Abstract
Saponins have the potential to favorably modulate rumen fermentation, but there is generally a lack of the chemical structures associated with the described effects. The activity of extracts from Calendula officinalis and Saponaria officinalis in the rumen was evaluated in vitro. The S. officinalis root extract, reduced CH4 production by 8.5% and increased total VFA concentration by 25.2%. C. officinalis and S. officinalis root extracts and the S. officinalis aerial part extract decreased the acetate to propionate ratio from 8.6 to 17.4%, according to the extract. An HPLC-ELSD analysis indicated that the saponin content ranged from 43.6 to 57.6 mg/g of dry matter (DM) in the C. officinalis extracts and from 224.0 to 693.8 mg/g of DM in the S. officinalis extracts, expressed as the hederacoside C equivalent. Identification of the saponin compounds present in the extracts by HPLC–MSn suggested that the saponin profile modulated the biological activities, showing the importance of determining the structure of saponins when evaluating extracts.
Key words:
Due to the complex symbiosis between micro-organisms and ruminants, carbohydrates are fermented in the rumen into volatile fatty acids (VFAs) and gases, including methane (CH4). VFAs represent up to 75% of ruminant energy requirements. The production of CH4 is responsible for 10–15% metabolizable energy loss,Citation1) and contributes significantly to anthropogenic greenhouse gas emissions worldwide.Citation2) Several feeding strategies have been described to mitigate CH4 emissions from ruminants. Plant secondary metabolites, including saponins, have shown promising results when incorporated into the diet.Citation3) Saponins are composed of a polar sugar moiety glycosidically linked to a non-polar aglycone (terpenoid or steroid) generally exhibiting antifungal, antibacterial, or antiprotozoal properties.Citation4) As most carbohydrates are metabolized by micro-organisms in the rumen, the inclusion of such compounds as saponins that are able to modulate the microbial population have the ability to modify VFA and CH4 production.
The effects of such commercially available saponin-containing plants, such as Yucca schidigera and Quillaja saponaria on CH4 and VFA production have been well documented.Citation3) In most of the studies, the saponin content is globally quantified, using non-specific methods, such as butanol extraction followed by gravimetric evaluation. The chemical structures of the saponins present in the resulting extracts are almost never known. Published data about saponin sources other than Y. schidigera and Q. saponaria are few, although saponins are widely distributed in plants.
The objective of this present study was to determine the effect of extracts from Calendula officinalis and Saponaria officinalis on rumen fermentation in respect of the VFA concentration, CH4 production, and protozoa population. These plants were selected because they are cultivable worldwide, they contain saponins, and they are not toxic when used as feed additives for ruminants.Citation5, 6) The observed biological activities were compared with the chemical profiles of the extracts which were obtained through individual saponin quantification.
Materials and methods
Plants and reagents
The plants were harvested between June 2009 and August 2010 in the experimental fields of the Iteipmai Technical Institute in Chemillé, France, to produce aqueous extracts from the aerial part of S. officinalis L. and floral head and root of C. officinalis L. Commercial powder of the S. officinalis L. root provided by Nor-Feed Sud (Beaucouzé, France) was used to produce a hydro-ethanolic extract. All solvents used for the saponin analysis were of HPLC grade, and distilled water was purified with a Milli-Q water purification system. Hederacoside C was purchased from Extrasynthèse (Lyon, France).
Preparation of the extracts
A 5 g amount of each powdered sample was extracted while stirring in 100 mL of either Milli-Q water or 50% ethanol for 1 h at room temperature. The solution was then centrifuged for 10 min at 3000 rpm and the supernatant was collected. When appropriate, ethanol was evaporated under vacuum with a rotary evaporator and a water bath at 40 °C to obtain an aqueous liquid extract, the aqueous extract was freeze-dried to obtain the dried extract that was used for the in vitro rumen fermentation. Each dried extract was dry-stored in the dark at room temperature in a vacuum-desiccator.
Identification of the saponin compounds by liquid chromatography–mass spectrometry
A freeze-dried sample was diluted in a solution of 50% acetonitrile and filtered through 0.2-μm nylon syringe membrane prior to HPLC injection. Each sample was analyzed with a Bruker Esquire 3000 Plus electrospray ionization-ion trap mass spectrometer coupled with a Waters 2790 separation module (HPLC–ESI–MSn). A Waters 2996 UV detector, recording the absorbance at 254 nm, was used to detect non-saponin compounds. A 10-μL amount of an extract diluted at 7.5 mg/mL was injected. Separation was conducted in a C18 Luna column (150 mm × 4.6 mm, 5-μm particle size, Phenomenex). The mobile phase was composed of water (A), acetonitrile (B), and acetic acid in acetonitrile 10% (C). Gradient elution of 90A/00B/10C to 43A/47B/10C in 47 min, then 00A/90B/10C to 60 min for C. officinalis, and 90A/00B/10C to 80A/10B/10C in 5 min, then 55A/35B/10C to 75 min, and finally 00A/90B/10C to 90 min for S. officinalis was conducted.
The MS conditions were as follows: collision gas, He; collision energy amplitude, 1.3 V; nebulizer and drying gas, N2 at 7 L/min; pressure of nebulizer gas, 30 psi; dry temperature, 340 °C; flow rate, 1.0 mL/min; solvent split ratio, 1:9; scan range, m/z 400–2000; and ionization in the negative mode. The elution order, mass-to-charge ratio, and fragmentation patterns (MS2, MS3, and MS4) of the detected compounds were compared with the data available in the literature in order to formulate structural assumptions, as described by Kowalczyk et al.Citation7) The product ions obtained by collision-induced dissociation allowed identifying of the carbohydrate units, prosapogenins, and sapogenins.
Quantification of the saponin compounds by liquid chromatography-evaporative light scattering detection
The method was modified from European pharmacopoeia and Kakigi et al.Citation9) An Agilent Series 1200 liquid chromatography coupled to an evaporative light scattering detector (ELSD) (Agilent Technologies, USA) was used for this analysis. The conditions for separation were similar to those described for the HPLC–MS analysis. The flow rate of the carrier gas, temperature of the drift tube, and gain of the detector were, respectively, were set at 2.5L/min, 60 °C, and 9. Each extract was dissolved in 50% acetonitrile, at 20 mg/mL for C. officinalis, 10 mg/mL for the S. officinalis aerial part, and 5 mg/mL for the S. officinalis root. The injection volume was 20 μL. Those saponins identified by HPLC–MS were quantified by HPLC-ELSD, using the hederacoside C as an external standard. The total saponin content was calculated as the sum of individual quantified saponins.
HPLC-ELSD method validation
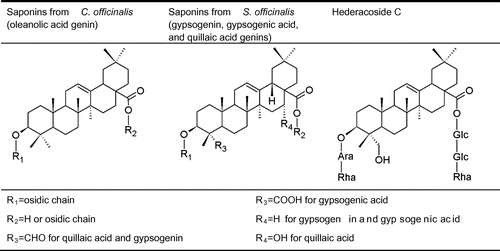
A standard stock solution was prepared by dissolving 15 mg of hederacoside C (Fig. ) in 10 mL of methanol to prepare calibration solutions (0.75, 0.38, 0.25, and 0.04 mg/mL) by appropriate dilution. The calibration curves were plotted after linear regression of the peak areas vs. concentrations in log base 10 units. The matrix effect was briefly investigated by comparing the estimated concentrations for compounds 25, 27, 29, 31, and 33 when injecting the extract of the S. officinalis root at 5 and 10 mg/mL.
Fig. 1. Structures of hederacoside C, saponins of C. officinalis, and saponins of S. officinalis.
Note: Glc, Glucose; Ara, arabinose; Rha, rhamnose.
The limit of detection (LOD) and the limit of quantification (LOQ) were determined on the basis of respective signal-to-noise ratios of 3:1 and 10:1. The intra-day precision was validated with 12 saponin compounds found at the beginning, the middle, and the end of chromatograms from the floral head (3, 10, and 12) and roots (5, 11, and 15) of C. officinalis, and from the aerial part (20, 28, and 41) and roots (23, 46, and 57) of S. officinalis. Inter-day precision was validated with six measurements on two consecutive days for the extraction of the aerial part from S. officinalis. The variation was calculated to evaluate repeatability.
In vitro rumen fermentation
The Hohenheim syringe-based in vitro gas method was used for rumen fermentation.Citation10, 11) Briefly, rumen fluid was collected before the morning feeding from two rumen-fistulated dry Holstein cows. The animals were housed according to European guidelines for animal welfare and fed twice daily at maintenance on a roughage-concentrated diet (70–30 w/w) of 7 kg of dry matter (DM) per day (98 g/kg of crude protein DM). The rumen fluid was filtered through a 1-mm sieve prior to being transferred to preheated thermos bottles. All sampling were quickly performed under vacuum. The rumen fluid was then added at a 1:2 ratio to a buffer medium kept at a temperature of 39 °C to obtain the incubation medium. This buffer medium was composed of a bicarbonate buffer, macromineral, micromineral, resazurin, and reducing solutions.Citation11) All handling were performed while continuously flushing with CO2.
Dry ray grass roughage and wheat seeds were ground in a Wiley mill to pass through a 1-mm screen and then blended (70–30, w/w DM) to prepare the basal feedstuff (960 g/kg of DM content). The respective dietary concentrations of crude proteins, crude fat, acid detergent fiber, neutral detergent fiber, starch, total sugars, and minerals were 89, 21, 250, 492, 172, 102, and 56 g/kg DM. The fermentation substrates were prepared by homogeneously blending the extracts (50 g/kg DM) and the basal feedstuff (950 g/kg DM) for six hours before the fermentation. Two hundred and fifty mg of each substrate was weighed and loaded into 100-mL glass syringes (Fortuna, Poulten & Graf, Wertheim, Germany) equipped with a Luer-lock valve. The control was composed only of the basal feedstuff. Two syringes without the substrate (blanks) were also prepared. The concentration in each plant extract was set at 0.4 mg/mL of the incubation medium (equivalent to 70 g/d/cow).
The incubation medium (30 mL) was dispensed through the valve of the preheated (39 °C) syringes using a peristaltic pump. After all the gas had been expelled, the syringes were placed for 24 h at 39 °C in a KS 4000i control-incubator shaker (IKA Werke, Staufen, Germany) at 50 rpm in triplicate. Aliquots of the incubated medium were then sampled and preserved with 1 mL of mercury chloride (II) at -20 °C for quantification of VFA. The fermentation gases were sampled in evacuated glass vials equipped with an Exetainer® gas-tight septum (Labco, Buckinghamshire, England) for quantification of the gases.
VFA, CH4, and total gas production
Aliquots were analyzed for their VFA content by gas chromatography according to Lecerf et al.Citation12) The total gas volume produced in the syringes was recorded after incubation. The CH4 proportion was determined by micro gas chromatography coupled with a CP-4900 thermal conductivity detector (Varian, Palo Alto, CA, USA). Separation was performed on a 5A molecular sieve with a detection threshold of 10 ppm. The gas was directly pumped from the vials, using helium as the carrier gas. The production of each gas was calculated as:
Enumeration of rumen protozoa
Samples from the incubation media were homogenized and mixed with a methyl green–formalin solution (50–50, v/v). The generic protozoa profile was microscopically determined using a 10-μL Agasse Lafont counting chamber (Preciss, France). Entodinium spp., large entodiniomorphids (e.g. Diplodinium spp., Eudiplodinium spp., Epidinium spp., and Polyplastron spp.), Dasytricha spp. and Isotricha spp. were identified according to Ogimoto and Imai.Citation13) The total rumen protozoa number was summed from the generic profile.
Statistical analysis
In vitro incubation was carried out on three replicates. Two analytical replicates from each technical replicate were used for measuring the biological activity (VFA, CH4, and protozoa). Means were compared by an unbalanced two-way analysis of variance with subsequent Duncan’s post-hoc multiple comparison test, using XLSTAT version 2011.2.04 (Addinsoft, USA). The following model was used: Yij = μ + Pi + εij, with Yij being the dependant variable, μ the least squares mean, and Pi the effect of the plant extract. Differences were considered significant at p < 0.05.
Results
Saponin content in the extracts
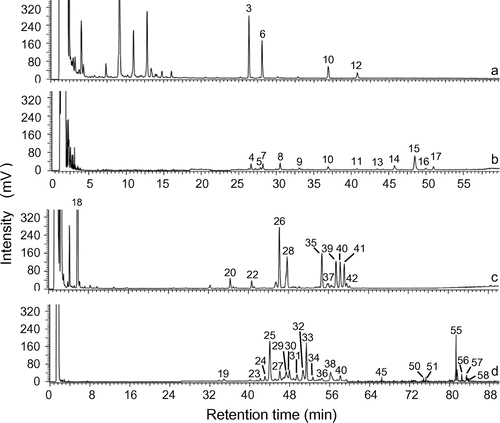
Fifty-four different saponins were detected in the four extracts by HPLC–MSn (Fig. ). The product ions obtained by collision-induced dissociation allowed identifying carbohydrate units, prosapogenins, and sapogenins. It was, thus, possible to formulate a structural hypothesis from the detected saponins and flavonoids by a comparison with literature data (Tables and ).
Fig. 2. HPLC-MS total ion chromatograms of the floral head (A) and root (B) extracts of C. officinalis and the aerial part (C), and root (D) extracts of S. officinalis.
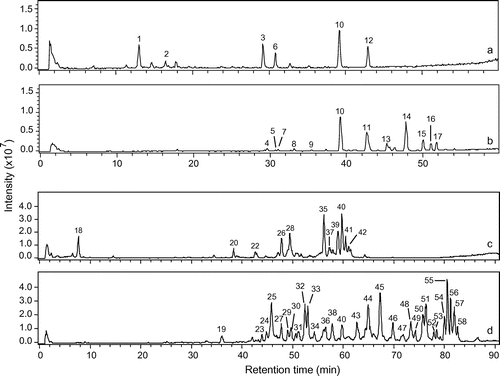
Table 1. Chromatographic, mass spectral data and general structures for the 17 major compounds detected by HPLC–MSn in the C. officinalis extracts.
Table 2. Chromatographic, mass spectral data and general structures for the 41 major compounds detected by HPLC–MSn in the S. officinalis extracts.
Structural identification could be attributed to 6 out of 14 saponins (3, 6, 10, 11, 12, and 14) detected in C. officinalis. These compounds were derived from oleanolic acid and possessed from 1 to 4 osidic units (Fig. ). Compounds 4, 9, 13, 15, 16, and 17 were unknown and probably acetylated saponins. Structural identification could be attributed to 4 out of 40 saponins (20, 26, 35, and 40) detected in S. officinalis. All the saponins were derived from either gypsogenic acid, quillaic acid, or gypsogenin, and most of them were possessed from 6 to 8 osidic units and acetyl groups (Fig. ).
The chemical footprints from the root and aerial parts differed qualitatively. Saponins from the root had a higher retention time than saponins from the aerial parts. Saponins from the C. officinalis floral head were only oleanolic acid glucuronides, while those from C. officinalis roots were oleanolic acid glucuronides and oleanolic acid glucosides. Saponins from the S. officinalis aerial part were mostly based on quillaic acid, while the majority of the saponins from S. officinalis root were based on gypsogenin. Only two saponins were found in the extracts of both the aerial part and root: compound 10 for C. officinalis and compound 40 for S. officinalis. Besides the saponins, three phenolic compounds were identified in the aerial parts. Two isorhamnetin derivatives were found in C. officinalis, and saponarin, a vitexin derivative, was detected in S. officinalis.
HPLC-ELSD quantification of saponins
The quantification method for saponins was developed by HPLC-ELSD. The linearity, limits of detection (LOD) and quantification (LOQ), and the intra-day and inter-day precision were evaluated. A linear dependence between the hederacoside C concentration and peak area on a log–log plot was apparent (y = 1700x + 4203; R = 0.997). LOD and LOQ were 12 and 33 μg/mL, respectively. The coefficients of variation of the retention times and of the peak areas were taken as measurements of precision for the intra- and inter-day variations. The results showed that the intra-day coefficient of variation was less than 0.56% for the retention times. The intra-day coefficient of variation for the peak areas of the major compounds (3, 10, 12, 15, 20, 23, 28, 41, 46, and 57) was less than 2.89%, the intra-day coefficient of variation for the ultra-minor compounds (5 and 11) was less than 7.85% (peak area), and the overall intra-day coefficient of variation of the total saponin content in the extracts was less than 2.15%. The average inter-day coefficient of variation for compounds 20, 28, and 41 was 0.17% for the retention times and 5.90% for the peak areas. These results were considered satisfactory for subsequent quantitative determination of the saponins as the hederacoside C equivalent in the extracts of C. officinalis and S. officinalis.
The saponin content ranged from 43.6 mg/g in the root extract to 57.6 mg/g in the floral head extract of C. officinalis, and from 224.0 mg/g in the aerial part extract to 693.8 mg/g in the root extract of S. officinalis (Table , Fig. ).
Table 3. Total gas and CH4 production, VFA concentration, and protozoa number from in vitro rumen fermentation, and concentration of saponins in the C. officinalis and S. officinalis extracts.
In vitro effect of the C. officinalis and S. officinalis extracts
The gas production, VFA concentration, and protozoa population were evaluated after 24 h of in vitro rumen incubation. No significant effect was apparent on total gas production and rumen protozoa when compared to the control with 0.4 mg of the plant extract/mL of the incubation medium (Table ). The total gas production ranged from 69.1 to 71.9 mL/250 mg of substrate, and the total rumen protozoa number from 3.12 to 3.55 × 105/mL.
CH4 production with the S. officinalis root extract was reduced from 10.7 to 9.7 mL/250 mg of the substrate, and the CH4 to total gas ratio was reduced from 146.5 to 139.8 mL/L (p < 0.05). The CH4 to VFA ratio differed significantly (p < 0.05) between the control (0.64 mmol/mmol) and incubation with the root extracts of both C. officinalis (0.50 mmol/mmol) and S. officinalis (0.46 mmol/mmol).
Significant differences (p < 0.05) in the VFA profile were noted. The acetate to propionate ratio was decreased with the aerial part extract of S. officinalis (2.59), root extracts of C. officinalis (2.58) and S. officinalis (2.33) when compared to the control (2.82).
Discussion
The objectives of this work were to evaluate the effects of the C. officinalis and S. officinalis extracts on rumen methanogenesis, and to analyze the chemical composition of the tested extracts. CH4 is derived from methanogenic Archaea metabolism in the rumen between 9 and 25% of the CH4 production originated from methanogenic Archaea interacting very closely with different hydrogen-producing organisms, including protozoa.Citation21) Saponins have been reported to reduce CH4 production in two different ways. Firstly, it is assumed from the anti-protozoa properties of saponins that CH4 production from methanogenic Archaea living in symbiosis with protozoa would be decreased.Citation4) Another mechanism of action involves the lower activity of such acetate producers, as protozoa and some bacteria (e.g. Ruminococcus flavefaciens),Citation22) and the higher activity of such a propionate producer as Selenomonas ruminantium.Citation23) Saponins might therefore, shift a part of the hydrogen flow from methanogenesis to propionate production. The root extract of S. officinalis in our model had the greatest lowering effect on CH4 production (8.5%) at a dosage approximately equivalent to 70 g/cow/day, but no statistically significant difference was apparent in the protozoa number. The decreased acetate-to-propionate ratio (17.4%) and CH4-to-total VFA ratio (27.9%) with the root extract of S. officinalis matches results already reported for extracts of Y. schidigera and Q. saponaria.Citation3, 4) Consequently, the effect of S. officinalis on CH4 production was certainly due to the realigned microbial fermentation towards propionate instead of CH4 production.
In our experiment, the extraction ratio of the C. officinalis floral head was 277.7 mg/g DM, and the concentration in saponins of this extract was 57.6 mg/g DM. Therefore, the concentration in total saponins of the C. officinalis aerial part was around 16.0 mg/g DM. The quantification of saponins in the aerial part of C. officinalis has been reported to range from 20 to 100 mg/g DM.Citation24) The extraction ratio of the S. officinalis root was 118.8 mg/g DM, and the concentration of saponins in this extract was 693.8 mg/g DM, indicating a concentration of total saponins of 82.4 mg/g DM in the root of S. officinalis. The quantification of saponins in the root of S. officinalis has been reported in the literature to range from 25 to 80 mg/g DM.Citation25) The quantitative data measured for the C. officinalis floral head and S. officinalis root are, therefore, consistent with the results from the previous research. The respective saponin contents in extracts of the C. officinalis floral head and S. officinalis root were estimated in a previous study by the gravimetric method to be 112.0 and 160.7 mg/g DM.Citation26) The value obtained by HPLC-ELSD was lower for the C. officinalis floral head, probably due to the affinity of flavonoid compounds to butanol, and was higher for S. officinalis, certainly due to the presence of highly glycosylated saponins which remained in the aqueous phase. The gravimetric method is not specific to saponins, as this finding is not consequently being unexpected.
The HPLC–MSn analysis allowed us to detect and to partially identify the saponins, while HPLC-ELSD was used for individual saponin quantification. As indicated in Figs. and , the relative intensity of saponins differed between the HPLC–MS and HPLC-ELSD chromatograms. For example, the analysis of the C. officinalis floral head extract showed higher responses with the MS detector for saponins 10 and 12 when compared with saponins 3 and 6, while the reverse results were apparent with the ELSD detector. Different results between UV, MS, and ELSD quantification have already been reported.Citation27) Among these detectors, ELSD is the most universal one used, because neither the optical properties of the compounds nor their ability to form charged species have an impact on the ELSD response. This detector is consequently well adapted to compounds with weak chromophores and without available standards, such as the saponins of S. officinalis and C. officinalis. Quantification by ELSD is almost independent of the structure of the compounds, allowing its use with a single standard. Hederacoside C was selected as the standard in this study due to its availability and its structure being close to that of saponins from S. officinalis and C. officinalis (Fig. ). However, quantification by HPLC-ELSD can be wrongly estimated due to a matrix effect.Citation28) The concentration of the saponins measured by the HPLC-ELSD quantification method in this work was on an average 1.80 higher when the amount of the injected extract was doubled. This difference in ratio may be attributed to a light matrix effect (9%). The representative profile for the saponin content is consequently consistent with the ELSD detector, and the matrix effect can be considered as negligible in respect of the purpose of this study, i.e. to quantify the saponin content of non-standardized extracts. Consequently, the developed HPLC-ELSD method allowed accurate quantification of total saponins in the extracts. This method is available for extracts of other saponin-containing plants, as long as the saponins can be separated by HPLC. It is also conceivable for the HPLC–MS profile to be less representative of the saponin content than the HPLC-ELSD profile, since the response factors are different for each compound in MS due to ionization and fragmentation. MS detection is also known to be highly sensitive to the matrix effect.Citation29)
The extracts from S. officinalis had a higher saponin concentration and biological activity than the extracts from C. officinalis. However, for such a parameter as the acetate-to-propionate ratio, the extracts from the aerial part of S. officinalis and from the roots of C. officinalis had a similar reducing effect (10%) despite the concentration of total saponins being four times higher in S. officinalis than C. officinalis. As the identified saponins were not the same, oleanolic acid-based saponins with few osidic units may be more effective for modulating rumen fermentation than quillaic acid with long osidic chains, showing the importance of determining the structures of saponins when evaluating the effect of extracts on the rumen fermentation pattern.
The results obtained from in vitro rumen fermentation depend on the microbial population of the rumen fluid and diet composition. When the activity of saponins is tested, variability also results from the saponin structure and total saponin content. In this study, pools of saponin compounds were successfully gathered with their capacity to modulate in vitro rumen fermentation in respect of CH4 and VFA. Long-term in vivo trials will now be necessary to confirm the potential of extracts from S. officinalis and C. officinalis as a feed additive to decrease methane production by ruminants.
Acknowledgments
We are grateful to Mathieu Wident (Iteipmai Technical Institute, Chemillé, France) for providing plants and to Oriane Partenay (University of Angers, France) for help with protozoa counting. This research was supported by funding from Région Pays de la Loire, France and ANR (National Research Agency) through the SAPONINES vs. GES project approved by the Vegepolys cluster (agreement 2008-00286).
Notes
Abbreviations: CH4, methane; DM, dry matter; ELSD, evaporative light scattering detection; HPLC, high performance liquid chromatography; LOD, limit of detection; LOQ, limit of quantification; MS, mass spectrometry; VFAs, volatile fatty acids.
References
- Jouany JP, Thivend P. Production of digestive methane in ruminants and impact on global warming. Manag. Av. 2008;20:259–274.
- Steinfeld H, Gerber P, Wassenaar T, Castel V, Rosales M, de Haan C. Livestock’s long shadow: environmental issues and options. Rome: FAO; 2006. p. 79–124.
- Wina E, Muetzel S, Becker K. The impact of saponins or saponin-containing plant materials on ruminant production. J. Agric. Food Chem. 2005;53:8093–8105.10.1021/jf048053d
- Patra AK, Saxena J. A review of the effect and mode of action of saponins on microbial population and fermentation in the rumen and ruminant production. Nutr. Res. Rev. 2009;22:204–219.10.1017/S0954422409990163
- Davidovic V, Joksimović Todorović M, Stojanović M, Reli R. Plant usage in protecting the farm animal health. Biotechnol. Anim. Husb. 2012;28:87–98.10.2298/BAH1201087D
- Szumacher-Strabel M, Zmora P, Pers-Kamczyc E, Pecio L, Moniuszko-Szajwaj B, Szczechowiak J, Nowak A, Szymczak M, Biechonski M, Cieslak A. Effect of Saponaria officinalis on rumen microbial population, rumen fermentation and methane and ammonia production in dairy cows. Adv. Anim. Biosci. 2013;4:492–492.
- Kowalczyk M, Pecio Ł, Stochmal A, Oleszek W. Qualitative and quantitative analysis of steroidal saponins in crude extract and bark powder of Yucca schidigera Roezl. J. Agric. Food Chem. 2011;59:8058–8064.10.1021/jf2022397
- European pharmacopoeia. 8th ed. Strasbourg: Directorate for the Quality of Medicines and Health Care of the Council of Europe (EDQM); 2014. Cimicifugae rhizoma; p. 3702–3707.
- Kakigi Y, Mochizuki N, Icho T, Hakamatsuka T, Goda Y. Analysis of terpene lactones in a Ginkgo leaf extract by high-performance liquid chromatography using charged aerosol detection. Biosci. Biotechnol. Biochem. 2010;74:590–594.
- Menke K, Steingass H. Estimation of the energetic feed value from chemical analysis and in vitro gas production using rumen fluid. Anim. Res. Dev. 1988;28:7–55.
- López S, Makkar HPS, Soliva CR. Screening plants and plant products for methane inhibitors. In: Vercoe PE, Makkar HPS, Schlink AC, editors. In vitro screening of plant resources for extra-nutritional attributes in ruminants: nuclear and related methodologies. Dordrecht: Springer; 2009. p. 191–231.
- Lecerf JM, Dépeint F, Clerc E, Dugenet Y, Niamba CN, Rhazi L, Cayzeele A, Abdelnour G, Jaruga A, Younes H, Jacobs H, Lambrey G, Abdelnour AM, Pouillart PR. Xylo-oligosaccharide (XOS) in combination with inulin modulates both the intestinal environment and immune status in healthy subjects, while XOS alone only shows prebiotic properties. Br. J. Nutr. 2012;1:1–12.
- Ogimoto K, Imai S. Atlas of rumen microbiology. Tokyo: Japan Scientific Societies Press; 1981. 231p.
- Vidal-Ollivier E, Elias R, Faure F, Babadjamian A, Crespin F, Balansard G, Boudon G. Flavonol glycosides from Calendula officinalis flowers. Planta Med. 1989;55:73–74.10.1055/s-2006-961831
- Yoshikawa M, Murakami T, Kishi A, Kageura T, Matsuda H. Medicinal flowers. III. Marigold. (1): hypoglycemic, gastric emptying inhibitory, and gastroprotective principles and new oleanane-type triterpene oligoglycosides, calendasaponins A, B, C, and D, from Egyptian Calendula officinalis. Chem. Pharm. Bull. 2001;49:863–870.10.1248/cpb.49.863
- Szakiel A, Ruszkowski D, Janiszowska W. Saponins in Calendula officinalis L. - structure, biosynthesis, transport and biological activity. Phytochem. Rev. 2005;4:151–158.10.1007/s11101-005-4053-9
- Vidal-Ollivier E, Balansard G, Faure R, Babadjamian A. Revised structures of triterpenoid saponins from the flowers of Calendula officinalis. J. Nat. Prod. 1989;52:1156–1159.10.1021/np50065a042
- Bukharov VG, Shcherbak SP. Triterpene glycosides from Saponaria officinalis. Chem. Nat. Compd. 1969;5:324–326.10.1007/BF00595069
- Jia Z, Koike K, Nikaido T. Major triterpenoid saponins from Saponaria officinalis. J. Nat. Prod. 1999;62:449–453.10.1021/np980434w
- Jia Z, Koike K, Nikaido T. Saponarioside C, the first α-d-galactose containing triterpenoid saponin, and five related compounds from Saponaria officinalis. J. Nat. Prod. 1998;61:1368–1373.10.1021/np980167u
- Newbold CJ, Ushida K, Morvan B, Fonty G, Jouany JP. The role of ciliate protozoa in the lysis of methanogenic archaea in rumen fluid. Lett. Appl. Microbiol. 1996;23:421–425.10.1111/j.1472-765X.1996.tb01350.x
- Patra AK, Stiverson J, Yu Z. Effects of quillaja and yucca saponins on communities and select populations of rumen bacteria and archaea, and fermentation in vitro. J. Appl. Microbiol. 2012;113:1329–1340.10.1111/jam.2012.113.issue-6
- Wang Y, McAllister TA, Yanke LJ, Xu ZJ, Cheeke PR, Cheng KJI. In vitro effects of steroidal saponins from Yucca schidigera extract on rumen microbial protein sysnthesis and ruminal fermentation. J. Sci. Food Agric. 2000;80:2114–2122.10.1002/(ISSN)1097-0010
- WHO. WHO monographs on selected medicinal plants. Vol. 2. Geneva, Switzerland: World Health Organisation; 2001. p. 35–44.
- Wichtl M, Anton R. Plantes thérapeutiques: Tradition, pratique officinale, science et thérapeutique [Herbal drugs: tradition, official practice, science and therapeutics]. Paris, France: Tec & Doc Lavoisier; 2003. p. 553–554.
- Budan A, Tessier N, Saunier M, Gillmann L, Hamelin J, Chicoteau P, Richomme P, Guilet D. Effect of several saponin containing plant extracts on rumen fermentation in vitro, Tetrahymena pyriformis and sheep erythrocytes. J. Food Agric. Environ. 2013;11:576–582.
- You Q, Chen F, Sharp JL, Wang X, You Y, Zhang C. High-performance liquid chromatography-mass spectrometry and evaporative light-scattering detector to compare phenolic profiles of muscadine grapes. J. Chromatogr. A. 2012;1240:96–103.10.1016/j.chroma.2012.03.086
- Liu Y, Shi XW, Liu EH, Sheng LS, Qi LW, Li P. More accurate matrix-matched quantification using standard superposition method for herbal medicines. J. Chromatogr. A. 2012;1254:43–50.10.1016/j.chroma.2012.07.020
- Trufelli H, Palma P, Famiglini G, Cappiello A. An overview of matrix effects in liquid chromatography–mass spectrometry. Mass Spectrom. Rev. 2011;30:491–509.10.1002/mas.v30.3