Abstract
Heat-processed Gynostemma pentaphyllum and its main dammaran-type saponins, gypenoside L, gypenoside LI, damulin B, and damulin A, possess non-small cell lung carcinoma A549 cell inhibitory activity. We established in this study a method by ultra-high performance liquid chromatography with tandem mass spectrometry for determination of the saponins and also investigated their content change in heat-processed G. pentaphyllum. The main saponins increased with increasing heating temperature and time. Further investigation showed that they were produced from gypenoside XLVI and gypenoside LVI by undergoing hydrolysis during the heat treatment.
Gynostemma pentaphyllum (Thunb.) Makino (Jiaogulan) has long been used as folk medicine and tea in Asia. G. pentaphyllum is a perennial creeping herb which belongs to the Cucurbitaceae family. It contains saponins,Citation1−4) flavonoids,Citation5) polysaccharides,Citation6,7) vitamins, and amino acids.Citation8) G. pentaphyllum has been reported to exhibit a variety of biological activities, including antioxidativeCitation9,Citation10) and immunostimulatory effects,Citation7) inhibitory effects on allergic reactions,Citation11) preventative effects on the gastrointestinal tract, kidney and liver,Citation12−14) anti-hyperlipidemic and hypoglycemic effects,Citation15,16) and antitumor effects.Citation17,18) The saponins in G. pentaphyllum, which are also named gypenosides, are usually known for their biological activities.Citation19)
Our research group has recently found that an ethanol extract from G. pentaphyllum, which had been heat processed by steaming the raw plant at high temperature (125 °C), showed more potent cytotoxicity against non-small cell lung carcinoma A549 cells than that of raw G. pentaphyllum.Citation20) Four dammarane-type saponins, gypenoside L (1), gypenoside LI (2), damulin B (3), and damulin A (4), were isolated from heat-processed G. pentaphyllum. All of them showed strong A549 cytotoxic activities.Citation21) However, their contents in heat-processed G. pentaphyllum have not been previously reported.
The separation of saponins has recently been carried out by high-performance liquid chromatography (HPLC) in combination with detectors like a photodiode array (PDA), evaporative light-scattering detection (ELSD), and by mass spectrometry (MS).Citation22−24) However, most of these methods have the disadvantages of a long run time, low sensitivity, low resolution, and high detection limit which do not meet the requirement of a speedy, accurate, and high-throughput analysis of samples in laboratories. Ultra-performance liquid chromatography coupled with tandem mass spectrometry (UPLC-MS) has gained considerable attention in recent years and has emerged as a prominent analytical tool for pharmaceutical and biomedical analyses because of its convenience, low time consumption, and better resolution and sensitivity.Citation25,26)
We developed in this present study a method for the simultaneous determination of saponins in G. pentaphyllum by UPLC-MS. In addition, the contents of four saponins isolated from raw and heat-processed G. pentaphyllum were determined by using this optimized UPLC-MS method, and the relationship between the amount of the active components and the heating temperature used for processing were investigated.
The leaves of G. pentaphyllum were purchased from Tong Ren Tang (Beijing, China). A voucher specimen (no. GP2011-01) has been deposited at the Isolation and Structure Identification Laboratory in Minzu University of China. Gypenoside L (1), gypenoside LI (2), damulin B (3), and damulin A (4) were isolated from heat-processed G. pentaphyllum, and their purities were above 98%.Citation21) The leaves of crude and heat-processed G. pentaphyllum (5.0 g) were extracted with 50 mL of 80% methanol by ultrasonication for 1.5 h and then evaporated. The residue was diluted to 5 mg/mL in methanol and passed through a 0.22-μm filter. Each prepared sample was sealed in a vial and kept in a refrigerator at 4 °C, until needed. A chromatographic analysis was performed using an ACQUITY UPLCTM system (Waters Corporation, USA), equipped with a binary solvent manager, sample manager, column oven, PDA, and tandem quadrupole (TQ) detector. Separation was carried out in an Aquity UPLC BEH C18 column (100 × 2.1 mm i.d., 1.7 μm; Waters, USA). The mobile phase was composed of water (A) and acetonitrile (B). The gradient program was solvent B from 38 to 54% for 10 min. The flow rate of the mobile phase was 0.25 mL/min, and the temperature was maintained at 25 °C. The detection wavelength was set at 203 nm and the injection volume for the UPLC analysis was 10 μL. Nitrogen was used as the desolvation gas at a flow rate of 550 L/h for the MS analysis. The cone gas flow rate was set at 50 L/h, the desolvation temperature was fixed at 350 °C, and the source temperature was set at 100 °C. The capillary voltage was 3000 V for the negative mode of ESI. The spectra were recorded in the negative mode at m/z 781 ± 0.5 and at 799 ± 0.5 in the single ion recording (SIR) mode. Data acquisition and quantification were performed by using MassLynx software V4.1.
The UPLC method was validated for its linearity, limits of detection (LOD) and quantification (LOQ), inter-day and intra-day precision, recovery and stability. The linearity of gypenoside L (1), gypenoside LI (2), damulin B (3), and damulin A (4) was, respectively, determined over the concentration ranges of 1–400, 0.5–200, 1–400, and 1–400 μg/mL. The calibration curves were acquired by analyzing six different concentrations of calibrators. The contents of the four saponins in the test samples were calculated by using regression parameters obtained from the standard curves. The samples with analyte concentrations exceeding the upper limit of linearity were diluted and then reanalyzed. The LODs and LOQs values were, respectively, calculated as three times and ten times the signal-to-noise (S/N) ratios. The analysis of the intra- and inter-day precision was, respectively, conducted by six repetitive injections on the same day and four consecutive days. The recovery test was performed by adding known amounts of standard reference solutions to each sample before extraction, before an analysis using the proposed method. The percentage recovery was calculated according to the following formula: recovery (%) = (measured amount − original amount in sample)/spiked amount × 100%. The stability of the samples was investigated by replicate injections of each sample solution stored at room temperature for 0, 2, 4, 6, 8, 10, and 12 h.
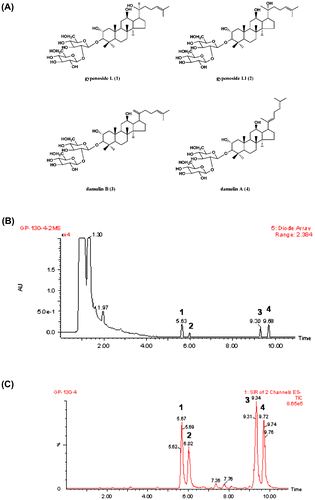
The UPLC-MS method was successfully developed in this study to analyze gypenoside L (1), gypenoside LI (2), damulin B (3), and damulin A (4) (Fig. (A)) in G. pentaphyllum with a TQ detector. The LC–MS data for the target constituents could be compared with those of the authentic standards. The desired compounds from G. pentaphyllum were identified by comparing both the retention times and mass spectra with those of the authentic standards. As a result, four marker constituents were sufficiently resolved under the proposed analytical conditions. The constituents were successfully separated by gradient elution in less than 10 min and there was no interference from other components in the matrix.
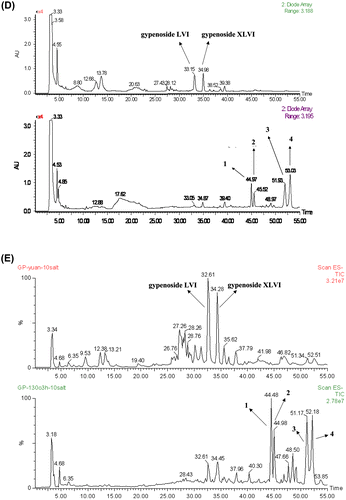
Fig. 1. Chemical structures of active saponins isolated from G. pentaphyllum (A), UPLC-UV chromatogram (B), and UPLC-MS chromatogram (C) for the methanol extract of heat-processed G. pentaphyllum, UPLC-UV chromatogram (D) and UPLC-MS chromatogram (E) for the methanol extract of G. pentaphyllum before and after heat processing.
The regression data, LOQs and LOD values for the components were determined by a statistical analysis of the results from UPLC-MS (Table ). All calibration curves showed good linear regression (r2 > 0.9977) within the tested ranges. The respective LOQs and LODs values were 0.43–0.63 ng and 0.10–0.20 ng by UPLC-MS. The relative standard deviation (RSD) was considered for the measurements of precision and accuracy. The overall intra- and inter-day variations were less than 4%, these results demonstrated that the developed method was reproducible with good precision. The accuracy was evaluated by a recovery test. The recoveries of the four tested compounds were 97.5–99.7% with RSDs of 1.3–3.9% (n = 6), these values indicated that the method was acceptable. The RSD values for the stability test were no more than 4% in each sample. The solution was therefore considered stable for at least 12 h at room temperature.
Table 1. Linear regression, LOQs and LODs, intra-day and inter-day precisions, recovery, and stability of four saponins.
This developed method was subsequently applied to the simultaneous determination of the four major compounds in the G. pentaphyllum samples. Fig. (B) and (C) show the representative UPLC-UV chromatogram at 203 nm (Fig. (B)) and selected ion chromatogram at m/z 799 ± 0.5 and 781 ± 0.5 (Fig. (C)) of the heat-processed G. pentaphyllum extract. The determined four compounds were well separated under the optimized conditions. They appeared at respective retention times of 5.67, 6.02, 9.34, and 9.72 min. In order to determine the relationship between the amount of the four compounds and the heat-processing temperature, we processed G. pentaphyllum at heating temperatures of 110, 120, and 130oC and analyzed each methanol extract. The contents of the four dammarane-type saponins were changed with the heating temperatures and processing time (Table ). Their respective contents were 6130 ± 37.7, 2463 ± 22.3, 5877 ± 29.6, and 11902 ± 96.3 μg/g in G. pentaphyllum heat processed at 130 °C for 3 h, whereas those from the raw plant were below 250 μg/g. Longer treatment times (>3 h) did not give any significant changes in the saponin contents (data not shown).
Table 2. Contents of four saponins in heat-processed G. pentaphyllum.
The phenomenon of some bioactivities being increased in heat-processed plants had been reported for ginseng due to hydrolysis of the glycosidic bonds of ginseng saponins.Citation27,28) It can, therefore, be inferred that gypenoside L (1), gypenoside LI (2), damulin B (3), and damulin A (4) were additionally generated from several saponins of G. pentaphyllum by undergoing hydrolysis during heat processing. So, we investigated the change of other chemical constituents during heat treatment. By modifying the chromatographic conditions, we found that the contents of the polar saponins (gypenoside XLVI and gypenoside LVI) were decreased in heat-processed G. pentaphyllum (Fig. (D) and (E)).Citation29) It can be suggested from these results that gypenoside L (1) and gypenoside LI (2) were each produced from these two decreased saponins by the loss of the glycosyl moiety, and further dehydration led to the increase of damulin B (3) and damulin A (4).
In this study, four dammarane-type saponins, gypenoside L (1), gypenoside LI (2), damulin B (3), and damulin A (4), from raw and heat-processed G. pentaphyllum were simultaneously determined by UPLC-MS in less than 10 min. The contents of these saponins from G. pentaphyllum were increased by heat processing, which led to enhanced cytotoxicity of heat-processed G. pentaphyllum. These findings may provide a potential approach for the development of therapeutic agents from natural products. Further study on the changes of other components of G. pentaphyllum by heat-processing is in progress.
Funding
This work was supported by program for Changjiang Scholars and Innovative Research Team in University (IRT0871) and NSFC [grant numbers 81181240306; 81274186].
Notes
Abbreviations: ELSD, evaporative light-scattering detector; HPLC, high-performance liquid chromatography; PDA, photodiode array; LOD, limit of detection; LOQ, limit of quantification; MS, mass spectrometry; RSD, relative standard deviation; SIR, single ion recording; S/N, signal-to-noise ratio; TQ, tandem quadrupole; UPLC-MS, ultra-high performance liquid chromatography with tandem mass spectrometry.
References
- Hu L, Chen Z, Xie Y. New triterpenoid saponins from Gynostemma pentaphyllum. J. Nat. Prod. 1996;59:1143–1145.10.1021/np960445u
- Liu X, Ye W, Mo Z, Yu B, Zhao S, Wu H, Che C, Jiang R, Mak TC, Hsiao WL. Five new Ocotillone-type saponins from Gynostemma pentaphyllum. J. Nat. Prod. 2004;67:1147–1151.10.1021/np034018+
- Piacente S, Pizza C, De Tommasi N, De Simone F. New dammarane-type glycosides from Gynostemma pentaphyllum. J. Nat. Prod. 1995;58:512–519.10.1021/np50118a005
- Yin F, Hu L, Lou F, Pan R. Dammarane-type glycosides from Gynostemma pentaphyllum. J. Nat. Prod. 2004;67:942–952.10.1021/np0499012
- Fang ZP, Zeng XY. Isolation and identification of flavonoids and organic acids from Gynostemma pentaphyllum Makino. Zhongguo Zhong Yao Za Zhi. 1989;14:676–678, 703.
- Song SL, Tang JB, Ji AG, Liang H, Zhu P, Wang WL. The extraction, purification and assaying of Gynostemma pentaphyllum polysaccharides. Zhong Yao Cai. 2006;29:595–598.
- Yang X, Zhao Y, Yang Y, Ruan Y. Isolation and characterization of immunostimulatory polysaccharide from an herb tea, Gynostemma pentaphyllum Makino. J. Agric. Food Chem. 2008;56:6905–6909.10.1021/jf801101u
- Zheng XJ. Composition analysis and dominance test of three kinds of raw variety of Gynostemma pentaphyllum. Zhongguo Zhong Yao Za Zhi. 2004;29:317–319.
- Wang P, Niu L, Guo XD, Gao L, Li WX, Jia D, Wang XL, Ma LT, Gao GD. Gypenosides protects dopaminergic neurons in primary culture against MPP(+)-induced oxidative injury. Brain Res. Bull. 2010;83:266–271.10.1016/j.brainresbull.2010.06.014
- Xie Z, Liu W, Huang H, Slavin M, Zhao Y, Whent M, Blackford J, Lutterodt H, Zhou H, Chen P, Wang TT, Wang S, Yu LL. Chemical composition of five commercial Gynostemma pentaphyllum samples and their radical scavenging, antiproliferative, and anti-inflammatory properties. J. Agric. Food Chem. 2010;58:11243–11249.10.1021/jf1026372
- Huang WC, Kuo ML, Li ML, Yang RC, Liou CJ, Shen JJ. Gynostemma pentaphyllum decreases allergic reactions in a murine asthmatic model. Am. J. Chin. Med. 2008;36:579–592.10.1142/S0192415X08005990
- Chen JC, Tsai CC, Chen LD, Chen HH, Wang WC. Therapeutic effect of gypenoside on chronic liver injury and fibrosis induced by CCl4 in rats. Am. J. Chin.Med. 2000;28:175–185.10.1142/S0192415X00000222
- Hesse C, Razmovski-Naumovski V, Duke CC, Davies NM, Roufogalis BD. Phytopreventative effects of Gynostemma pentaphyllum against acute indomethacin-induced gastrointestinal and renal toxicity in rats. Phytother. Res. 2007;21:523–530.10.1002/(ISSN)1099-1573
- Rujjanawate C, Kanjanapothi D, Amornlerdpison D. The anti-gastric ulcer effect of Gynostemma pentaphyllum Makino. Phytomedicine. 2004;11:431–435.10.1016/j.phymed.2003.07.001
- Megalli S, Aktan F, Davies NM, Roufogalis BD. Phytopreventative anti-hyperlipidemic effects of gynostemma pentaphyllum in rats. J. Pharm. Pharm. Sci. 2005;8:507–515.
- Megalli S, Davies NM, Roufogalis BD. Anti-hyperlipidemic and hypoglycemic effects of Gynostemma pentaphyllum in the Zucker fatty rat. J. Pharm. Pharm. Sci. 2006;9:281–291.
- Ky PT, Huong PT, My TK, Anh PT, Kiem PV, Minh CV, Cuong NX, Thao NP, Nhiem NX, Hyun JH, Kang HK, Kim YH. Dammarane-type saponins from Gynostemma pentaphyllum. Phytochemistry. 2010;71:994–1001.10.1016/j.phytochem.2010.03.009
- Lu KW, Tsai ML, Chen JC, Hsu SC, Hsia TC, Lin MW, Huang AC, Chang YH, Ip SW, Lu HF, Chung JG. Gypenosides inhibited invasion and migration of human tongue cancer SCC4 cells through down-regulation of NFkappaB and matrix metalloproteinase-9. Anticancer Res. 2008;28:1093–1099.
- Li L, Jiao L, Lau BH. Protective effect of gypenosides against oxidative stress in phagocytes, vascular endothelial cells and liver microsomes. Cancer Biother. 1993;8:263–272.10.1089/cbr.1993.8.263
- Piao XL, Wu Q, Yang J, Liu HM. A549 cell inhibitory activity from heat-processed Gynostemma pentaphyllum. J. Minzu Univ. Chin. 2012;21:49–53.
- Piao XL, Wu Q, Yang J, Park SY, Chen DJ, Liu HM. Dammarane-type saponins from heat-processed Gynostemma pentaphyllum show fortified activity against A549 cells. Arch. Pharm. Res. 2013;36:874–879.10.1007/s12272-013-0086-6
- Guo S, Duan JA, Tang Y, Qian Y, Zhao J, Qian D, Su S, Shang E. Simultaneous qualitative and quantitative analysis of triterpenic acids, saponins and flavonoids in the leaves of two Ziziphus species by HPLC-PDA-MS/ELSD. J. Pharm. Biomed. Anal. 2011;56:264–270.10.1016/j.jpba.2011.05.025
- Kao TH, Huang SC, Inbaraj BS, Chen BH. Determination of flavonoids and saponins in Gynostemma pentaphyllum (Thunb.) Makino by liquid chromatography-mass spectrometry. Anal. Chim. Acta. 2008;626:200–211.10.1016/j.aca.2008.07.049
- Kwon SW, Han SB, Park IH, Kim JM, Park MK, Park JH. Liquid chromatographic determination of less polar ginsenosides in processed ginseng. J. Chromatogr. A. 2001;921:335–339.10.1016/S0021-9673(01)00869-X
- Kokkonen M, Jestoi M. Determination of ergot alkaloids from grains with UPLC-MS/MS. J. Sep. Sci. 2010;33:2322–2327.10.1002/jssc.v33:15
- Ortega N, Romero MP, Macià A, Reguant J, Anglès N, Morelló JR, Motilva MJ. Obtention and characterization of phenolic extracts from different cocoa sources. J. Agric. Food Chem. 2008;56:9621–9627.10.1021/jf8014415
- Keum YS, Park KK, Lee JM, Chun KS, Park JH, Lee SK, Kwon H, Surh YJ. Antioxidant and anti-tumor promoting activities of the methanol extract of heat-processed ginseng. Cancer Lett. 2000;150:41–48.10.1016/S0304-3835(99)00369-9
- Kim WY, Kim JM, Han SB, Lee SK, Kim ND, Park MK, Kim CK, Park JH. Steaming of ginseng at high temperature enhances biological activity. J. Nat. Prod. 2000;63:1702–1704.10.1021/np990152b
- Liu HM, Yang J, Chen DJ, Piao XL. Isolation and characterization of gypenosides from Gynostemma pentaphyllum. J. Food Saf. Qual. 2013;4:283–288.