kim YS. Determination of the kinetic properties of platycodin D for the inhibition of pancreatic lipase using a 1,2-diglyceride-based colorimetric assay. Arch. Pharm. Res. 2004;27:968–972.
- Xu BJ, Han LK, Zheng YN, Lee JH, Sung CK. In vitro inhibitory effect of triterpenoidal saponins from platycodi Radix on pancreatic lipase. Arch. Pharm. Res. 2005;28:180–185.
- Zhao HL, Sim JS, Shim SH, Ha YW, Kang SS, Kim YS. Antiobese and hypolipidemic effects of platycodin saponins in diet-induced obese rats: evidences for lipase inhibition and calories intake restriction. Int. J. Obesity. 2005;29:983–990.
- Zheng Q, Koike K, Han LK, Okuda H, Nikaido T. New biologically active triterpenoid saponins from Scabiosa tschiliensis. J. Nat. Prod. 2004;67:604–613.
- Han LK, Zheng YN, Yoshikawa M, Okuda H, Kimura Y. Anti-obesity effects of chikusetsusaponins isolated from Panax japonicus rhizomes. BMC Complement. Altern. Med. 2005;5:9–18.
- Han LK, Takaku T, Li J, Kimura Y, Okuda H. Anti-obesity action of oolong tea. Int. J. Obesity. 1999;23:98–105.
- Hu XQ, Wang YM, Wang JF. Dietary saponins of sea cucumber alleviated orotic acid-induced fatty liver in rats via PPARα and SREBP-1c signaling. Lipids Health Dis. 2010;9:1–9.