Abstract
The effects of mechanical stress during emulsification on the oxidation of methyl linoleate were investigated by four methods (high-pressure homogenization, high-speed stirring, ultrasonic homogenization, and membrane emulsification). The oxidation rates and induction periods were almost constant, regardless of the emulsification method, except for membrane emulsification, by which the induction period was prolonged when a cellulose acetate membrane was used.
Emulsions are widely used in many foods, and there are various methods for preparing emulsions. Four emulsification methods, namely high-speed stirring, high-pressure homogenization, ultrasonic homogenization, and membrane emulsification, are commonly used. Some methods in the emulsification process subject the oil to mechanical stress which causes deterioration of the oil.Citation1) However, some results have shown that oil deterioration can be suppressed in emulsions.Citation2−5) There are therefore no definite conclusions regarding the loss of oil quality, including its oxidation, during emulsification. This uncertainty arises because food-based materials containing various components are used for evaluating the oxidation of oil.
In this study, the four emulsification methods were applied to mixtures of water and methyl linoleate without an emulsifier to evaluate the effect of each emulsification method on lipid deterioration. The degree of oxidation of bulk methyl linoleate was determined after the emulsification to evaluate the influence of mechanical stress applied during emulsification on the oxidation rate constant and induction period.
Emulsification was performed by adding methyl linoleate (>95% purity), purchased from Tokyo Chemical Industry (Tokyo, Japan), to distilled water at a final concentration of 10% (w/v), and vortexing the mixture for 10 s with a TM-351 test tube mixer (Iwaki Glass, Tokyo, Japan). The mixture was then rapidly subjected to one of the four emulsification methods (high-pressure homogenization, high-speed stirring, ultrasonic homogenization, and membrane emulsification).
High-pressure homogenization was carried out by homogenizing the mixture of water and methyl linoleate with a PEL-20 Nanomizer high-pressure device (Yoshida Kikai, Aichi, Japan). Homogenization was performed at 40, 81, and 152 MPa, with two passes through the homogenizer. The effect of the number of passes was also evaluated for 1, 2, 4, and 8 passes at 81 MPa.
Homogenization by high-speed stirring was carried out with a PT20SK Polytron homogenizer (Kinematica, Luzern, Switzerland). A 40-mL amount of the mixture of water and methyl linoleate was first transferred to a 50-mL beaker and stirred. The stirring speeds were 3 × 103, 8 × 103, and 1.5 × 104 rpm, with a stirring time of 2.0 min. The effect of the stirring time was also evaluated by setting times of 0.50, 2.0, and 8.0 min, with a stirring speed of 8 × 103 rpm.
Ultrasonic homogenization was performed using a Nissei US-300T ultrasonic device (Nihon Seiki, Tokyo, Japan), using an ultrasonic tip diameter of 20 mm. The ultrasonic tip was inserted into 40 mL of the mixture of water and methyl linoleate. The intensity of the ultrasonic wave was controlled by adjusting the output dial to the levels 1.0, 5.0, and 9.0, with a treatment time of 2.0 min, to investigate the influence of the intensity. The effect of the treatment time (0.50, 2.0, and 8.0 min) was also examined with an output level of 5.0.
Membrane emulsification was finally performed. The mixture of water and methyl linoleate was transferred to a 50-mL plastic syringe connected to a DISMIC-25CS cellulose acetate membrane filter (0.80 μm pore size) or to a mixed cellulose ester membrane filter (0.80 μm pore size; Toyo Roshi, Tokyo, Japan). The syringe interior was pressurized with nitrogen at 0.20 MPa. The number of passes through the membrane filter was 1, 2, or 4.
The effect of the amount of a membrane on the oxidation was also investigated. A cellulose acetate membrane was taken from a DISMIC cellulose acetate membrane filter. The cellulose acetate membrane (2.5–40 mg) or mixed cellulose ester membrane (2.5–40 mg) was immersed in 4 g of methyl linoleate placed in a 50-mL amber vial. The vial was filled with nitrogen and then kept at 4 °C in the dark for 18 h, with occasional stirring.
The mixture, which had been subjected to each emulsification method, was then separated into its oil and aqueous phases. The oil phase was collected and centrifuged at 1.5 × 104 rpm for 10 min with an MC-150 high-speed microcentrifuge (Tomy, Tokyo, Japan). Methyl linoleate was then oxidized. The oil phase (440 mg) was dissolved in 25 mL of methanol, before placing 100 μL of the solution in a flat-bottomed glass cup (1.5 cm I.D. × 3.0 cm); 120–240 samples were prepared. Methanol was then removed under reduced pressure. The cups were placed in a plastic container (300 mm width × 150 mm height × 150 mm depth), with a dry-air flow of 5 mL/min after passing the air through silica gel. The plastic container was stored at 55 °C in a DN-400 oven (Yamato Scientific, Tokyo, Japan).
The cups were periodically removed from the container, and 1.0 mL of a methanol solution of methyl myristate (1.455 g/L), as the internal standard for a gas chromatographic analysis, was added to the cup. Unoxidized methyl linoleate was determined using a GC-2014 gas chromatograph (Shimadzu, Kyoto, Japan) equipped with a flame ionization detector and a DB-1ht column (0.25 mm I.D. × 30 m; Agilent Technologies, CA, USA). The respective temperatures of the injector, column, and detector were 230, 205, and 240 °C.
Fig. shows the time-course characteristics of the changes in the unoxidized fractions of methyl linoleate emulsified by different methods. With high-pressure homogenization, the oxidation of methyl linoleate with different numbers of passes was similar to that of methyl linoleate without emulsification (Fig. (A)). Two other emulsification methods, i.e., high-speed stirring and ultrasonic emulsification, showed that the effects of stirring speed and ultrasonic power on oxidation were minor (Fig. (B) and (C)). Although a rise in temperature would occur during emulsification, its effects were not also significant. The mechanical stress on methyl linoleate during emulsification would therefore not have affected the stability of methyl linoleate.
Fig. 1. Oxidation of methyl linoleate treated by various emulsification methods.
Note: Mixtures of methyl linoleate and water were emulsified by (A) high-pressure homogenization, (B) high-speed stirring, (C) ultrasonic homogenization, and (D) membrane filtration, using a cellulose acetate membrane. High-pressure homogenization was performed one to four times at 81 MPa: (△) 1, (□) 2, (♢) 3, and (○) 4 times. High-speed stirring was performed for 0.50–8.0 min at 8 × 103 rpm: (△) 0.50, (□) 2.0, and (♢) 8.0 min. Ultrasonic homogenization was carried out at output levels of 1.0–9.0 for 2.0 min: (△) 1.0, (□) 5.0, and (♢) 9.0. Membrane emulsification was performed by using a cellulose acetate membrane with one to four passes through the membrane: (△) 1, (□) 2, and (♢) 4 passes. Symbol (●) in A–D represents the results obtained by performing oxidation without emulsification; all curves are calculated results.
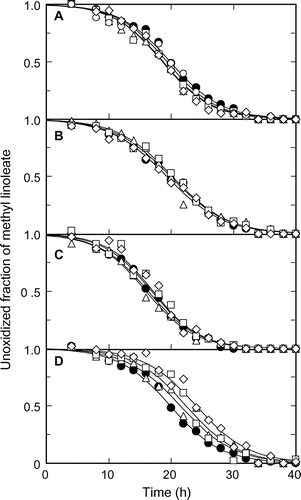
In contrast, membrane emulsification affected the oxidation behavior of methyl linoleate when the cellulose acetate membrane was used (Fig. (D)). The induction period for oxidation significantly increased with increasing number of passes of the emulsion through the membrane filter. These results indicate that some radicals, which had initially been present in methyl linoleate, had been adsorbed to the membrane, delaying the oxidation.
The oxidation of n-6 unsaturated fatty acids and their esters, including methyl linoleate, can be expressed by the autocatalytic kinetic equation:Citation6)(1)
where Y is the fraction of unoxidized methyl linoleate, t is the time, k is the oxidation rate constant, and Y0 is Y at t = 0, reflecting the initial state of methyl linoleate. The Y0 value decreases with increasing initial concentration of the radicals in methyl linoleate. Based on Equation (Equation1(1) ), the k and Y0 values can, respectively, be estimated from the slope and intercept of the line obtained by plotting ln (1 − Y)/Y vs. t. The calculated results in Fig. coincide well with the experimental results, indicating that the autocatalytic oxidation model represented the entire oxidation process of methyl linoleate, even after emulsification.
Table shows the relative oxidation rate constants, k/k*, and ()/(1 − Y0) values under the various emulsifying conditions, where k* and
are the respective rate constant and the parameter for the oxidation of methyl linoleate without emulsification. None of the emulsification conditions such as the number of homogenization steps, homogenization pressure, and stirring time had any significant effect on k/k* and (
)/(1 − Y0) during high-pressure homogenization, high-speed stirring, and ultrasonic emulsification. These results show that the mechanical stress during these emulsification procedures did not change the initial state of methyl linoleate under the tested conditions.
Table 1. Dependence of the Relative Oxidation Rate Constant and the Parameter, (1−)/(1−Y0), on the Emulsifying Conditions.
Moreover, k/k* did not change after membrane emulsification, even after increasing the number of passes through the membrane. However, the value for ()/(1 − Y0) clearly increased with increasing number of passes when the cellulose acetate membrane was used. A possible reason for this increase was adsorption of the radicals initially present in methyl linoleate to the membrane. However, the value for (
)/(1 − Y0) did not depend much on the number of passes when a mixed cellulose ester membrane was used. Although the reason for this is not clear at present, the chemical structures of the membranes probably affected the adsorption behavior.
The effect of the weight ratio of the membrane to methyl linoleate on the latter’s oxidation was then investigated. No significant change in ()/(1 − Y0) was apparent when the concentration of the cellulose acetate membrane was ≤2.5 mg/g of oil, or when a mixed cellulose ester membrane was used. However, the value for (
)/(1 − Y0) increased when the cellulose acetate membrane concentration was 10 mg/g of oil. These results show that a cellulose acetate membrane concentration of at least 2.5–10 mg/g of oil was needed for adsorption of the radicals in methyl linoleate.
In conclusion, the mechanical stress caused during emulsification did not affect the oxidation behavior of methyl linoleate. The relative oxidation rate constant was almost constant, regardless of the type of emulsification method. However, the induction period and the value for ()/(1 − Y0) increased in the presence of a cellulose acetate membrane. This increase was probably caused by the adsorption of radicals present in the membrane before its emulsification.
Acknowledgments
T.M. expresses sincere gratitude for a scholarship from the Ministry of Education, Culture, Sports, Science and Technology of the Japanese government. This study was performed under the project entitled Creation of Innovative Technology for Marine Products Industry, Program for Revitalization Promotion of the Japan Science and Technology Agency (JST).
References
- Kuhn KR, Cunha RL. Flaxseed oil – whey protein isolate emulsions: effect of high pressure homogenization. J. Food Eng. 2012;111:449–457.
- Poliseli-Scopel FH, Hernádez-Herrero M, Guamis B, Ferragut V. Comparison of ultra high pressure homogenization and conventional thermal treatments on the microbiological, physical and chemical quality of soymilk. LWT – Food Sci. Technol. 2012;46:42–48.
- Atarés L, Marshall LJ, Akhtar M, Murray BS. Structure and oxidative stability of oil in water emulsions as affected by rutin and homogenization procedure. Food Chem. 2012;134:1418–1424.
- Kim JY, Seo TR, Lim ST. Preparation of aqueous dispersion of β-carotene nano-composites through complex formation with starch dextrin. Food Hydrocolloid. 2013;33:256–263.
- Rodriguez-Turienzo L, Cobos A, Diaz O. Effects of edible coatings based on ultrasound-treated whey proteins in quality attributes of frozen Atlantic salmon (Salmo salar). Innov. Food Sci. Emerg. Technol. 2012;14:92–98.
- Adachi S, Ishiguro T, Matsuno R. Autoxidation kinetics for fatty acids and their esters. J. Am. Oil Chem. Soc. 1995;72:547–551.
