Abstract
We designed a cyclic compression system using readily available six-well culture plates to investigate the influence of mechanical stress on skin-like structures. The effects of cyclic mechanical stress on protein expression by cells were easily examined, and hence, this system should be useful for further analysis of skin responses to mechanical stress.
Mechanical stress regulates cell behavior. It has been proposed that in vivo, mechanical stimulation of skin, by massage, for example, affects arterial blood pressure,Citation1) the autonomic nervous system,Citation2) and wound healing.Citation3) Many mechanical stimulation studies employ and hence intricate culture systems, the relationship between extracellular matrix material and the response of skin cells to mechanical stress remains unknown. In this study, we used a cyclic compression system to investigate the influence of mechanical stress on skin-like structures.
Cell-containing collagen gels at neutral pH were prepared by mixing a type 1 collagen solution (IAC-30; Koken, Tokyo), diluted by Dulbecco’s Modified Eagle’s Medium (Sigma–Aldrich, ST. Louis, MO) to a final density of 0.1% with 10% fetal bovine serum (Sigma–Aldrich, lot No. 106K0366), with normal human dermal fibroblasts (NB1RGB cells; Riken, Tsukuba) at densities ranging from 2.5 × 105 to 1.0 × 106 cells/gel.Citation4) The cell-containing collagen gel (2.0 mL) was poured into a nontreated plate (Nunc, Roskilde, Denmark), a tissue culture plate (Nunclon Δ Surface plate; Nunc), or a low-adherence plate (12-well Lipidure®-Coat Multi-Dish A-12MD; NOF, Tokyo) that was coated with 2-methacryroyloxyethyl-phosphoryl-choline (MPC). A tissue culture plate is a plate that is corona discharge-treated to promote cell adhesion. A normal plate is a common plate that is not treated. The plates were then incubated for 48 h to produce shrunken collagen gels, and the area of each collagen gel was measured. The 0.1% collagen gels (5.0 mL) of the low-adherence plate containing 2.5 × 106–1.0 × 106 cells/gel, incubated for 72 h, were subjected to cyclic mechanical compression by means of FX-4000C™ (Flexcell International, Hillsborough, NC) as follows: condensed collagen gels were placed into the wells of a compression plate (BioPressTM; Flexcell International, Hillsborough, NC, diameter 13 mm) with 2.0 mL of medium supplemented with 10% fetal bovine serum. The plates were then subjected to cyclic mechanical compression (40 kPa and 1 Hz for 6 h). All compression experiments were conducted at 37 °C in a 5% CO2 incubator. After compression, the plates were incubated for 48 h to produce culture supernatants. The hyaluronic acid in the culture medium was extracted as described in a previous report.Citation5) The hyaluronic acid content was analyzed by cellulose acetate membrane electrophoresis by the standard method.Citation6) The molecular weight of hyaluronic acid in the culture medium was determined by agarose gel electrophoresis. Hyaluronic acid markers (1,500, 1,000, 700, 500, and 50 kDa; Kewpie, Tokyo) were loaded as standards. The gels were stained with Stains-All (Sigma–Aldrich, ST. Louis, MO) and analyzed by image analysis software (Scion Image; Scion, Frederick, MD). Before compression, the nuclei of the dermal fibroblasts within the collagen gels were labeled with DAPI and viewed under a fluorescence microscope. All experiments were run independently at least twice, and similar results were obtained.
The areas of cell-containing collagen gels on the various types of plates were measured after 48 h of incubation (Fig. (A)). The areas of the gels in the low-adherence plates were smaller than those of the gels in the other plates. Furthermore, the standard deviations of gel areas in the low-adherence plates were smaller than those of the other plates. The phosphocholine group on the surface of the wall of a low-adherence plate can prevent collagen adhesion to the wall and subsequently prevent disorder of the collagen gels during shrinkage. The Lipidure® (NOF, Tokyo) coating material of the low-adherence plate did not cause cell damage or damage the protein conformation of the gel.Citation7,8) The plate wall resembles the surface of a human cell membrane, and cells do not adhere to this plate surface.Citation9,10) The shrunken collagen gels in the low-adherence plate were precisely circular or cylindrical in the shape, whereas the gels in the other plates were skewed, and inner circular lines were observed within the gel (Fig. (B)). These lines were due to collagen gel accumulation in specific areas of the gel that brought about different heights in the collagen gel and to the attachment of collagen to the wall of the plate at an early stage of gel shrinkage. When a variety of cells is embedded in a three-dimensional collagen gel, the gel is condensed by the cell cytoskeleton and becomes stiff. This shrunken collagen gel has a dermal tissue-like structure, and it can be applied in the screening of many active compounds.Citation11) Several methods have been proposed of creating a circular-shaped shrunken collagen gel, including the use of mineral oil,Citation12) a Teflon membrane,Citation4) and a rotator,Citation4) but we wished to avoid such artificial methods and instead to use a low-adherence plate to control the gel shape. Since the collagen gels in the nontreated plate and the tissue culture plate were not circular in the shape, we measured the area of the gels (Fig. ), but the gels in the low-adherence plates were circular in the shape. We measured their diameters, since the diameter of the condensed collagen gel had to be below 13 mm in order to fit into the wells of the BioPressTM Plate. We examined the effects of various incubation times and various cell densities on gel diameter (Fig. (A)). In accord with previous reports,Citation4) the speed of gel condensation, as measured by the analysis of gel diameter, was found to decrease with increasing cell numbers and incubation duration. Shrunken gels within which 1.0 × 106 fibroblasts incorporated were labeled with DAPI after 48 h of incubation in low-adherence plate. Labeling of the cells indicated a homogeneous distribution of fibroblasts within the gel (Fig. (B)). We selected a 72-h incubation with 1.0 × 106 cells/gel for a subsequent study, since the diameters were 9.0 mm on average. After 42 h of incubation, the molecular weight of hyaluronic acid in the culture supernatant was higher in the mechanically compressed group than in the noncompressed control (Fig. (A)). The total level of hyaluronic acid in the supernatant was also higher in the compressed group than in the noncompressed control group (Fig. (B)). Compression induced an increase in the mRNA expression of HAS2, NFKBIZ, CREB5, and IL-1 B in the fibroblasts soon after compression (data not shown), and this is known to be characteristic of hyaluronic acid synthesis.Citation13,14) This study therefore indicates that mechanical stress of human fibroblasts embedded in a collagen gel affects gene expression and hyaluronic acid synthesis. Human skin is mechanically stressed in daily life, and skin cells are further subjected to compression, stretching, and shearing forces. Recently, cell-stretching systems have been created to study these phenomena, and studies that have investigated the relationship between skin cells and stretching have been conducted.Citation15,16) Some studies have also investigated the mechanism by which skin is protected from breakdown following mechanical stress.Citation17) Many of these studies have applied stretching to monolayer cells, but the extracellular matrix that surrounds skin cells has not been taken into account. Shrunken collagen gel scaffolds are easy to obtain, but far as we know, no mechanical stress analysis of them has been done. Hence, the relationship between extracellular matrix materials and the responses of skin cells to mechanical stress remains unknown. Our results suggest that skin massage can affect hyaluronic acid synthesis through an as yet unidentified pathway. We acknowledge that compression might also affect the permeability of the collagen gel, and that fetal bovine serum and the medium used for culture can influence fibroblasts. Our system should be useful in further analysis of skin responses to mechanical stress.
Fig. 1. Area change over time and images of cell-containing collagen gels on different types of culture plates.
Notes: Fibroblasts at a density of 1.0 × 106 cells/well containing 0.1% collagen gel (2.0 mL) was poured into a nontreated plate, a tissue culture plate, and a low-adherence plate, and the plates were incubated. The area of each collagen gel was measured by image analyzer. The gel areas were determined automatically by image analysis. (A) Areas of collagen gels (mean ± SD, n = 4) on the indicated plates in relation to incubation duration. The collagen gel area was significantly smaller on the low-adherence plates than on the other two types of plates. aa, p < 0.01 by Tukey’s test versus nontreated plates. bb, p < 0.01 by Tukey’s test versus tissue culture plates. (B) Images of representative gels on each plate after 48 h of incubation. The shrunken collagen gels in the low-adherence plate were precise circular and cylindrical shapes, whereas the gels in the other plates were skewed, and inner circular lines were observed within the gel. Bar, 1 mm.
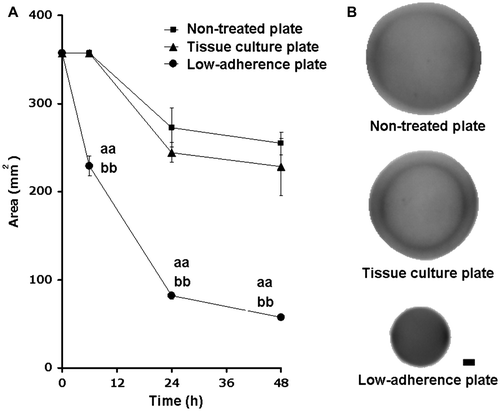
Fig. 2. Effects of cell density and incubation time on the diameters of the cell-containing collagen gels.
Notes: The 0.1% collagen gel (5.0 mL) with fibroblasts (at densities ranging from 2.5 × 105 to 1.0 × 106 cells/gel) was poured into a 12-well Lipidure®-Coat Multi-Dish (NOF, Tokyo) and incubated at 37 °C in 5% CO2 for the indicated times. (A) Percentage changes in gel diameter between 12-h incubation (from 0 to 72 h) are shown below: 26.9, 30.6, 11.7, 8.5, 7.6, 8.2% for gels at a density of 2.5 × 105 cells/gel, 51.0, 19.4, 10.8, 3.4, 4.9, 7.4% for gels a density of 5.0 × 105 cells/gel, and 61.9, 15.6, 11.1, 0.0, 10.0, 0.0% for gels a density of 1.0 × 106 cells/gel. Data are means ± SD (n = 6). (B) A shrunken gel within which 1.0 × 106 fibroblasts were incorporated was labeled with DAPI after 48-h incubation in a low-adherence plate. The labeling of the cells indicates a homogeneous distribution of the fibroblasts within the collagen gel. Bar, 1 mm.
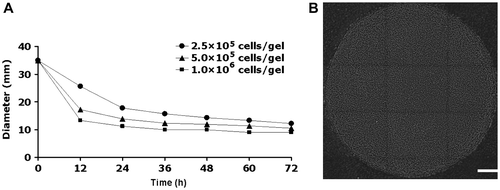
Fig. 3. Effect of compression on hyaluronic acid in the culture supernatant of cell-containing collagen gels.
Notes: Collagen gels containing 1.0 × 106 cells/gel were subjected to cyclic mechanical compression (40 kPa and 1 Hz for 6 h) by the FX-4000CTM Flexcercell® Compression PlusTM System (Flexcell International, Hillsborough, NC). After 42-h incubation, the hyaluronic acid in the culture medium was extracted. Hyaluronic acid markers were loaded as standards. The gels were stained with Stains-All and analyzed by image analysis software. (A) The molecular weight of hyaluronic acid in the culture medium was determined by agarose gel electrophoresis. The molecular weight of the hyaluronic acid in the culture supernatant was higher in the mechanically compressed group than in the noncompressed control. (B) Hyaluronic acid levels were analyzed by cellulose acetate membrane electrophoresis. The total level of hyaluronic acid in the supernatant was higher in the compressed group than in the noncompressed control group.
Acknowledgements
Lipidure is a registered trademark of NOF Corporation in Japan. All other trademarks are the property of their respective owners.
Notes
Abbreviation: MPC, 2-methacryroyloxyethyl-phosphoryl-choline.
References
- Kimura A, Ohsawa H, Sato A, Sato Y. Somatocardiovascular reflexes in anesthetized rats with the central nervous system intact or acutely spinalized at the cervical level. Neurosci. Res. 1995;22:297–305.10.1016/0168-0102(95)00907-B
- Holey LA, Dixon J, Selfe J. An exploratory thermographic investigation of the effects of connective tissue massage on autonomic function. J. Manipulative Physiol. Ther. 2011;34:457–462.10.1016/j.jmpt.2011.05.012
- Timmenga EJ, Andreassen TT, Houthoff HJ, Klopper PJ. The effect of mechanical stress on healing skin wounds: an experimental study in rabbits using tissue expansion. Br. J. Plast. Surg. 1991;44:514–519.10.1016/0007-1226(91)90008-8
- Nishiyama T, Tominaga N, Nakajima K, Hayashi T. Quantitative evaluation of the factors affecting the process of fibroblast-mediated collagen gel contraction by separating the process into three phases. Collagen Rel. Res. 1988;8:259–273.10.1016/S0174-173X(88)80045-1
- Ohara H, Iida H, Ito K, Takeuchi Y, Nomura Y. Effects of Pro-Hyp, a collagen hydrolysate-derived peptide, on hyaluronic acid synthesis using in vitro cultured synovium cells and oral ingestion of collagen hydrolysates in a guinea pig model of osteoarthritis. Biosci. Biotechnol. Biochem. 2010;74:2096–2099.10.1271/bbb.100193
- Hata R, Nagai Y. A micro colorimetric determination of acidic glycosaminoglycans by two dimensional electrophoresis on a cellulose acetate strip. Anal. Biochem. 1973;52:652–656.10.1016/0003-2697(73)90075-4
- DeFife KM, Yun JK, Azeez A, Stack S, Ishihara K, Nakabayashi N, Colton E, Anderson JM. Adhesion and cytokine production by monocytes on poly(2-methacryloyloxyethyl phosphorylcholine-co-alkyl methacrylate)-coated polymers. J. Biomed. Mater. Res. 1995;29:431–439.10.1002/(ISSN)1097-4636
- Ishihara K, Nomura H, Mihara T, Kurita K, Iwasaki Y, Nakabayashi N. Why do phospholipid polymers reduce protein adsorption? J. Biomed. Mater. Res. 1998;39:323–330.10.1002/(ISSN)1097-4636
- Wataya T, Ando S, Muguruma K, Ikeda H, Watanabe K, Eiraku M, Kawada M, Takahashi J, Hashimoto N. Minimization of exogenous signals in ES cell culture induces rostral hypothalamic differentiation. Proc. Nat. Acad. Sci. 2008;105:11796–11801.10.1073/pnas.0803078105
- Yasuda E, Seki Y, Higuchi T, Nakashima F, Noda T, Kurosawa H. Development of cystic embryoid bodies with visceral yolk-sac-like structures from mouse embryonic stem cells using low-adherence 96-well plate. J. Biosci. Bioeng. 2009;107:442–446.10.1016/j.jbiosc.2008.12.004
- Bell E, Ivarsson B, Merrill C. Production of a tissue-like structure by contraction of collagen lattices by human fibroblasts of different proliferative potential in vitro. Proc. Nat. Acad. Sci. 1979;76:1274–1278.10.1073/pnas.76.3.1274
- Vernon RB, Gooden MD. An improved method for the collagen gel contraction assay. In Vitro Cellular Dev. Biol. Anim. 2002;38:97–101.10.1290/1071-2690(2002)038<0097:AIMFTC>2.0.CO;2
- Saavalainen K, Pasonen-Seppanen S, Dunlop TW, Tammi R, Tammi MI, Carlberg C. The human hyaluronan synthase 2 gene is a primary retinoic acid and epidermal growth factor responding gene. J. Biol. Chem. 2005;280:14636–14644.10.1074/jbc.M500206200
- Stylianou E, Saklatvala J. Interleukin-1. Int. J. Biochem. Cell Biol. 1998;30:1075–1079.10.1016/S1357-2725(98)00081-8
- Russell D, Andrews PD, James J, Lane EB. Mechanical stress induces profound remodelling of keratin filaments and cell junctions in epidermolysis bullosa simplex keratinocytes. J. Cell Sci. 2004;117:5233–5243.10.1242/jcs.01407
- Shelton JC, Bader DL, Lee DA. Mechanical conditioning influences the metabolic response of cell-seeded constructs. Cells Tissues Organs. 2003;175:140–150.10.1159/000074630
- Sanders JE, Goldstein BS, Leotta DF. Skin response to mechanical stress: adaptation rather than breakdown--a review of the literature. J. Rehabil. Res. Dev. 1995;32:214–226.
