Abstract
The metabolism of quercetin was investigated in Mythimna separata larvae. Quercetin 4′-O-sulfate was mainly identified in the frass when 6th instar larvae were fed artificial diets containing 1% quercetin. In the case of the 3rd instar larvae, a larger amount of quercetin was detected in the frass. M. separata larvae had different metabolic strategies for quercetin at different developmental stages.
Quercetin is one of the most abundant flavonoids in plants and is assumed to have protective roles against herbivory and pathogens. A previous study has shown that quercetin could inhibit the growth of Heliothis virescens, Helicoverpa zea, and Pectinophora gossypiella,Citation1) reduce the pupation rate of Helicoverpa armigera, and lead to larval mortality of Spodoptera eridania.Citation2) Furthermore, a recent study has revealed that quercetin could differentially induce the activities of the detoxification enzymes, GSTs, COE, and P450s.Citation3)
In this study, oriental armyworm Mythimna separata larvae were fed artificial diets containing 1% quercetin to investigate how M. separata larvae would cope with the toxic compound. The metabolism of quercetin in insects is still unclear and requires further investigation, although quercetin 5-O-glucoside has been identified from orally administrated quercetin in the Bombyx mori silkworm.Citation4) These results may help us understand the ecological interactions between herbivorous insects and host plants and the physiological adaptations that insects have evolved against plants to obtain fundamental knowledge for designing strategies aimed at pest control.
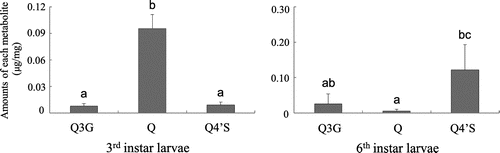
Typical chromatograms for extracts from the frass of 3rd instar and 6th instar M. separata larvae that had been fed the artificial diet containing 1% quercetin are shown in Fig. . As previously described,Citation4,Citation5) O-monoglucosides of quercetin as well as O-monosulfates were baseline-separated in a C18 column by HPLC. Three distinct peaks were observed in the frass of both M. separata larvae. By comparing the chromatographic behavior and mass spectra with those of authentic compounds, compounds 1 (tR 3.6 min, m/z 463 [M–H]−), 2 (tR 7.5 min, m/z 301), and 3 (tR 9.4 min, m/z 381) were, respectively, identified as quercetin 3-O-glucoside, quercetin, and quercetin 4′-O-sulfate. The amounts of quercetin 3-O-glucoside, quercetin, and quercetin 4′-O-sulfate in the frass of larvae fed the artificial diet containing 1% quercetin are shown in Fig. . Quercetin was mainly excreted as a sulfate conjugate in the frass of 6th instar larvae, whereas it was excreted in an unchanged form in the frass of 3rd instar larvae (Fig. ), and of 1st, 2nd, 4th, and 5th instar larvae (data not shown).
Fig. 1. Structures of quercetin (A) and quercetin 4′-O-sulfate (B), and a typical chromatogram of quercetin metabolites in the frass of 3rd and 6th instar M. separata larvae (C).
Note: LC/MS selected ion chromatograms ([M–H]−) of quercetin 3-O-glucoside (m/z 463), quercetin (m/z 301), quercetin 4′-O-sulfate (m/z 381), and the total ion chromatogram (TIC) are shown.
![Fig. 1. Structures of quercetin (A) and quercetin 4′-O-sulfate (B), and a typical chromatogram of quercetin metabolites in the frass of 3rd and 6th instar M. separata larvae (C).Note: LC/MS selected ion chromatograms ([M–H]−) of quercetin 3-O-glucoside (m/z 463), quercetin (m/z 301), quercetin 4′-O-sulfate (m/z 381), and the total ion chromatogram (TIC) are shown.](/cms/asset/cf7e2fdc-ce43-4273-bf55-89e1fe7ca6db/tbbb_a_877835_f0001_b.gif)
Fig. 2. Amounts of quercetin metabolites identified in the frass of 3rd (left) and 6th (right) instar larvae fed the 1% quercetin diet (mean ± SD, n = 5).
Note: Quercetin 3-O-glucoside (Q3G), quercetin (Q), and quercetin 4′-O-sulfate (Q4′S) are shown. Different letters represent significant differences (p < 0.05, Tukey–Kramer HSD test for multiple comparisons).
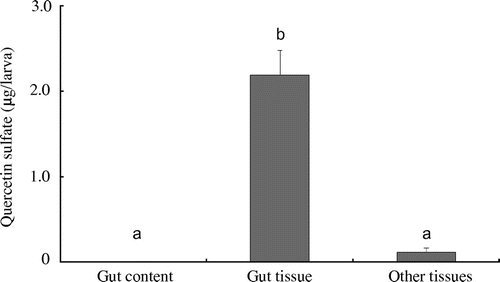
The amounts of quercetin 4′-O-sulfate produced by the gut contents, gut tissues, and other tissue preparations of the 6th instar larvae are shown in Fig. . In the case of the gut tissue preparation, the amount of quercetin 4′-O-sulfate produced was estimated to be 2.2 ± 0.2 μg (mean ± SD, n = 5), which was higher than that produced by the other tissue preparations (0.11 ± 0.05 μg, mean ± SD, n = 5). In the case of the gut content preparations, no quercetin 4′-O-sulfate was detected in the incubated mixture. The enzymatic activity of the gut tissue of 6th instar larvae (21.9 ± 2.9 μg/mg of proteins, mean ± SD, n = 5) was significantly (p < 0.001, Student’s t-test) higher than that of the 3rd instar larvae (1.1 ± 0.5 μg/mg of proteins, mean ± SD, n = 5).
Fig. 3. Amounts of quercetin 4′-O-sulfate produced by in vitro incubation with the substrates and homogenates of each part of 6th instar larvae (mean ± SD, n = 5).
Note: Different letters represent significant differences (p < 0.05, Tukey–Kramer HSD test for multiple comparisons).
Sulfate conjugation is known to be a major metabolic pathway for the detoxification of endogenous and exogenous phenolic compounds. This reaction is catalyzed by phenol sulfotransferase which has been identified in several mammalian organs.Citation5,Citation6) This enzyme catalyzes the transfer of sulfate from 3′-phosphoadenosine 5′-phosphosulfate (PAPS) to various alcohol groups. In this study, quercetin was metabolized to a sulfate conjugate in the 6th instar larvae. Yang and Wilkinson have shown that a sulfotransferase system was present in the gut tissues of southern armyworm larvae and was active in the sulfation of p-nitrophenol and such steroids of the insect as α-ecdysone.Citation7) It has been suggested that the insect system utilized PAPS as the sulfate donor in the sulfation of various acceptors, similar to that in the mammalian system.Citation7) According to Yang and Wilkinson, the gut, malpighan tubules, and fat body exhibited sulfotransferase activity. The sulfation of flavonoids in insects may be important in detoxication, excretion, or both.
Quercetin was excreted mainly in an unchanged form in the frass in 3rd instar M. separata larvae fed on the artificial diet containing quercetin, and a nearly undetectable amount of quercetin sulfate was detected after in vitro incubation of the gut tissues of the 3rd instar larvae. M. separata larvae may have different metabolic strategies for quercetin at different development stages. In this study, we show the stage-specific enzymatic activity of sulfotransferase in the gut tissues of M. separata larvae. Further studies are necessary to understand the ecological meaning(s) of the stage-specific enzymatic activity in M. separata larvae.
Experimental
Caterpillar rearing
Larvae of the oriental armyworm, M. separata, were reared on an artificial diet (Insecta-LFS, Nihon Nosan Kogyo, Yokohama, Japan) at 24 °C under a 16 h/8 h (light/dark) cycle. To investigate the metabolites of quercetin, 1st to 6th instar larvae were separated into solo cups, and individually fed an artificial diet containing 1% quercetin (w/w; Wako Pure Chemical Industries, Osaka, Japan) for 2 days. Quercetin and its metabolites in the frass excreted for 2 days were analyzed by LC/MS.
LC/MS analysis of quercetin and its metabolites
Quercetin and its metabolites were extracted from the frass of larvae by methanol/water (2/1, v/v) at 60 °C for 1 h. The crude extract was centrifuged for 5 min at 14,000 g, and the resulting supernatant was evaporated to dryness. The residue was dissolved in 1 mL of water and applied to a Sep-Pak C18 cartridge (Waters, MA, USA). Quercetin and its metabolites were eluted from the cartridge by methanol and analyzed by an LCMS2010 instrument (Shimadzu, Kyoto, Japan) equipped with an LC-10ADvp pump (Shimadzu, Kyoto, Japan). The CDL temperature was 250 °C, voltage was 1.8 kV, nebulizer gas flow was 1.5 mL/min, and detection was done in the negative ESI mode. A Mightysil RP-18 GP column (50 × 2.0 i.d. mm, Kanto Chemical Co., Tokyo, Japan) was eluted at 0.2 mL/min with a gradient of 30–90% acetonitrile in water containing 0.05% ammonia formate over 25 min. The column temperature was maintained at 60 °C.
In vitro assay for quercetin sulfation. Sixth instar larvae were anesthetized by immersing in tepid water and then dissected in saline. Gut tissue containing the whole gut content was removed from the larva, and then the gut content was carefully separated from the gut tube. The gut tissue and gut content were separately homogenized in 300 μL of ice-cold 25 mM Tris–HCl at pH 7.5. Each homogenate was centrifuged for 5 min at 14,000 g. PAPS was used as the sulfate donor for in vitro sulfation as previously reported.Citation8) In the present study, the standard assay mixture consisted of 0.67 mM of quercetin dissolved in DMSO, 2 mM PAPS, 6.7 mM MgCl2, and a crude protein extract prepared from the gut tissue or gut content in a 25 mM Tris–HCl buffer at pH 7.5 in a total volume of 150 μL. The reaction was initiated by adding the homogenate and the mixture incubated for 60 min at 37 °C, before aliquots of the reaction mixture were taken and processed as described for the LC/MS analysis. Quercetin and its metabolites were identified by comparing with authentic samples. Quercetin and quercetin 3-O-glucoside were purchased from Sigma-Aldrich Japan (Tokyo, Japan). Quercetin 4′-O-sulfate was synthesized as described next. Protein contents were determined by the BCA protein assay method (Pierce, Rockford, IL, USA), using bovine serum albumin as the standard.
Preparation of quercetin 4′-O-sulfate
Quercetin 4′-O-sulfate was synthesized as reported with modifications.Citation5) Quercetin dissolved in dioxane was reacted with a 10-fold molar excess of the sulfur trioxide-N-triethylamine complex at 40 °C for 90 min. The reaction solution was decanted off, and the residue was dissolved in methanol. The solution was analyzed using HPLC. The resulting chromatogram showed the presence of quercetin and eight peaks, some of which were surmised to be quercetin sulfates. Quercetin 4′-O-sulfate was isolated by HPLC after pre-purification in a Sep-Pak C18 cartridge. A Cosmosil 5C18-ARII column (250 × 10 i.d. mm, Nacalai Tesque, Kyoto, Japan) was eluted at 2.0 mL/min with a 10 min gradient of 25–45% methanol in water containing 0.1 M ammonium acetate. The gradient conditions were then changed from 45 to 55% over 10 min, followed by a 10 min gradient from 55 to 100%, and finally held for 5 min at 100%. The column temperature was maintained at 40 °C. Quercetin 4′-O-sulfate was eluted at 26 min. The 1H NMR data are similar to those previously reported.Citation5) 1H-NMR (DMSO-d6) δ: 6.21 (1H, d, J = 2.0 Hz, H-6), 6.46 (1H, d, J = 2.0 Hz, H-8), 7.38 (1H, d, J = 8.8 Hz, H-5′), 7.59 (1H, dd, J = 8.8 Hz, 2.0 Hz, H-6′), 7.71 (1H, d, J = 2.0 Hz, H-2′).
NMR analysis
1H-NMR (400 MHz) spectra were recorded with TMS as an internal standard in DMSO-d6 using a Bruker AC400 spectrometer. Chemical shifts are given as δ values.
Funding
This study was partly supported by grant-aid for scientific research [grant number 22380068], [grant number 24120006] from the Ministry of Education, Culture, Sports, Science and Technology of Japan. TA was the recipient of a research fellowship for young scientists (No. 20.3494) from the Japan Society for the Promotion of Science.
Notes
Abbreviations: CDL, curved desolvation line; COE, carboxylesterases; DMSO, dimethyl sulfoxide; ESI, electron spray ionization; GSTs, glutathione S-transferases; LC/MS, liquid chromatography/mass spectrometry; NMR, nuclear magnetic resonance; P450s, cytochrome P450 monooxygenases; PAPS, 3′-phosphoadenosine 5′-phosphosulfate; TMS, trimethylsilane.
References
- Shaver TN, Lukefahr MJ. Effect of flavonoid pigments and gossypol on growth and development of the bollworm, tobacco budworm and pink bollworm. J. Econ. Entomol. 1969;62:643–646.
- Lindroth RL, Peterson SS. Effects of plant phenols on performance of southern armyworm larvae. Oecologia. 1988;75:185–189.
- Zhang Y, Ma H, Feng D, Lai X, Chen Z, Xu M, Yu Q, Zhang Z. Induction of detoxification enzymes by quercetin in the silkworm. J. Econ. Entomol. 2012;105:1034–1042.
- Hirayama C, Ono H, Tamura Y, Konno K, Nakamura K. Regioselective formation of quercetin 5-O-glucoside from orally administered quercetin in the silkworm, Bombyx mori. Phytochemistry. 2008;69:1141–1149.
- Jones DJL, Jukes-Jones R, Verschoyle RD, Farmer PB, Gescher A. A synthetic approach to the generation of quercetin sulfates and the detection of quercetin 3′-O-sulfate as a urinary metabolite in the rat. Bioor. Med. Chem. 2005;13:6727–6731.
- Sekura RD, Duffel MW, Jakoby WB. Aryl sulfotransferases. Methods Enzymol. 1981;77:197–206.
- Yang RSH, Wilkinson CF. Enzymic sulphation of p-nitrophenol and steroids by larval gut tissues of the southern armyworm (Prodenia eridania Cramer). Biochem. J. 1972;130:487–493.
- Banerjee RK, Roy AB. The sulfotransferases of guinea pig liver. Mol. Pharmacol. 1966;2:56–66.
