Abstract
Sulfated glycosaminoglycans and sulfated lipids are involved in the biological functions of human matrix metalloproteinase 7 (MMP-7). In this study, the effects of heparin and cholesterol sulfate (CS) on the activity and stability of MMP-7 in the hydrolysis of a synthetic substrate, (7-methoxycoumarin-4-yl)acetyl-l-Pro-l-Leu-Gly-l-Leu-[N3-(2,4-dinitrophenyl)-l-2,3-diaminopropionyl]-l-Ala-l-Arg-NH2, were examined. Heparin increased activity by decreasing Km, and the Km values for 0 and 50 μM heparin were 57 ± 8 and 19 ± 5 μM, respectively. CS decreased activity in a non-competitive inhibitory manner with a Ki value of 11 ± 3 μM. In thermal incubation at 50−70 °C, heparin increased relative activity (the ratio of kcat/Km of MMP-7 with incubation to that without it), while CS decreased relative activity. These results indicate that heparin increases the activity and stability of MMP-7, while CS decreases them.
Graphical Abstract
Interaction of MMP-7 with Heparin and CS (A) MMP-7 catalyzes the hydrolysis. (B) Heparin activates MMP-7 activity. (C) CS inhibits MMP-7 activity non-competitively. (D) Heparin and CS interact with MMP-7 non-competitively.

Matrix metalloproteinases (MMPs) are a family of zinc endopepeptidases. They are anchored outside the cell and are believed to take part in both normal and pathological processes by degrading extracellular matrix (ECM) and non-ECM molecules.Citation1–4) Human matrix metalloproteinase 7 (MMP-7, matrilysin) [EC 3.4.24.23] is the smallest MMP. It consists of 173 amino acid residues with two zinc ions and two calcium ions. Like all other MMPs, it has consensus sequence HEXXHXXGXXH, in which three histidine residues chelate a catalytic zinc ion, and a turn containing methionine (Met-turn).Citation5) On the other hand, unlike all other MMPs, it lacks a hinge region and a hemopexin domain. MMP-7 has been detected in various lesions, including prostate,Citation6) colon,Citation7) brain,Citation8) stomach,Citation9) lung,Citation10) and breast,Citation11) and it is involved in tumor invasion and metastasis.Citation4)
The development of MMP-7 inhibitors is thought to be of potential therapeutic benefit. We have reported that alcohols,Citation12) synthetic MMP inhibitors thiorphan and R-94138,Citation13) and lignansCitation14) inhibit MMP-7 activity in a competitive manner, while green tea catechinsCitation15,16) and a fluorescent probe, 8-alininonaphthalene 1-sulfonate (ANS),Citation17) inhibit it in a non-competitive manner. MMP-7 exhibits a broad bell-shaped pH profile of MMP-7 activity with acidic and alkaline pKa (pKe1 and pKe2) values of about 4 and 10.Citation18) We have reported that Glu198 is probably the ionizable group for pKe1, and that there are two water molecules in the active site, one bound to the main-chain nitrogen of Ala162 and the other to the main-chain carbonyl oxygen of Pro217, and either of the two might be a candidate for the ionizable group for pKe2.Citation18–Citation21)
MMP-7 has biological functions through naturally occurring sulfated glycosaminoglycans such as heparin and sulfated lipids such as cholesterol sulfate (CS). Heparin contains glucosamine and glucuronic acid or iduronic acid as disaccaride unit. The disaccharide units of heparin bear an average of 3.5 negatively charged groups. It is localized on the surface of various cells and is involved in the activation or inhibition of a number of proteinases and protein proteinase inhibitors. Heparin is widely used as an anticoagulant drug in hospitals. It has been reported that in zymographic detection, heparin increases MMP-7 activity.Citation22,23) CS is a highly amphipathic molecule containing a sulfate group, a sterol ring, and hydrophobic side chains. It is widely distributed in various body fluids and in tissues and cells, including erythrocytes, platelets, skin, hair, adrenals, lung, and the brain. It has been reported that under physiological conditions, MMP-7 binds to the CS present in the cellular membranes of colon cancer cells and causes proteolysis.Citation24−Citation26) These reports on heparin and CS have revealed that they are involved in the biological functions of MMP-7. Here, we describe the effects of heparin and CS on the activity and stability of MMP-7 in the hydrolysis of a synthetic peptide, (7-methoxycoumarin-4-yl)acetyl-l-Pro-l-Leu-Gly-l-Leu-[N3-(2,4-dinitrophenyl)-l-2,3-diaminopropionyl]-l-Ala-l-Arg-NH2 [MOCAc-PLGL(Dpa)AR]. Our results indicate that heparin increases activity and stability while CS decreases them.
Materials and methods
Materials
MOCAc-PLGL(Dpa)AR (molecular mass 1093.2 Da)Citation27) and (7-methoxycoumarin-4-yl)acetyl-l-Pro-l-Leu-Gly (MOCAc-PLG) (501.54 Da) were purchased from Peptide Institute (Osaka, Japan).
Dinitrophenyl-l-Arg-l-Pro-l-Leu-l-Ala-l-Leu-l-Trp-l-Arg-l-Ser (Dnp-RPLALWRS) was from Bachem Holding (Budendorf, Switzerland). Their concentrations were determined by the denoted molecular weights. Porcine intestinal heparin (average molecular mass 5000 Da) was from Nacalai Tesque (Kyoto, Japan). CS (466.72 Da) was from Sigma (St. Louis, MO). All other chemicals were from Nacalai Tesque.
Expression and purification of MMP-7
Expression in Escherichia coli and purification of recombinant MMP-7 were carried out as described previously.Citation28,29) Briefly, mature MMP-7 (Tyr78-Lys250) was expressed in BL21(DE3) cells in the form of inclusion bodies, solubilized with 6 M guanidine HCl, refolded with 1 M l-arginine, and purified by sequential ammonium sulfate precipitation and heparin affinity column-chromatography procedures of the refolded products. The concentration of MMP-7 was determined spectrophotometrically using the molar absorption coefficient at 280 nm, ε280, of 31,800 M−1 cm−1.Citation28,29)
Fluorometric analysis of the MMP-7-catalyzed hydrolysis of MOCAc-PLGL(Dpa)AR
Fluorometric analysis was carried out as described previously.Citation30) In the experiment with heparin, pre-incubation (270 μL) was initiated by mixing 20 μL of the MMP-7 solution (4.0 μM in 50 mM HEPES-NaOH buffer, 10 mM CaCl2 at pH 7.5 (buffer A), 0–250 μL of the heparin solution (5.0 mM in buffer A), and 0–250 μL of buffer A. In the experiment with CS, pre-incubation (270 μL) was initiated by mixing 20 μL of the MMP-7 solution (4.0 μM in buffer A), 0–250 μL of the CS solution (5.0 mM in methanol), and 0–250 μL of methanol. After pre-incubation at 25 °C for 0−60 min, the reaction was initiated by adding 2214 μL of buffer A and 16 μL of MOCAc-PLGL(Dpa)AR solution (234 μM) dissolved in DMSO (total volume 2500 μL). The initial concentrations of enzyme, substrate, and DMSO were 32 nM, 1.5 μM, and 0.64% v/v, respectively. The reaction was measured by following the increase in fluorescence intensity at 393 nm with excitation at 328 nm with a Shimadzu RF-5300 fluorescence spectrophotometer (Shimadzu, Kyoto, Japan) for 1 min at 25 °C. The peptide bond of the Gly-l-Leu residues was cleaved with MMP-7, and the amount of the product, MOCAc-PLG, was estimated by fluorescence intensity by comparison with that of the MOCAc-PLG solution.
The reaction was carried out under pseudo-first-order conditions, and the initial concentration (1.5 μM) of the substrate was much lower than Km (60 μM).Citation14) Hence the Michaelis–Menten equation was, then, expressed as follows (Equation (1)):(1)
where vo, kcat, [E]o, and [S]o mean the initial reaction rate, the molecular activity, the initial enzyme concentration, and the initial substrate concentration, respectively.
HPLC analysis of the MMP-7-catalyzed hydrolysis of MOCAc-PLGL(Dpa)AR
HPLC analysis was done as described previously.Citation30) In the experiment on heparin, pre-incubation (238 μL) was initiated by mixing 4 μL of the MMP-7 solution (2.0 μM in buffer A), 0, 2.5, 5.0, 10, 15, or 20 μL of the heparin solution (2.5 mM in buffer A), and 234, 231.5, 229, 224, 219, or 214 μL of buffer A. In the experiment on CS, pre-incubation (238 μL) was initiated by mixing 4.0 μL of the MMP-7 solution (2.0 μM in buffer A), 0, 1.0, 2.5, 5.0, or 10 μL of the CS solution (2.5 mM in methanol), 10, 9.0, 7.5, 5.0, or 0 μL of methanol, and 224 μL of buffer A. After pre-incubation at 25 °C for 10 min, the reaction was initiated by adding 12 μL of the substrate solution (0−2.8 mM) dissolved in DMSO (total volume 250 μL) at 25 °C. The initial concentrations of enzyme, substrate, and DMSO were 32 nM, 0−140 μM, and 5%, respectively. The reaction was stopped at appropriate times, by mixing 100 μL of the reaction solution with 400 μL of 1% trifluoroacetic acid (TFA). This mixture (100 μL) was then applied to reversed-phase HPLC done on a TSKgel ODS-80Ts column (4.6 mm inner diameter × 150 mm) (Tosoh, Tokyo) equilibrated with 0.1% TFA. A linear gradient was generated from 20 to 70% v/v acetonitrile at a retention time of 5 min over 20 min at a flow rate of 1.0 mL/min. The absorption of elutes was detected at 335 nm. The substrate and its two products, MOCAc-PLG and L(Dpa)AR, were separated, and they were evaluated by the respective peak areas. vo was determined from the time course of the production of MOCAc-PLG. The kinetic parameters, kcat and Km, were determined based on the Michaelis–Menten equation using the non-linear least-squares method. The HPLC apparatus, consisting of a solvent delivery system CCPM, UV monitoring system UV-8010, computer control system PX-8010, and integrator Chromatocorder 21, was from Tosoh.
HPLC analysis of the MMP-7-catalyzed hydrolysis of Dnp-RPLALWRS
A reaction (105 μL) was initiated by mixing 3.4 μL of the MMP-7 solution (1.0 μM in buffer A), 0 or 2.1 μL of the CS solution (0.5 mM in methanol), 2.1 or 0 μL of methanol, 94.9 μL of buffer A, and 4.6 μL of the substrate solution (0−11.5 mM) dissolved in DMSO at 25 °C. The initial concentrations of the enzyme, the substrate, and DMSO were 32 nM, 0−500 μM, and 5%, respectively. The reaction was stopped at an appropriate time by mixing 100 μL of the reaction solution with 400 μL of 1% TFA and subjected to reversed-phase HPLC as described above. Absorption of elutes was detected at 335 nm. The substrate and its two products, Dnp-RPLA and LWRS, were separated. vo was determined from the time course of the decrease in Dnp-RPLALWRS.
Thermal inactivation of MMP-7
In the experiment on heparin, thermal incubation (1,250 μL) was initiated by mixing 20 μL of the MMP-7 solution (4.0 μM in buffer A), 0, 12.5, 25, or 50 μL of the heparin solution (5.0 mM in buffer A), and 1,230, 1,217.5, 1,205, or 1,180 μL of buffer A. In the experiment on CS, thermal incubation (1,250 μL) was initiated by mixing 20 μL of the MMP-7 solution (4.0 μM in buffer A), 0, 12.5, 25, or 50 μL of the CS solution (5.0 mM in methanol), 125, 112.5, 100, or 75 μL of methanol, and 1,105 μL of buffer A. After incubation at 25, 50, 60, or 70 °C for 10 min, the reaction was initiated by adding 1,234 μL of buffer A and 16 μL of the substrate solution.
Results
Activation of MMP-7 activity by heparin
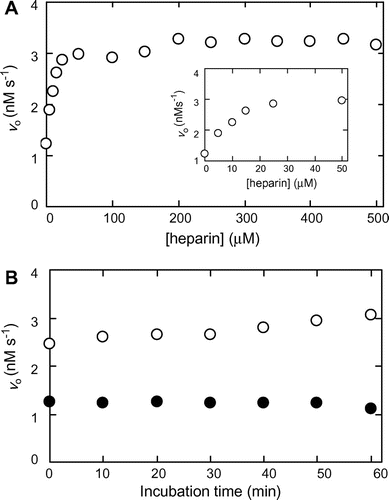
Fig. (A) shows the effects of increasing concentrations of heparin on MMP-7 activity in the hydrolysis of MOCAc-PLGL(Dpa)AR. MMP-7 and heparin were pre-incubated at 25 °C at pH 7.5 for 30 min, followed by the reaction at 25 °C at pH 7.5. The initial reaction rate without heparin was 1.2 nM s−1. It increased with increasing concentrations of heparin, and almost reached the maximum (3.0 nM s−1) at 50 μM. Fig. (B) shows the effects of pre-incubation duration on MMP-7 activity. MMP-7 was pre-incubated with heparin for 0−60 min, followed by the reaction. The initial reaction rates with and without 100 μM heparin were in the range of 2.5−3.1 and 1.1−1.2 nM s−1, respectively, indicating that the duration of pre-incubation did not affect MMP-7 activity.
Fig. 1. Activation of MMP-7-catalyzed hydrolysis of MOCAc-PLGL(Dpa)AR by heparin.
Note: Pre-incubation of MMP-7 was carried out in the presence and the absence of heparin at 25 °C at pH 7.5, and the reaction was carried out with 32 nM MMP-7 and 1.5 μM MOCAc-PLGL(Dpa)AR at 25 °C at pH 7.5. (A) Effect of heparin concentration on the initial reaction rate, vo. Pre-incubation was carried out for 30 min, and the reaction was carried out with 0−500 μM heparin. (B) Effect of pre-incubation time on vo. Pre-incubation was carried out for 0−60 min, and the reaction was carried out with 0 (solid circle) or 100 (hollow circle) μM heparin.
Inhibition of MMP-7 activity by CS
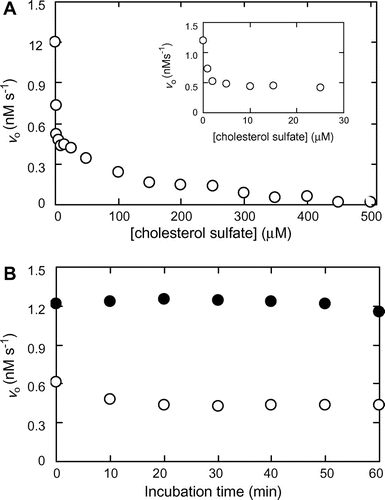
Fig. (A) shows the effects of increasing concentration of CS on MMP-7 activity. MMP-7 and CS were pre-incubated at 25 °C at pH 7.5 for 30 min, followed by the reaction at pH 7.5 at 25 °C. The initial reaction rate decreased with increasing concentrations of CS, and diminished almost to zero at 450 μM. The plot appears to exhibit a biphasic profile, suggesting that the mechanisms of inhibition are different between CS concentrations in the range of 0–25 and 100–500 μM. The CS concentration giving 50% inhibition (IC50 value) was 1.6 μM. Fig. (B) shows the effects of pre-incubation on MMP-7 activity. MMP-7 was pre-incubated with or without CS for 0−60 min, followed by the reaction. The initial reaction rates with and without and 25 μM CS were in the range of 0.4−0.6 and 1.1−1.2 nM s−1, respectively, indicating that the duration of pre-incubation did not affect MMP-7 activity.
Fig. 2. Inhibition of MMP-7-catalyzed hydrolysis of MOCAc-PLGL(Dpa)AR by CS.
Note: Pre-incubation of MMP-7 was carried out in the presence and the absence of CS at 25 °C at pH 7.5, and the reaction was carried out with 32 nM MMP-7 and 1.5 μM MOCAc-PLGL(Dpa)AR at 25 °C at pH 7.5. (A) Effect of CS concentration on the initial reaction rate, vo. Pre-incubation was carried out for 30 min, and the reaction was carried out with 0−500 μM CS. (B) Effect of pre-incubation time on vo. Pre-incubation was carried out for 0−60 min, and the reaction was carried out with 0 (solid circle) or 25 (hollow circle) μM CS.
Activation of MMP-7 by heparin and inhibition of MMP-7 by CS
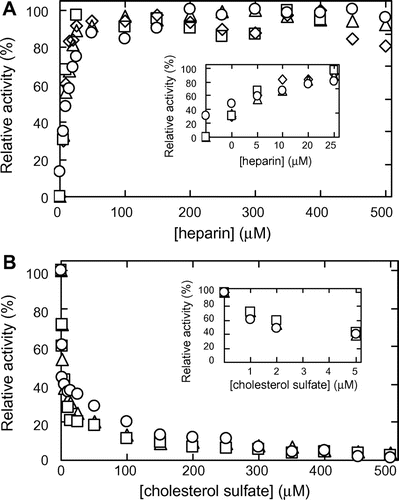
Fig. (A) shows the effects of increasing concentrations of heparin on MMP-7 activity in the hydrolysis of MOCAc-PLGL(Dpa)AR in the presence of 0–100 μM CS. MMP-7, heparin, and CS were pre-incubated at 25 °C at pH 7.5 for 30 min followed by the reaction at 25 °C at pH 7.5. The initial reaction rates for 0 μM heparin with 1, 10, and 100 μM CS were 0.94, 0.52, and 0.26 nM s−1, respectively, 78, 43, and 22%, respectively, of that for 0 μM CS and 0 μM heparin (1.2 ± 0.1 nM s−1). Relative activity as given in Fig. (A) was defined as the ratios of the initial reaction rate at the heparin concentration indicated to the maximum value. In the case without CS (Fig. (A)), relative activities with 1, 10, and 100 μM CS increased with increasing concentration of heparin and almost reached the maximum (2.7, 1.4, and 0.4 nM s−1, respectively) at 50 μM heparin, 90, 47, and 8% of that without CS (3.0 nM s−1). The heparin concentrations (EC50 values) giving 50% activation at 0, 1, 10, and 100 μM CS were 13, 10, 7.6, and 8.7 μM, respectively. Fig. (B) shows the effects of increasing concentrations of CS on MMP-7 activity in the hydrolysis of MOCAc-PLGL(Dpa)AR in the presence of 0–500 μM heparin. The initial reaction rates at 0 μM CS with 10 and 500 μM heparin were 1.9 and 2.9 nM s−1, respectively, 160 and 240% respectively of that with 0 μM CS and 0 μM heparin (1.2 ± 0.1 nM s−1). Relative activity given in Fig. (B) was defined as the ratio of the initial reaction rate at the CS concentration indicated to that without it. In the case without heparin (Fig. (A)), relative activities with 10 and 500 μM heparin decreased with increasing concentrations of CS, and reached 0.72 and 1.2 nM s−1, respectively, at 5 μM CS, 150 and 250% of that without heparin (0.48 nM s−1). The CS concentrations (IC50 values) giving 50% inhibition at 0, 10, and 500 μM heparin were 3.1, 3.6, and 3.9 μM, respectively. Considering that the EC50 values were in the range of 7.6–13 μM heparin, the dose-response curves for the activation of MMP-7 by heparin in the presence of various concentrations of CS were not much different. Considering that the IC50 values were in the range of 3.1–3.9 μM CS, the dose-response curves of the inhibition of MMP-7 by CS in the presence of various concentrations of heparin were similar. Hence we suggest that the binding of heparin and CS to MMP-7 is non-competitive.
Fig. 3. Effects of heparin and CS on the MMP-7-catalyzed hydrolysis of MOCAc-PLGL(Dpa)AR.
Note: Pre-incubation of MMP-7 was carried out in the presence and the absence of heparin and CS at 25 °C at pH 7.5 for 30 min, and the reaction was carried out with 32 nM MMP-7, 1.5 μM MOCAc-PLGL(Dpa)AR, 0−100 μM heparin, and 0−500 μM CS at 25 °C at pH 7.5. (A) Activation of the MMP-7 activity by heparin in the presence of CS. The relative activity of MMP-7 was defined as the ratio of the initial reaction rate at the heparin concentrations indicated to the maximum value (3.3 nM s−1 for 0 μM CS (hollow circle); 2.9 nM s−1 for 1 μM CS (hollow triangle); 1.4 nM s−1 for 10 μM CS (hollow square); and 0.49 nM s−1 for 100 μM CS (hollow diamond). (B) Inhibition of MMP-7 activity by CS in the presence of heparin. The relative activity of MMP-7 was defined as the ratio of the initial reaction rate at the CS concentrations indicated to that without it (1.2 nM s−1 for 0 μM heparin (hollow circle); 1.9 nM s−1 for 10 μM heparin (hollow triangle); and 2.9 nM s−1 for 500 μM heparin (hollow square)).
Manner of activation of heparin in MMP-7 activity
To determine kcat and Km for MMP-7 in the hydrolysis of MOCAc-PLGL(Dpa)AR separately, initial reaction rates in the presence and the absence or presence of heparin were measured. It was necessary to increase the concentration of MOCAc-PLGL(Dpa)AR to 140 μM, higher than the Km value (60 μM),Citation14) and fluorescence detection was not available because of the inner filter effect of the substrate. Hence, the products were detected by reversed-phase HPLC.Citation30)
The effects of heparin and CS on the solubility of MOCAc-PLGL(Dpa)AR were examined. MOCAc-PLGL(Dpa)AR (0−140 μM) was incubated with heparin (200 μM) and with CS (100 μM) without MMP-7. After centrifugation, the supernatant was applied to reversed-phase HPLC (Supplemental Fig. 1; see Biosci. Biotechnol. Biochem. Web site). The peak area of MOCAc-PLGL(Dpa)AR increased proportionally with increasing MOCAc-PLGL(Dpa)AR concentrations in the presence of heparin and of CS and in their absence, indicating that neither 200 μM heparin nor 100 μM CS affected the solubility of MOCAc-PLGL(Dpa)AR.
The initial reaction rates in the hydrolysis of MOCAc-PLGL(Dpa)AR in the presence and the absence of various concentrations of heparin were measured. All plots showed saturated profiles (Fig. (A)), and the kcat and Km values for MMP-7 were determined separately (Fig. B). The Km values at 25−200 μM heparin were in a range of 17−21 μM, 30−37% of that without heparin (57 ± 8 μM). The kcat values at 25−200 μM heparin were 1.2 s−1, 75% of that without heparin (1.6 ± 0.1 s−1). Consequently, the kcat/Km values at 25−200 μM heparin were 200−250% of that without heparin. This indicates that heparin increased activity by decreasing Km.
Fig. 4. HPLC analysis of activation of the MMP-7-catalyzed hydrolysis of MOCAc-PLGL(Dpa)AR by Heparin.
Note: MMP-7 was pre-incubated in the presence or absence of heparin at pH 7.5 at 25 °C for 10 min. The reaction was carried out with 32 nM MMP-7, 0–140 μM MOCAc-PLGL(Dpa)AR, and 0−200 μM heparin at 25 °C at pH 7.5, and stopped at an appropriate time.( A) Effects of the initial substrate concentrations, [S]o, on vo. The solid line represents best fit of the Michaelis–Menten equation by the non-linear least-squares method. Symbols for heparin concentration (μM): 0, hollow circle; 25, hollow triangle; and 200, hollow square. (B) Effects of the heparin concentration on Km and kcat.
![Fig. 4. HPLC analysis of activation of the MMP-7-catalyzed hydrolysis of MOCAc-PLGL(Dpa)AR by Heparin.Note: MMP-7 was pre-incubated in the presence or absence of heparin at pH 7.5 at 25 °C for 10 min. The reaction was carried out with 32 nM MMP-7, 0–140 μM MOCAc-PLGL(Dpa)AR, and 0−200 μM heparin at 25 °C at pH 7.5, and stopped at an appropriate time.( A) Effects of the initial substrate concentrations, [S]o, on vo. The solid line represents best fit of the Michaelis–Menten equation by the non-linear least-squares method. Symbols for heparin concentration (μM): 0, hollow circle; 25, hollow triangle; and 200, hollow square. (B) Effects of the heparin concentration on Km and kcat.](/cms/asset/0aaa25d2-8615-437b-8f54-57fd95367466/tbbb_a_878213_f0004_b.gif)
Manner of inhibition of CS in MMP-7 activity
The initial reaction rates for the hydrolysis of MOCAc-PLGL(Dpa)AR in the presence and the absence of various concentrations of CS were measured. All the plots showed saturated profiles (Fig. (A)). The plot of 1/vo vs. 1/[S]o (Lineweaver–Burk plot) in the presence and the absence of CS showed non-parallel lines intersecting at the X-axis, indicating that the Km value was 62 ± 8 μM and that inhibition was non-competitive (Fig. (B)). The apparent kcat values (kcat,app) observed for 0, 10, 25, 50, and 100 μM CS were determined to be 1.6 ± 0.2, 0.71 ± 0.09, 0.57 ± 0.07, 0.26 ± 0.03, and 0.18 ± 0.02 s−1, respectively. Based on this, the reaction rate can be described by Equation (2):(2)
Fig. 5. HPLC analysis of inhibition of the MMP-7-catalyzed hydrolysis of MOCAc-PLGL(Dpa)AR by CS.
Note: MMP-7 was pre-incubated in the presence and the absence of CS at 25 °C at pH 7.5 for 10 min. The reaction was carried out with 32 nM MMP-7, 0–140 μM MOCAc-PLGL(Dpa)AR, and 0 (hollow circle), 10 (hollow triangle), 25 (solid triangle), 50 (hollow square), or 100 (solid square) μM CS at 25 °C at pH 7.5, and stopped at an appropriate time. (A) Effects of the initial substrate concentrations, [S]o, on vo. Solid line represents the best fit of the Michaelis–Menten equation by the non-linear least-squares method. (B) Lineweaver–Burk plot.
![Fig. 5. HPLC analysis of inhibition of the MMP-7-catalyzed hydrolysis of MOCAc-PLGL(Dpa)AR by CS.Note: MMP-7 was pre-incubated in the presence and the absence of CS at 25 °C at pH 7.5 for 10 min. The reaction was carried out with 32 nM MMP-7, 0–140 μM MOCAc-PLGL(Dpa)AR, and 0 (hollow circle), 10 (hollow triangle), 25 (solid triangle), 50 (hollow square), or 100 (solid square) μM CS at 25 °C at pH 7.5, and stopped at an appropriate time. (A) Effects of the initial substrate concentrations, [S]o, on vo. Solid line represents the best fit of the Michaelis–Menten equation by the non-linear least-squares method. (B) Lineweaver–Burk plot.](/cms/asset/bc73a063-69f1-4398-9faf-af09c84a4ab0/tbbb_a_878213_f0005_b.gif)
where [I]o and Ki are the initial inhibitor concentration and the inhibitor constant, respectively. The Ki value of CS was calculated to be 11 ± 3 μM from Equation (2).
The initial reaction rates in the hydrolysis of Dnp-RPLALWRS in the presence and the absence of 10 μM CS were measured. All the plots showed saturated profiles (Fig. (A)). The Lineweaver–Burk plot in the presence and the absence of 10 μM CS showed non-parallel lines intersecting at the Y-axis, indicating that the kcat value was 47 ± 1 s−1 and that the inhibition was competitive (Fig. (B)). The apparent Km values (Km,app) observed for 0 and 10 μM CS were determined to be 0.26 ± 0.02 and 0.92 ± 0.04 mM, respectively. This coincides with a previous report that CS inhibited MMP-7 activity competitively in the hydrolysis of Dnp-RPLALWRS with Km,app values of 0.26 mM for 0 μM CS and 1.2 mM for 10 μM CS.Citation25) According to the rate equation for competitive inhibition, the Ki value for CS was calculated to be 3.9 ± 0.7 μM (Equation (3)): (3)
Fig. 6. HPLC analysis of inhibition of the MMP-7-catalyzed hydrolysis of Dnp-RPLALWRS by CS.
Note: MMP-7 was pre-incubated in the presence and the absence of CS at 25 °C at pH 7.5 for 10 min. The reaction was carried out with 32 nM MMP-7, 0–0.5 mM Dnp-RPLALWRS, and 0 (hollow circle) or 10 (solid circle) μM CS at 25 °C at pH 7.5, and stopped at an appropriate time. (A) Effects of the initial substrate concentrations, [S]o, on vo. Solid line represents best fit of the Michaelis–Menten equation by the non-linear least-squares method. (B) Lineweaver–Burk plot.
![Fig. 6. HPLC analysis of inhibition of the MMP-7-catalyzed hydrolysis of Dnp-RPLALWRS by CS.Note: MMP-7 was pre-incubated in the presence and the absence of CS at 25 °C at pH 7.5 for 10 min. The reaction was carried out with 32 nM MMP-7, 0–0.5 mM Dnp-RPLALWRS, and 0 (hollow circle) or 10 (solid circle) μM CS at 25 °C at pH 7.5, and stopped at an appropriate time. (A) Effects of the initial substrate concentrations, [S]o, on vo. Solid line represents best fit of the Michaelis–Menten equation by the non-linear least-squares method. (B) Lineweaver–Burk plot.](/cms/asset/32676f13-c0b5-4170-9ced-f99b97d29bad/tbbb_a_878213_f0006_b.gif)
Stabilization of MMP-7 by heparin and destabilization of MMP-7 by CS
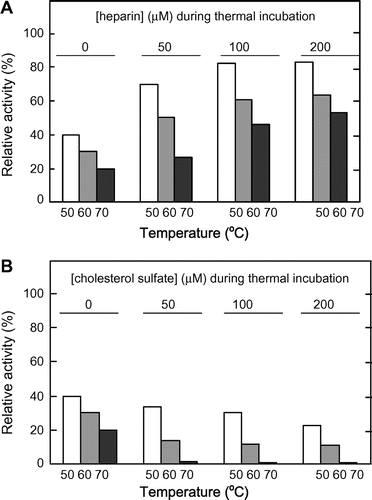
Fig. shows the remaining activities of MMP-7 in hydrolyzing MOCAc-PLGL(Dpa)AR after thermal treatment at 50–70 °C for 10 min. The relative activities, defined as the ratio of the initial reaction rate with incubation at the indicated temperature for 10 min to that without it, of MMP-7 without heparin or CS decreased with increasing temperatures. At all temperatures examined, the relative activities of MMP-7 increased with increasing heparin concentrations (Fig. (A)) while they decreased with increasing CS concentrations (Fig. (B)). This indicates that heparin increased MMP-7 stability while CS decreased it.
Fig. 7. Irreversible thermal inactivation of MMP-7.
Note: MMP-7 was incubated at 25, 50, 60, or 70 °C at pH 7.5 with 0, 50, 100, or 200 μM heparin or CS for 10 min, and the hydrolyzing reaction was carried out with 0, 25, 50, and 100 μM heparin or CS, respectively, at 25 °C at pH 7.5. The relative activity of MMP-7 was defined as the ratio of the initial reaction rate with thermal incubation for 10 min at 50, 60, or 70 °C to that at 25 °C (1.0 nM s−1 for thermal incubation without heparin or CS; 2.6, 2.8, and 3.0 nM s−1 for that with 50, 100, and 200 μM heparin; and 0.36, 0.15, and 0.07 nM s−1 for that with 50, 100, and 200 μM CS, respectively).
Discussion
Mechanism of activation of MMP-7 by heparin
Heparin binds numerous proteins including enzymes and inhibitors involved in the clotting cascade.Citation31) In this study, we found that heparin increased the activity (Figs. and ) and stability (Fig. ) of MMP-7. Activation was brought about by a decrease in Km. MMP-7 has 11 Lys and eight Arg residues. According to the X-ray structure (Protein Data Bank accession number No.),Citation5) all Lys and Arg residues except for Arg143 and Arg180 are located on the reverse side when MMP-7 is viewed with the active site at the center (Fig. ). The pI value of MMP-7 is 5.9,Citation4) suggesting that MMP-7 is positively charged at neutral and alkaline pH. Although the amino acid residues of MMP-7 involved in binding to heparin are unknown, we speculate that MMP-7 binds to heparin through its basic region far from the active site.
Fig. 8. Overall structure of MMP-7.
Note: The MMP-7-hydroxymate inhibitor complex was based on Protein Data Bank No. 1MMQ.Citation5) MMP-7 residues (Tyr78-Lys243) are represented by a CPK model. Lys residues (dark gray), Arg residues (light gray), Ile106, and Trp132 are indicated. Arg244, Ser245, Asn246, Ser247, Arg248, Lys249, and Lys250 are not shown, because they are not present in 1MMQ. The hydroxymate inhibitor is represented by a ball-and-stick model. (A) MMP-7 viewed with the active site at the center. (B) MMP-7 viewed from the opposite side.
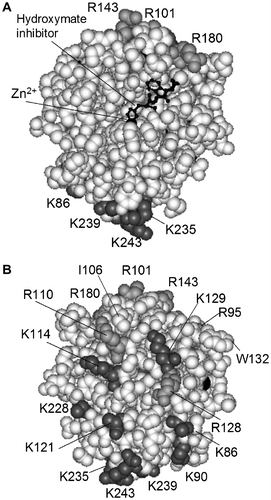
It has been reported that the heparin-binding site of protein kinase CK2 consists the four consecutive residues (Lys74-Lys75-Lys76-Lys77) and three residues (Arg191, Arg195, and Lys198),Citation32) and that of phospholipase A2 is Lys49.Citation32) They are located in the vicinity of their active sites, and heparin inhibits their activities competitively.Citation32,33) MMP-7, which binds with negatively charged liposomes, retains activity whereas MMP-7, which binds with positively charged ones, loses activity.Citation34) Hence, it can be said that heparin does not decrease enzyme activities except when the heparin-binding sites are located near the active sites. Identification of the binding sites of MMP-7 for heparin is an important research subject.
Mechanism of stabilization of MMP-7 by heparin
Heparin stabilizes basic fibroblast growth factor (bFGF),Citation35) tissue transglutaminase,Citation36) and tripeptidyl-peptidase I,Citation37) and destabilizes cytochrome c.Citation38) The mechanism of stabilization of MMP-7 by heparin is unknown. Considering that not only heparin but also other sulfated glycosaminoglycans such as heparan sulfate and chondroitin sulfate increase enzyme stability,Citation37) we speculate that the negative charge density of heparin protects MMP-7 against inactivation by heating. Heparin protects bFGF against inactivation by heating it more strongly at high temperaturesCitation35) and protects tissue transglutaminase against inactivation by heating and also degradation by other proteases.Citation36) This evidence suggests that under physiological conditions, heparin is involved in the stabilization of MMP-7.
Mechanism of inhibition of MMP-7 by CS
CS is present in cellular membranes and also circulates in the plasma and gastric juices.Citation39) In cellular membranes, CS is oriented parallel to the fatty acid chains of phospholipids with the sulfate group on the surface. In plasma and gastric juices, it is associated with other proteins.Citation39) It regulates the activity of a variety of enzymes.Citation39,40) It activates factor XII, triggering the intrinsic blood coagulation system. On the other hand, it inhibits thrombin and plasmin, suppressing blood clotting.
In this study, we found that CS inhibited the activity of MMP-7 in hydrolyzing MOCAc-PLGL(Dpa)AR (Figs. and ) and Dnp-RPLALWRS (Fig. ), and decreased the stability (Fig. ) of MMP-7. The manner of inhibition was non-competitive for MOCAc-PLGL(Dpa)AR (Fig. ), and competitive for Dnp-RPLALWRS (Fig. ). It has been reported that CS (10 μM) competitively inhibited the activity of MMP-7 in hydrolyzing Dnp-RPLALWRS, suggesting that CS reduces the affinity between Dnp-RPLALWRS and MMP-7.Citation25) Our results suggest that CS inhibits the activity in hydrolyzing MOCAc-PLGL(Dpa)AR without reducing the affinity between MOCAc-PLGL(Dpa)AR and MMP-7. The reason for this discrepancy is unknown, and calls for further study, but we speculate that the binding of CS imparts a long-range interaction to the active site and affects substrate binding. Regarding the difference in the manner of inhibition of MMP-7 by CS depending on the substrate, the difference in the interaction modes of CS with substrates must be considered.
MMP-7 binds to the surface CS of colon cancer cells and this binding is essential for MMP-7-catalyzed proteolysis.Citation24Citation–Citation26) We speculate that the difference in the effects on MMP-7 activity as between free CS and membrane-bound CS can be explained by the fact that both the sulfate group and the hydrophobic side chain of free CS are involved in the inhibition of MMP-7, in view of the following evidence: (i) ANS, but not sodium sulfate, inhibits MMP-7 activity;Citation21) and (ii) in the crystal structure of the complex of retinoic acid-related orphan receptor α (BORα) and CS, both the sulfate group and the hydrophobic side chain of CS are completely surrounded by amino acid residues of BORα.Citation41) The amino acid residues of MMP-7 involved in binding to the surface CS of cancer cells have been identified. They are Ile106, Arg110, Arg128, Trp132, Arg248, Lys249, and Lys250, located on the side opposite to the active site (Fig. ).Citation25) Identification of the binding sites of MMP-7 for free CS is an important research subject.
Supplemental material
The supplemental material for this paper is available at http://dx.doi.org/10.1080/09168451.2014.878213.
Fig. S1. HPLC Analysis of MOCAc-PLGL(Dpa)AR.
Download PDF (121.4 KB)Notes
Abbreviations: CS, cholesterol sulfate; DMSO, dimethyl sulfoxide; Dnp-RPLALWRS, dinitrophenyl-l-Arg-l-Pro-l-Leu-l-Ala-l-Leu-l-Trp-l-Arg-l-Ser; HEPES, 2-[4-(2-hydroxyethyl)-1-piperazinyl] ethanesulfonic acid; MOCAc-PLG, (7-methoxycoumarin-4-yl)acetyl-l-Pro-l-Leu-Gly; MOCAc-PLGL(Dpa)AR, (7-methoxycoumarin-4-yl)acetyl-l-Pro-l-Leu-Gly-l-Leu-[N3-(2,4-dinitrophenyl)-l-2,3-diaminopropionyl]-l-Ala-l-Arg-NH2.
References
- Woessner JF Jr. Matrix metalloproteinases and their inhibitors in connective tissue remodeling. FASEB J. 1991;5:2145–2154.
- Matrisian LM. The matrix degrading metalloproteinases. BioEssays. 1992;14:455–463.
- Nagase H, Woessner JF Jr. Matrix metalloproteinases. J. Biol. Chem. 1999;274:21491–21494.
- Woessner JF Jr, Taplin CJ. Purification and properties of a small latent matrix metallo-proteinase of the rat uterus. J. Biol. Chem. 1988;263:16918–16925.
- Browner MF, Smith WW, Castelhano AL. Matrilysin-inhibitor complexes: common themes among metalloproteases. Biochemistry. 1995;34:6602–6610.
- Pajouh MS, Nagle RB, Breathnach R, Finch JS, Brawer MK, Bowden GT. Expression of metalloproteinase genes in human prostate cancer. J. Cancer Res. Clin. Oncol. 1991;117:144–150.
- Yoshimoto M, Itoh F, Yamamoto H, Hinoda Y, Imai K, Yachi A. Expression of MMP-7 (PUMP-1) mRNA in human colorectal cancers. Int. J. Cancer. 1993;54:614–618.
- Nakano A, Tani E, Miyazaki K, Yamamoto Y, Furuyama J. Matrix metalloproteinases and tissue inhibitors of metalloproteinases in human gliomas. J. Neurosurg. 1995;83:298–307.
- Adachi Y, Itoh F, Yamamoto H, Matsuno K, Arimura Y, Kusano M, Endo T, Hinoda Y, Oohara M, Hosokawa M, Imai K. Matrix metalloproteinase matrilysin (MMP-7) participates in the progression of human gastric and esophageal cancers. Int. J. Oncol. 1998;13:1031–1035.
- Muller D, Breathnach R, Engelmann A, Millon R, Bronner G, Flesch H, Dumont P, Eber M, Abecassis J. Expression of collagenase-related metalloproteinase genes in human lung or head and neck tumours. Int. J. Cancer. 1991;48:550–556.
- Happner kJ, Matrisian LM, Jensen RA, Rodgers WH. Expression of most matrix metalloproteinase family members in breast cancer represents a tumour-induced host response. Am. J. Pathol. 1996;149:273–282.
- Muta Y, Oneda H, Inouye K. Inhibitory effects of alcohols on the activity of human matrix metalloproteinase 7 (matrilysin). Biosci. Biotechnol. Biochem. 2004;68:2649–2652.
- Oneda H, Inouye K. Interactions of human matrix metalloproteinase 7 (matrilysin) with the inhibitors thiorphan and R-94138. J. Biochem. 2001;129:429–435.
- Muta Y, Oyama S, Umezawa T, Shimada M, Inouye K. Inhibitory effects of lignans on the activity of human matrix metalloproteinase 7 (matrilysin). J. Agric. Food Chem. 2004;52:5888–5894.
- Oneda H, Shiihara M, Inouye K. Inhibitory effects of green tea catechins on the activity of human matrix metalloproteinase 7 (matrilysin). J. Biochem. 2003;133:571–576.
- Miyake T, Yasukawa K, Inouye K. Analysis of the mechanism of inhibition of human matrix metalloproteinase 7 (MMP-7) activity by green tea catechins. Biosci. Biotechnol. Biochem. 2011;75:1564–1569.
- Samukange V, Yasukawa K, Inouye K. Interaction of 8-anilinonaphthalene 1-sulphonate (ANS) and human matrix metalloproteinase 7 (MMP-7) as examined by MMP-7 activity and ANS fluorescence. J. Biochem. 2012;151:533–540.
- Muta Y, Oneda H, Inouye K. Anomalous pH-dependence of the activity of human matrilysin (matrix metalloproteinase-7) as revealed by nitration and amination of its tyrosine residues. Biochem. J. 2005;386:263–270.
- Muta Y, Inouye K. Tyr219 of human matrix metalloproteinase 7 (MMP-7) is not critical for catalytic activity, but is involved in the broad pH-dependence of the activity. J. Biochem. 2011;150:183–188.
- Takeharu H, Yasukawa K, Inouye K. Thermodynamic analysis of ionizable groups involved in the catalytic mechanism of human matrix metalloproteinase 7 (MMP-7). Biochim. Biophys. Acta. 2011;1814:1940–1946.
- Morishima A, Yasukawa K, Inouye K. A possibility of a protein-bound water molecule as the ionizable group responsible for pKe at the alkaline side in human matrix metalloproteinase 7 activity. J. Biochem. 2012;151:501–509.
- Yu WH, Woessner JF Jr. Heparan sulfate proteoglycans as extracellular docking molecules for matrilysin (matrix metalloproteinase 7). J. Biol. Chem. 2000;275:4183–4191.
- Yu WH, Woessner JF Jr. Heparin-enhanced zymographic detection of matrilysin and collagenases. Anal. Biochem. 2001;293:38–42.
- Yamamoto K, Higashi S, Kioi M, Tsunezumi J, Honke K, Miyazaki K. Binding of active matrilysin to cell surface cholesterol sulfate is essential for its membrane-associated proteolytic action and induction of homotypic cell adhesion. J. Biol. Chem. 2006;281:9170–9180.
- Higashi S, Oeda M, Yamamoto K, Miyazaki K. Identification of amino acid residues of matrix metalloproteinase 7 essential for binding to cholesterol sulfate. J. Biol. Chem. 2008;283:35735–35744.
- Yamamoto K, Miyazaki K, Higashi S. Cholesterol sulfate alters substrate preference of matrix metalloproteinase-7 and promotes degradations of pericellular laminin-332 and fibronectin. J. Biol. Chem. 2010;285:28862–28873.
- Knight CG, Willenbrock F, Murphy G. A novel coumarin-labelled peptide for sensitive continuous assays of the matrix metalloproteinases. FEBS Lett. 1992;296:263–266.
- Muta Y, Yasui N, Matsumiya Y, Kubo M, Inouye K. Expression in Escherichia coli, refolding, and purification of the recombinant mature form of human matrix metalloproteinase 7 (MMP-7). Biosci. Biotechnol. Biochem. 2010;74:2515–2517.
- Oneda H, Inouye K. Refolding and recovery of recombinant human matrix metalloproteinase 7 (matrilysin) from inclusion bodies expressed by Escherichia Coli. J. Biochem. 1999;126:905–911.
- Oneda H, Inouye K. Effects of dimethyl sulfoxide, temperature, and sodium chloride on the activity of human matrix metalloproteinase 7 (matrilysin). J. Biochem. 2000;128:785–791.
- Kjellen L, Lindahl U. Proteoglycans: structures and interactions. Annu. Rev. Biochem. 1991;60:443–475.
- Gambetti S, Dondi A, Cervellati C, Squerzanti M, Pansini FS, Bergamini CM. Interaction with heparin protects tissue transglutaminase against inactivation by heating and by proteolysis. Biochimie. 2005;87:551–555.
- O’Farrell F, Loog M, Janson IM, Ek P. Kinetic study of the inhibition of CK2 by heparin fragments of different length. Biochim. Biophys. Acta. 1999;1433:68–75.
- Ganguly B, Banerjee J, Elegbede AI, Klocke DJ, Mallik S, Srivastava DK. Intrinsic selectivity in binding of matrix metalloproteinase-7 to differently charged lipid membranes. FEBS Lett. 2007;581:5723–5726.
- Vemuri S, Beylin I, Sluzky V, Stratton P, Eberlein G, Wang YJ. The stability of bFGF against thermal denaturation. J. Pharm. Pharmacol. 1994;46:481–486.
- Bugs MR, Bortoleto-Bugs RK, Cornélio ML. The interaction between heparin and Lys49 phospholipase A2 reveals the natural binding of heparin on the enzyme. Int. J. Biol. Macromol. 2005;37:21–27.
- Golabek AA, Walus M, Wisniewski KE, Kida E. Glycosaminoglycans modulate activation, activity, and stability of tripeptidyl-peptidase I in vitro and in vivo. J. Biol. Chem. 2005;280:7550–7561.
- Bágel’ová J, Antalík M, Bona M. Studies on cytochrome c-heparin interactions by differential scanning calorimetry. Biochem. J. 1994;297:99–101.
- Strott CA, Higashi Y. Cholesterol sulfate in human physiology: what’s it all about? J. Lipid Res. 2003;44:1268–1278.
- Nakae H, Hiroi H, Momoeda M, Koizumi M, Iwamori M, Taketani Y. Inhibition of cell invasion and protease activity by cholesterol sulfate. Fertil. Steril. 2010;94:2455–2457.
- Kallen J, Schlaeppi JM, Bitsch F, Delhon I, Fournier B. Crystal structure of the human RORα ligand binding domain in complex with cholesterol sulfate at 2.2 Å. J. Biol. Chem. 2004;279:14033–14038.
