Abstract
We have reported that new N-glycans carrying the TF-antigen occurred on a major royal jelly glycoprotein, and we have identified the glycosylation site to which the antigenic N-glycan is linked, but an appropriate procedure has not been established to prepare non-labeled immunoreactive glycopeptides in large amounts for functional analysis. In this study, we developed an effective method of preparing Asn-glycopeptide bearing TF-antigen.
Previously,Citation1) we reported that complex type N-glycans bearing the TF-antigen unit (Galβ1-3GalNAc) occur on royal jelly glycoproteins, which suggests that honeybee cells carry a specific biosynthetic mechanism to form honeybee-specific N-glycoproteins. We also found that the N-glycan containing TF-antigen unit is linked to Asn (28) at the N-terminal region of major royal jelly glycoprotein 1.Citation2,3) Since the occurrence of complex type N-glycans harboring the TF-antigen unit on royal jelly glycoprotein suggested that a β-1,3-galactosyltransferase (β1-3GalT) must function in the cephalic cells of the honeybee, we screened the glycosyltransferase activity with GalNAc2GlcNAc2Man3GlcNAc2-PA as receptor substrate. As the result of screening, we confirmed that the β1-3GalT activity in the microsomal fraction prepared from the cephalic portion of honeybees,Citation4) and identified the bee β1-3GalT gene (Kimura Y, Sakamura S, Fujiyama K, Kimura M, Okihara S, Sugimoto H, and Yamada H, Abstract the 82nd Annual Meeting of the Japanese Biochemical Society, 4P-163/4T20a-3, 2009). To analyze the physiological functions and distribution of the royal jelly glycoprotein-specific N-glycans expressed in honeybee cephalic cells, the preparation of an antibody against unique N-glycans is prerequisite. For this purpose, in this study, we established a new method of preparing an N-glycopeptide harboring the T-antigen unit in large amounts.
Crude royal jelly (12 g, Yamada Bee Farm, Okayama, Japan) was reduced and carboxymethylated by the method of Crestfield et al.Citation5) and the alkylated royal jelly proteins were digested by means of actinase E (200 mg, Waken-yaku, Kyoto, Japan) in 430 mL of 0.1 M NH4HCO3 at 37 °C over 48 h. The actinase digest was concentrated to about 100 mL by rotary evaporation and centrifuged at 8000 g for 30 min to remove insoluble materials. The resulting supernatant (about 100 mL) was divided to four fractions (25 mL each), and each fraction was subjected to gel filtration on a Sephadex G-25 superfine column (4 × 85 cm) equilibrated with 0.1 N NH4OH. The glycopeptide fraction (3 mL/tube) was collected at a flow rate of 1 mL/min. This (elution volume from 420 to 770 mL), detected by the Phenol-H2SO4 methodCitation6), was pooled and concentrated to about 400 mL by rotary evaporation. It was digested again by actinase E (100 mg) in 0.1 M NH4HCO3 at 37 °C over 48 h. After gel filtration through a Sephadex G-25 superfine column (4 × 85 cm), it was pooled and concentrated by rotary evaporation.
Peanut agglutinin, which is specific for O-glycans having Galβ1-3GalNAc, was not available for the purification of N-glycans having TF-antigen at the non-reducing end. Hence, to exclude high-mannose type N-glycopeptides, the glycopeptide fraction was applied to a Con A Sepharose 4B column (3.2 × 21 cm) equilibrated with 20 mM Trsi–HCl buffer, pH 8.0. The non-absorbed fractions obtained (200 mL) were pooled and applied to the Con A Sepharose 4B column. Lectin affinity chromatography was run four times to exclude the high-mannose type and hybrid type N-glycopeptides linked to royal jelly glycoproteins as much as possible.Citation7) After lyophilization, the glycopeptide fraction was dissolved in 50% acetonitrile/water (4 mL), and mixed with 25 mL of settled Shodex Asahipak NH2P-50 4E resin (Showa Denko, Tokyo) equilibrated with 10 mL of 80% acetonitrile/water in a centrifuge tube at room temperature. After incubation for 10 min and centrifugation (3,500 g, 5 min) at 20 °C, the supernatant was discarded and the resin was washed twice with 80% acetonitrile/water (12.5 mL). After centrifugation at 20 °C, the resulting supernatant (80% acetonitrile fraction) was removed, and the resin was washed with 0.1% TFA/water (50 mL) to elute the glycopeptide absorbed. After centrifugation (3500 g, 5 min) at 20 °C, the supernatant (0.1% TFA fraction) was removed and the resin was further washed with 0.1 N NH4OH (50 mL). After centrifugation (3500 g, 5 min) at 20 °C, the resulting supernatant (0.1 N NH4OH fraction) was removed. The three fractions were concentrated to dryness, and the amino acid compositions and structures of the N-glycans linked to the glycopeptides were analyzed as described in our previous paper.Citation8) Since the glycopeptides were hydrolyzed in 5.7 N HCl at 110 °C for 12 h,Citation9,10) the recovery of hexosamines (GalNAc and GlcNAc) was low. As shown in Fig. (3) and (4), Asp and GlcNAc were detected as main components from the glycopeptide recovered in the 0.1% TFA fraction and the 80% acetonitrile fraction, suggesting that the purified glycopeptide is a glycosylated Asn and the Asn-glycopeptide. Small amounts of GalNH2 (due to degradation under strong acid conditions) were also detected in both fractions, indicating that occurrence of TF-antigen unit. On the contrary, as shown in Fig. (2), the glycopeptide recovered from the 0.1 N NH4OH fraction gave several amino acids in addition to the Asn residue, GlcNH2, and GalNH2, suggesting that it might be an oligopeptide, but is not an Asn-glycopeptide. In subsequent structural analysis, the glycopeptide recovered in the 0.1% TFA fraction was used, since the yield of Asn-glycopeptide was high. The structures of the N-glycans linked to the purified Asn-glycopeptide were analyzed by size-fractionation (SF-) HPLC with a Shodex Asahipak NH2P-50 column (0.46 × 25 cm), as described in our previous paper.Citation1,Citation2) To confirm the predominant occurrence of TF-antigen among the Asn-glycopetides purified, N-glycans were released from the Asn-glycopeptide by hydrazinolysis, followed by N-acetylation, and they were pyridylaminated. Since the occurrence of high-mannose type N-glycans was confirmed (Fig. (1)), the glycopeptides were treated with Endo-H (840 U, New England Biolabs, Ipswich, MA) in 0.5 M Na-acetate buffer, pH 5.5, at 37 °C for 24 h to exclude the remaining oligomannose type N-glycans. After Endo-H digestion, four major N-glycans (a, b, c, and d) and four other minor peaks were obtained (Fig. (2)). The elution position of peak a coincided with that of authentic GlcNAc2Man3GlcNAc2-PA.Citation1,Citation4) Peak c was converted to peak e (GalNAc1GlcNAc1Man3GlcNAc2-PA), and peak d was converted to peak b (GalNAc1GlcNAc2Man3GlcNAc2-PA) by means of β1-3 specific galactosidase (Xanthomonas manihotis, Sigma, St. Louis, MO), suggesting the occurrence of the Galβ1-3GalNAc unit in these PA-sugar chains. By means of jack bean β-hexosaminidase (JB β-HexNAc’ase, Seikagau Kogyo, Tokyo), almost all the products in the β-Gal’ase digest were converted to M3 (Fig. (4)). The JB HexNAc’ase digest (Man3GlcNAc2-PA) was finally converted to M1 (Man1GlcNAc2-PA) by jack bean α-mannosidase (JB α-Man’ase, Seikagau Kogyo, Tokyo) (Fig. (5)). Based on these structural analyses, we deduced that the structures of peaks c and d were Gal1GalNAc1GlcNAc1Man3GlcNAc2-PA and Gal1GalNAc1GlcNAc2Man3GlcNAc2-PA, respectively.
Fig. 1. Amino acid analysis of glycopeptides bearing the TF-antigen unit.
Note: (1) Authentic amino acids and hexosamines (GlcNH2 and GalNH2); (2) hydrolysate of peptides recovered in the 0.1 N NH4OH fraction; (3) hydrolysate of glycopeptides recovered in the 0.1% TFA fraction; (4) hydrolysate of glycopeptides recovered in the 80% acetonitrile fraction. The amino acids and N-acetyl-hexosamines in the purified glycopeptides were analyzed as PITC-derivatives by reverse-phase HPLC after acid hydrolysis in 5.7 N HCl at 110 °C over 12 h. The column used was a Cosmosil 5C22-AR (0.46 × 25 cm). Several peaks, marked with asterisks, were not amino acids, but unknown derivatives produced during PITC labeling.
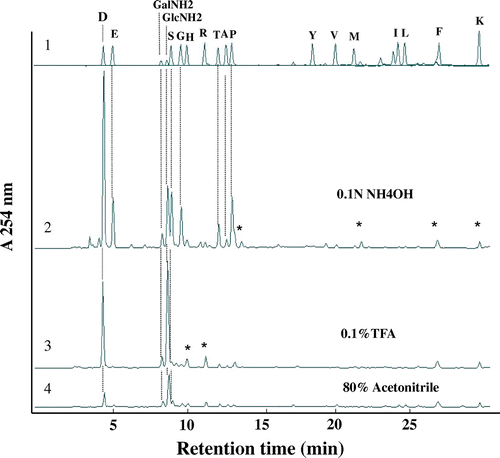
Fig. 2. SF-HPLC of PA-sugar chains obtained from glycopeptides containing TF-antigen.
Note: (1) SF-HPLC of PA-sugar chains obtained from Asn-glycopeptides purified by hydrophilic partitioning by means of Shodex Asahipak NH2-P 50 resin; (2) Endo-H digest of 1; (3) β1-3/6 specific galactosidase (β-Gal’ase) digest of 2; (4) Jack bean β-hexosaminidase (JB β-HexNAc’ase) digest of 3; (5) Jack bean α-mannosidase (α-Man’ase) digest of 4. M1-9, elution positions of authentic Man1~9GlcNAc2-PA. ○, galactose; ●, mannose; □, GalNAc; ■, GlcNAc.
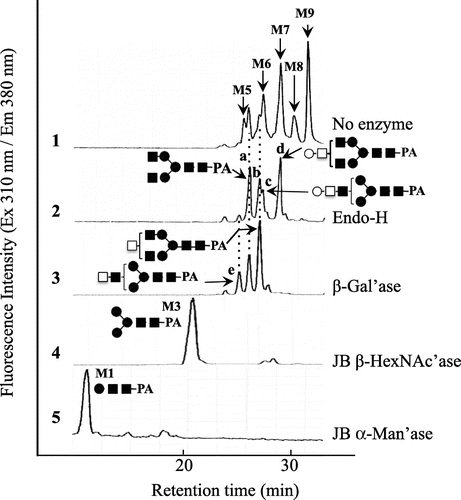
Although at this time there is no effective method of excluding agalactosylated N-glycans (peaks a and b) from Endo-H treated Asn-glycopeptides (high-mannose type free Asn-glycopeptide) purified by hydrophilic partition by means of Shodex Asahipak NH2P-50 4E resin,Citation8) we found that the major N-glycans linked to the glycopeptide were mono-galactosylated and carried the TF-antigen. Thus, the Asn-glycopeptide prepared from royal jelly in this study should prove useful in preparing an antibody against the TF-antigen (Galβ1-3GalNAc) unit for functional analysis of royal jelly-specific complex type N-glycans in a subset of Kenyon cells in the mushroom bodies of the honeybee brain.Citation2,Citation11)
In conclusion, from 12 g of crude royal jelly proteins, 100.1 mg of Asn-glycopeptide carrying the TF-antigen unit (as Endo-H treated Asn-glycopeptide) was prepared by the procedure described above. Preparation of an antibody against the N-glycan containing TF-antigen will be described elsewhere.
Acknowledgments
This work was supported by Basic Research C from the Ministry of Education, Culture, Sports, Science and Technology of Japan [24580494 to M. M., 24580495 to Y. K., 23400944 to M. K.], and Program to disseminate tenure tracking system from the Ministry of Education, Culture, Sports, Science and Technology of Japan [FY 2011-2013 to M. M.].
Notes
Abbreviations: RP-HPLC, reversed-phase HPLC; SF-HPLC, size-fractionation HPLC; PA-, pyridylamino; Endo-H, endo-β-N-acetylglucosaminidase from Streptomyces plicatus; β-Gal’ase, β-galactosidase; JB HexNAc’ase, jack bean β-hexosaminidase; JB Man’ase, jack bean mannosidase; Hex, hexose; HexNAc, N-acetylhexosamine; Pen, pentose; Man, D-mannose.
References
- Kimura Y, Ushijima T, Maeda M, Hama Y, Kimura M, Okihara K, Sugimoto H, Yamada H. Tumor antigen occurs in N-glycan of royal jelly glycoproteins: honeybee cells synthesize T-antigen unit in N-glycan moiety. Biosci. Biotechnol. Biochem. 2006;70:2583–2587.10.1271/bbb.60331
- Kimura Y, Nagai H, Miyamoto M, Kimura M, Yonekura M. Identification of a royal jelly glycoprotein that carries unique complex-type N-glycans harboring the T-antigen (Galβ1-3GalNAc) unit. Biosci. Biotechnol. Biochem. 2010;74:2148–2150.10.1271/bbb.100472
- Schmitzová J, Klaudiny J, Albert Š., Schröder W, Schreckengost W., Hanes J, Júdová J. A family of major royal jelly proteins of the honeybee Apis mellifera L. Cell. Mol. Life Sci. 1998;54:1020–1030.10.1007/s000180050229
- Kimura Y, Sakamura S, Ushijima T, Hama Y, Kajiura H, Fujiyama K, Okihara K, Hashimoto K, Sugimoto H. Evidence for new β1-3 galactosyltransferase activity involved in biosynthesis of unusual N-glycan harboring T-antigen in Apis mellifera. Biosci. Biotechnol. Biochem. 2007;71:1111–1114.10.1271/bbb.70081
- Crestfield AM, Moore S, Stein WH. The preparation and enzymatic hydrolysis of reduced and S-carboxymethylated proteins. J. Biol. Chem. 1963;238:622–627.
- Dubois M, Gilles KA, Hamilton J. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956;28:350–356.10.1021/ac60111a017
- Kimura Y, Miyagi C, Kimura M, Nitoda T, Kawai N, Sugimoto H. Structural features of N-glycans linked to royal jelly glycoproteins: structures of high-mannose type, hybrid type, and biantennary type glycans. Biosci. Biotechnol. Biochem. 2000;64:2109–2120.10.1271/bbb.64.2109
- Maeda M, Takeda N, Mano A, Yamanishi M, Kimura M, Kimura Y. Large-scale preparation of Asn-glycopeptide carrying structurally homologous antigenic N-glycan. Biosci. Biotechnol. Biochem. 2013;77:1269–1274.10.1271/bbb.130076
- Heinrikson R, Meredith SC. Amino acid analysis by reverse-phase high-performance liquid chromatography: precolumn derivatization with phenylisothiocyanate. Anal. Biochem. 1984;136:65–74.10.1016/0003-2697(84)90307-5
- Bidlingmeyer BA, Cohen SA, Tarvin TL. Rapid analysis of amino acids using pre-column derivatization. J. Chromatogr. 1984;336:93–104.10.1016/S0378-4347(00)85133-6
- Kucharski R, Maleszka R. A royal jelly protein is expressed in a subset of Kenyon cells in the mushroom bodies of the honey bee brain. Naturwissenschaften. 1998;85:343–346.10.1007/s001140050512
