Abstract
A Vigna nakashimae (VN) extract has been shown to have antidiabetic and anti-obesity effects. However, the mechanism underlying the effect of a VN extract on hepatic inflammation and endoplasmic reticulum (ER) stress remains unclear. In the present study, we investigated how a VN extract protects against the development of non-alcoholic fatty liver disease (NAFLD). A VN extract for 12 weeks reduced the body weight, serum metabolic parameters, cytokines, and hepatic steatosis in high-fat diet (HFD)-fed mice. A VN extract decreased HFD-induced hepatic acetyl CoA carboxylase and glucose transporter 4 expressions. In addition to the levels of high-mobility group box 1 and receptor for advanced glycation, the hepatic expression of ATF4 and caspase-3 was also reduced by a VN extract. Thus, these data indicate that a chronic VN extract prevented NAFLD through multiple mechanisms, including inflammation, ER stress, and apoptosis in the liver.
Nonalcoholic fatty liver disease (NAFLD) is commonly associated with obesity and is accompanied by chronic inflammation and endoplasmic reticulum (ER) stress.Citation1) Obesity-induced insulin resistance, oxidative stress, ER stress, and inflammation have been shown to have synergistic effects on the development of NAFLD.Citation2) NAFLD is also closely linked to metabolic syndrome and diabetes mellitus.Citation3)
NAFLD is characterized by an increase in intrahepatic triglyceride (TG) that leads to damage to hepatocytes.Citation4) Chronic high-fat diet (HFD)-induced insulin resistance results in lipogenesis and an increased release of free fatty acid (FFA) that can be taken in by hepatocytes, consequently leading to hepatic fat storage and inflammation.Citation5) Hepatocyte-derived cytokines aggravate hepatic inflammation and result in apoptosis.Citation6) High-mobility group box 1 (HMGB1) has the capacity to induce pro-inflammatory cytokine release through both toll-like receptors (TLRs) and the receptor for advanced glycation end products (RAGE).Citation7) In particular, HFD-induced TLR4 signaling contributes to obesity-dependent insulin resistance.Citation8) However, the role of hepatic RAGE levels in the pathogenesis of NAFLD remains unclear.
In addition to hepatic inflammation, the ER is emerging as an important organelle in the regulation of lipid metabolism in hepatocytes. ER stress has been observed in NAFLD.Citation9) In obese and NAFLD humans and rodent models, the induction of ER stress is linked to hepatic insulin resistance and inflammation in obesity.Citation10,11) Upon ER stress, activating transcription factor 4 (ATF4) activates numerous ER stress response genes.Citation12) However, sustained or massive ER stress leads to apoptosis.Citation13)
Species in the genus Vigna include important foods in Asia and Africa. They are rich sources of protein, carbohydrates, oil, potassium, and vitamin B. Recent studies have demonstrated that Vigna species, Vigna mungo and Vigna angularis, had antihyperglycemic effects and improved cholesterol in diabetic rats and HFD-fed mice.Citation14,15) In particular, Vigna nakashimae (VN), which is cultivated in South Korea, has been reported to have respective anti-obesity and antidiabetic effects on HFD-fed mice and db/db mice.Citation16,Citation17) Yeo et al. have demonstrated that VN dose-dependently inhibited α-glucosidase activity compared with the effect of acarbose.Citation16) Saponin from V. mungo (L.) Hepper has been shown to have a hypolipidemic effect on type 2 diabetic rats.Citation18,19) Although it has been established that VN had anti-obesity and antidiabetic effects, the pathological mechanisms acting in NAFLD are less clear.
We investigated in the present study the protective effects of a long-term VN extract administration on hepatic inflammation and apoptosis in mice with HFD-induced obesity. We evaluated the anti-inflammatory effects of VN through HMGB1/RAGE signaling and its anti-apoptotic effects through ER stress-mediated hepatocyte death in NAFLD.
Materials and methods
Animals and obesity model
Male C67BL/6J mice (3-week old) were purchased from Koatech (Pyeongtaek, South Korea) and maintained in the animal facility at Gyeongsang National University. The experiments were performed in accordance with the National Institutes of Health Guidelines on the Use of Laboratory Animals. The University Animal Care Committee for Animal Research of Gyeongsang National University approved the study protocol (GLA-101104-M0108). The mice were individually housed with an alternating 12-h light/dark cycle. The mice, starting at four weeks of age, were randomly assigned to three groups (n = 10 mice per group). The mice were fed for 12 weeks with either an HFD (45%, Research Diets, New Brunswick, NJ, USA) or standard diet (normal diet, ND). Based on previous studies, the mice were dosed daily with 250 mg/kg of the VN extract in either the ND or HFD feed.Citation16) The mice were weighed three times monthly and just prior to their sacrifice at 16 weeks of age.
Plant material
VN (IT178464) was grown in an experimental field of the National Institute of Crop Science, RDA (Miryang, South Korea) in 2010.
Preparation of the VN extract
Dried seeds of VN (1 kg) were pulverized with a grinder and extracted with 10 L of 80% ethanol for 3 d while continuously shaking at room temperature and then filtered. The 80% ethanol extract was concentrated in vacuum at 40 °C to give a brownish residue. The crude residue was then freeze-dried and ground into powder (98 g). The extract of VN was analyzed with an Acquity UPLC system coupled on-line with a triple quadrupole mass spectrometric detector (Waters, MA, USA). We identified four saponin peaks (soyasaponin Bb, AzII, soyasaponin αg, and AzIII) from the extract of VN by mass spectrometry.
Glucose tolerance test (GTT) and insulin tolerance test (ITT)
The mice were fasted overnight (16 h) before GTT. D-Glucose (2 g/kg; Sigma-Aldrich, St. Louis, MO, USA) was intraperitoneally injected, and blood samples were taken before and 30, 60, 90, and 120 min after injecting glucose. The blood glucose levels were measured with an Accu-Chek glucometer (Roche Diagnostics, Mannheim, Germany). ITT was performed on the mice at around 2 pm. The mice were injected with Humulin-R insulin (0.75 U/kg; Eli Lilly, Indianapolis, IN, USA) in 0.1 mL of 0.9% normal saline. A drop of blood was taken from the tail vein before and 15, 30, 45, and 60 min after injecting the insulin for determining the blood glucose levels with the Accu-Chek glucometer.
Measurement of the serum metabolic parameters
To analyze the serum, all mice were intramuscularly anesthetized with zoletil (5 mg/kg; Virbac Laboratories, Carros, France). Blood samples (n = 10 mice per group) were transcardially extracted through the apex of the left ventricle with a 1-mL syringe and allowed to clot for 2 h at room temperature. After centrifugation, the serum samples were removed and stored at −80 °C until needed for analysis. The serum AST, ALT, FFA, total cholesterol, and TG levels were determined by using enzymatic colorimetric assays from Green Cross Reference Laboratory (Yongin-si, South Korea). The serum insulin, leptin, IL-1β, and IL-6 levels were determined by using the Bio-Plex Suspension Array System (Bio-Rad Laboratories, CA, USA).
Liver TG colorimetric assay
After extracting the serum, the livers were stored at −80 °C until needed for analysis. The liver TG concentrations (n = 6 mice per group) were measured by using the TG colorimetric assay kit (Cayman Chemical Company, Ann Arbor, MI, USA) according to the manufacturer’s protocol.
Tissue collection and sample preparation
To analyze the tissue, mice (n = 4 per group) were anesthetized with zoletil (Virbac Laboratories) and then transcardially perfused with heparinized saline and the with 4% paraformaldehyde in 0.1 mL of PBS. Six hour after postfixing in the same fixative, the livers were processed for paraffin embedding and sectioned (5 μm).
Oil Red O staining
Oil Red O staining is commonly used to identify lipid deposits. To determine the hepatic lipid accumulation, frozen 5-μm sections of the liver were stained for 10 min with 0.5% Oil Red O (Sigma), washed, and then counter-stained for 45 s with Mayer’s hematoxylin (Sigma). The sections were visualized under a BX51 optical microscope (Olympus, Tokyo, Japan), and digital images were captured and documented.
Western blot analysis
To extract the protein, frozen liver tissue was homogenized in a lysis buffer. The antibodies specific to the following targets were used: acetyl CoA carboxylase (ACC) and cleaved caspase-3 from Cell Signaling Technology (Danvers, MA, USA), glucose transporter 4 (GLUT4) and HMGB1 from Abcam (Cambridge, UK), and ATF4 and RAGE from Santa Cruz Biotechnology (Santa Cruz, CA, USA). The membranes were probed with each antibody or anti-β-actin (Sigma) and then visualized by using an enhanced chemiluminescence substrate (Pierce, Rockford, IL, USA). The Multi-Gauge v3.0 image analysis program (Fujifilm, Tokyo, Japan) was used to measure the band density.
Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay
A TUNEL analysis was performed by using an in situ cell death detection kit (Roche Molecular Biochemicals, Mannheim, Germany) according to the manufacturer’s protocol.
Statistics
Differences between ND and HFD with or without VN supplementation were determined by two-way ANOVA and a subsequent Bonferroni post hoc analysis. Values are expressed as the mean ± standard error of the mean (SEM). p values less than 0.05 were considered statistically significant.
Results
Effects of the VN extract on the body weight and blood glucose in the HFD-fed mice
To examine the anti-obesity effect of VN on the HFD-fed mice, we measured the body weight during the 12 weeks of supplementation. Fig. (A) shows that the body weight of the HFD-fed mice gradually increased when compared to the normal diet (ND)-fed mice, whereas the body weight of the HFD-fed mice was significantly reduced by the VN extract during the final four weeks (p < 0.05). In addition, we found that the weights of the liver and epididymal fat pads in the HFD-fed mice were also reduced by VN supplementation (Table ). To determine the effect of the VN extract on the blood glucose levels in the mice fed with HFD, we measured the fasting blood glucose levels every 4 weeks. After eight weeks, there was a significant increase of blood glucose in the HFD-fed mice when compared with the ND-fed mice (Fig. (B)).
Fig. 1. Effects of the VN extract on body weight and blood glucose in HFD-fed mice.
Notes: (A) Body weight. The HFD-fed mice with VN had significantly lower body weight than the HFD-fed mice after 12 weeks. (B) Blood glucose. During the final 4 weeks, the HFD-fed mice with VN had significantly lower fasting blood glucose levels than the HFD-fed mice. Data (n = 10 mice per group) are presented as the mean ± SEM.
*p < 0.05 vs. ND mice; †p < 0.05 vs. HFD mice.
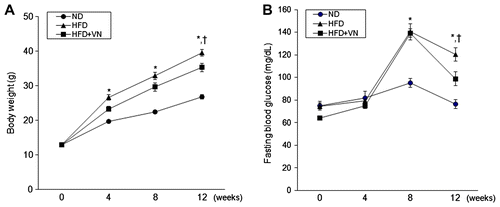
Table 1. Effects of extract of VN on body, liver, and epididymal fat weight in HFD-fed mice.
Effects of the VN extract on insulin resistance in the HFD-fed mice
To determine whether the VN extract exerted an antidiabetic effect on HFD-induced obesity, we performed insulin and glucose tolerance tests (Fig. ). The HFD-fed mice were significantly more glucose intolerant following a 12-week HFD challenge (Fig. (A)). However, administering the VN extract enhanced the glucose tolerance. Consistent with the effects of VN on glucose tolerance, the reduction in glucose levels during ITT was greater in the HFD-fed mice with the VN extract (Fig. (B)). Hyperinsulinemia and hyperleptinemia in the HFD-fed mice were significantly reduced by VN supplementation (Table ).
Fig. 2. Effects of the VN extract on glucose and insulin tolerance in HFD-fed mice.
Notes: (A) Blood glucose levels after injecting D-Glucose (2 g/kg) in the ND-, HFD-, and HFD + VN-fed mice. (B) Blood glucose levels after the insulin treatment (0.75 U/kg). The blood glucose levels of the HFD + VN-fed mice were significantly lower than those of the HFD-fed mice. Data (n = 10 mice per group) are presented as the mean ± SEM.
*p < 0.05 vs. ND mice; †p < 0.05 vs. HFD mice.
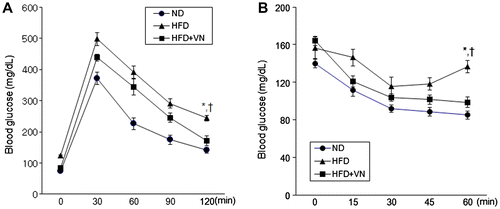
Table 2. Effects of Extract of VN on Serum Metabolic Parameters in HFD-Fed Mice.
Effects of the VN extract on serum cytokines and metabolic parameters in the HFD-fed mice
We measured the serum concentrations of IL-1β and IL-6 by ELISA (Table ) which were significantly increased in the HFD-fed mice when compared to the ND-fed mice. However, the levels of these inflammatory cytokines were reduced by the VN administration. Furthermore, to investigate if the VN extract affected the hepatic function in the HFD-fed mice, we measured the serum levels of AST and ALT by ELISA (Table ). The concentrations of these two hepatic enzymes were higher in the HFD-fed mice than in the ND-fed mice, whereas treating with VN resulted in a significant decrease (p < 0.05). The serum total cholesterol, FFA, and TG levels in the obese mice were also significantly lower after the VN administration (p < 0.05). However, the serum FFA and TG levels in the HFD-fed mice did not significantly increase when compared to those in the ND-fed mice (Table ).
Effect of the VN extract on hepatic steatosis in the HFD-fed mice
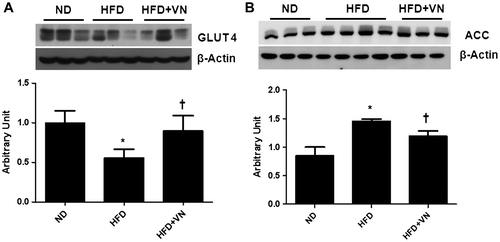
Hematoxylin and eosin and Oil Red O staining revealed that the hepatocytes of the HFD-fed mice were distended with lipid droplets (Fig. (A)). The VN treatment reduced these lipid droplets in the liver of the HFD-fed mice. Furthermore, the effect of the VN extract on the hepatic TG level in the HFD-treated mice was confirmed by using the liver TG colorimetric assay (Fig. (B)). As was apparent by Oil Red O staining, the HFD-fed mice had significantly more TG than the ND-fed mice. After the VN administration, a significant decrease in the quantity of TG was apparent (p < 0.05). To investigate the effect of the VN extract on hepatic GLUT4 and ACC expression, the expression levels of these proteins were assessed by western blot ting (Fig. ). After the long-term VN extract treatment, the GLUT4 level was significantly increased in the liver of the HFD-fed mice (Fig. (A)), while the ACC level was significantly decreased (Fig. (B)).
Fig. 3. Effect of the VN extract on hepatic steatosis in HFD-fed mice.
Notes: (A) Representative microphotographs of hematoxylin and eosin and Oil Red O staining of the hepatic lipid accumulation. The scale bar shows 100 μm. (B) Concentration of hepatic TG. Data (n = 6 mice per group) are presented as the mean ± SEM.
*p < 0.05 vs. ND mice; †p < 0.05 vs. HFD mice.
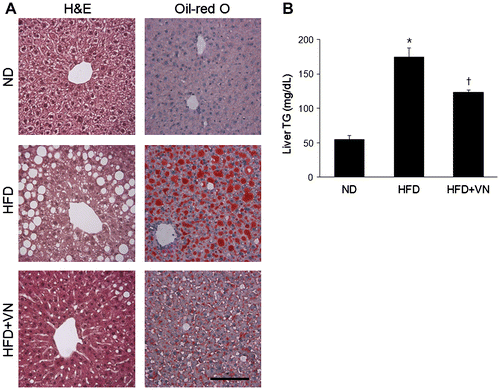
Fig. 4. Effect of the VN extract on hepatic energy metabolism in HFD-fed mice.
Notes: (A) Western blotting for GLUT 4 expression in the liver of each group (n = 3–4 mice per group). Quantification of GLUT4 expression from the Western blotting analysis. (B) Western blotting for ACC expression in the liver of each group. Quantification of ACC expression from the Western blotting analysis corrected to its basal level. Data are presented as the mean ± SEM.
*p < 0.05 vs. ND mice; †p < 0.05 vs. HFD mice.
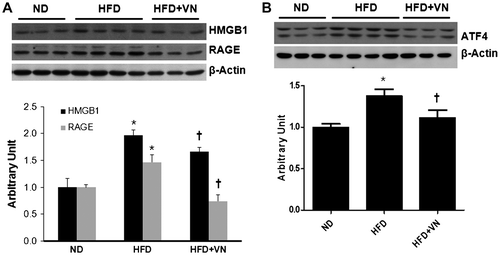
Effect of the VN extract on hepatic inflammation and ER stress in the HFD-fed mice
The effect of the VN extract on hepatic HMGB1 and RAGE expression was evaluated in HFD-fed mice by western blotting. Hepatic HMGB1 and RAGE expression was significantly higher in the HFD-fed mice than in the ND-fed mice, whereas the VN extract significantly decreased HMGB1 and RAGE expression (p < 0.05) (Fig. (A)). Western blotting revealed that the hepatic ATF4 expression level in response to ER stress was significantly higher in the HFD-treated mice than in the ND-fed mice (Fig. (B)). However, ATF4 expression was significantly decreased by the VN treatment (p < 0.05).
Fig. 5. Effects of the VN extract on hepatic inflammation and ER stress in HFD-fed mice.
Notes: (A) Western blotting for the hepatic HMGB1 and RAGE expression levels in each group (n = 3–4 mice per group). Quantification of the HMGB1 and RAGE expression levels from the western blotting analysis. (B) Western blotting for hepatic ATF4 expression in each group. Quantification of ATF4 expression from the Western blotting analysis. Data are presented as the mean ± SEM.
*p < 0.05 vs. ND mice; †p < 0.05 vs. HFD mice.
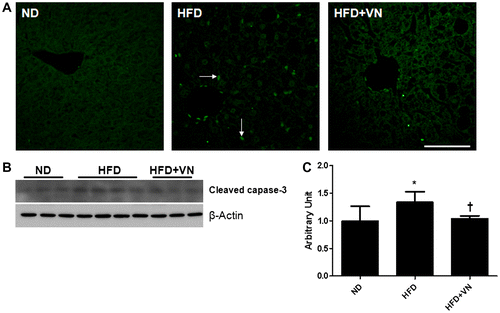
Effect of the VN extract on hepatic apoptosis in the HFD-fed mice
To investigate the effect of the VN extract on HFD-induced hepatic apoptosis, a TUNEL assay and western blotting analysis of the cleaved caspase-3 levels were performed (Fig. ). We found that TUNEL-positive cells were distributed throughout the hepatic tissue of the HFD-fed mice, whereas the number of apoptotic cells was reduced by administering the VN extract (Fig. (A)). Western blotting revealed that the hepatic cleaved caspase-3 expression level was significantly higher in the HFD-treated mice than in the ND-fed mice (Fig. (B) and (C)). However, the cleaved caspase-3 level was significantly decreased by the VN treatment (p < 0.05).
Fig. 6. Effect of the VN extract on hepatic apoptosis in HFD-fed mice.
Notes: (A) Representative microphotographs showing the TUNEL analysis. Arrows indicate TUNEL-positive cells. The scale bar shows 50 μm. (B) Western blotting for hepatic cleaved caspase-3 expression in each group (n = 3–4 mice per group). (C) Quantification of cleaved caspase-3 expression from the Western blotting analysis. Data are presented as the mean ± SEM.
*p < 0.05 vs. ND mice; †p < 0.05 vs. HFD mice.
Discussion
NAFLD is emerging as one of the most common liver diseases and accelerates inflammation and insulin resistance.Citation20) Recent studies have reported that VN had antidiabetic and anti-obesity effects by inhibiting α-glucosidase activity and the peroxisome proliferator-activated receptor in db/db mice with a 15-d supplementation regimen, and in HFD-fed mice with a 40-day supplementation regimen.Citation16,17) We found in the present study that long-term VN administration ameliorated hepatic steatosis and inflammation by inhibiting HMGB1 and RAGE expression in HFD-fed mice with a 12-week supplementation regimen. Administration of the VN extract significantly reduced the body weight and insulin resistance in HFD-fed mice (p < 0.05). In addition, serum hepatic enzymes, liver TG, and pro-inflammatory cytokines were reduced by VN supplementation. These findings indicate that the VN extract may be useful for preventing and treating obesity-induced NAFLD.
High hepatic cholesterol and serum FFA levels are risk factors for insulin resistance, hepatic steatosis, and liver fibrosis.Citation21) Serum FFA released by excessive visceral fat is transported to the liver and contributes to hepatic steatosis, insulin resistance, and inflammation.Citation22,23) An accumulation of cholesterol in hepatocytes plays an important role in lipid-induced hepatic inflammation.Citation24,25) HFD-induced hepatic lipid droplets and the TG level were reduced in the current study by VN supplementation. Supplementation also reduced the levels of serum cytokines, including IL-1β and IL-6. In patients with NAFLD, the levels of pro-inflammatory cytokines TNF-α and IL-6 are elevated when compared with normal subjects.Citation26) These cytokines stimulate hepatic lipogenesis and impair insulin signaling.Citation27) IL-1β-deficient mice have exhibited less hepatic inflammation and steatosis than wild-type mice.Citation5) Circulating IL-6 is associated with insulin resistance and the impairment of insulin signaling in hepatocytes.Citation28) Our findings are consistent with evidence that the serum TG, IL-6, TNF-α, and liver TG levels were reduced by VN supplementation.Citation17) Furthermore, the serum leptin level was increased in patients with obesity and insulin resistance.Citation29) However, this trend was reversed by the VN treatment, removing a major driver of NAFLD. Our data therefore show that the VN extract prevented an elevation in HFD-induced inflammatory cytokines and in the hepatic TG level and enhanced insulin tolerance.
As well as lipid accumulation in the liver, several other mechanisms are involved in the development of NAFLD.Citation21) The HMGB1 and RAGE levels in this present study were particularly elevated in the HFD-fed mice and reduced by VN supplementation. Recently established as a pro-inflammatory mediator, HMGB1 has been associated with many inflammatory diseases, including NAFLD.Citation6,30) An increased FFA level and gut-derived endotoxin in NAFLD have elevated HMGB1 expression.Citation30,Citation31) HMGB1 activates TLR4 signaling in hepatocytes at the onset of NAFLD.Citation32) A previous study has demonstrated that TLR4 deficiency significantly inhibited body weight gain compared with HFD-fed wild-type mice.Citation6) RAGE signaling plays an important role in regulating inflammatory cytokines, oxidative stress, and the endothelial dysfunction in type 2 diabetes.Citation33,34) We found that hepatic RAGE expression was significantly induced by HFD, whereas this induction was significantly inhibited by the VN extract (p < 0.05). This result is consistent with a recent finding that pentoxifylline alleviated HFD-induced NAFLD in rats by inhibiting RAGE expression.Citation7) The activation of RAGE by AGE induces pro-inflammatory molecules through NF-κB signaling, including vascular cell adhesion molecule-1, TNF-α, and IL-6.Citation35) Although the precise molecular mechanisms by which the VN extract attenuated inflammation in NAFLD was not investigated, the results of this study suggest that the VN extract reduced hepatic inflammation by decreasing HMGB1 and RAGE expression.
Long-term VN supplementation reduced lipid accumulation in the liver of the HFD-fed mice. This effect is mediated by the inhibition of ACC. In our previous study, α-lipoic acid was shown to increase ACC expression in OLETF rats.Citation36) This finding is also consistent with the inactivation of ACC by the VN extract.Citation17) In addition to ACC, GLUT4 plays an important role in maintaining glucose homeostasis.Citation37) The impairment of glucose uptake by hepatocytes in obesity is closely associated with the reduction of cellular GLUT4 expression.Citation38) The decreased expression of GLUT4 was reversed in the VN extract-treated HFD-fed mice, and the blood glucose levels were well controlled. The data indicate that the VN extract-treated obese mice had significantly improved insulin sensitivity and glucose utilization through the elevation of GLUT4 expression.
ER stress is an important link between hepatic steatosis, insulin resistance, and the metabolic syndrome.Citation39) ER stress in type 2 diabetes can be induced by the overproduction of protein to process excessively supplied materials like lipids and glucose.Citation40) An association between ER stress signaling and NAFLD has been proven through the genetic modulation of eukaryotic initiation factor 2 alpha (elF2α).Citation41) As a downstream target of elF2α, ATF4 transcriptionally activates ER stress response genes, including those genes responsible for apoptosis.Citation11) In another study, a VN extract dose-dependently reduced the ER stress induced by thapsigargin in HepG2 cells.Citation16) Our study also confirms that the VN extract attenuated HFD-induced ATF4 expression in the liver (p < 0.05). Saturated fatty acid-induced ER stress and apoptosis in human hepatocytes occur through ATF4 signaling.Citation42) In patients with NAFLD, there has been a highly significant increase in TUNEL-positive cells when compared with normal subjects.Citation43) This is consistent with our result that the HFD-induced cleaved caspase-3 level was reduced by the VN extract. These data therefore indicate that the VN extract may have protected hepatocytes from apoptosis by inhibiting HFD-induced ER stress.
In conclusion, a long-term VN extract administration prevented NAFLD by preventing hepatic steatosis, inflammation, ER stress, and apoptosis. The VN extract might therefore be useful for treating or preventing NAFLD by targeting various pathways in the progression of NAFLD.
Acknowledgments
This study was carried out with the support of the Cooperative Research Program for Agriculture Science and Technology Development (project No. PJ00900001) of the Rural Development Administration, Republic of Korea. No competing financial interests exist.
Notes
Abbreviations: ACC, acetyl CoA carboxylase; ATF4, activating transcription factor 4; ER, endoplasmic reticulum; FFA, free fatty acid; GLUT4, glucose transporter 4; HFD, high-fat diet; HMGB1, high-mobility group box 1; NAFLD, nonalcoholic fatty liver disease; RAGE, receptor for advanced glycation end products; TLR, Toll-like receptor; VN, Vigna nakashimae.
References
- Angulo P. N. Engl. J. Med. 2002;346:1221–1231.10.1056/NEJMra011775
- Day CP. Clin. Med. 2006;6:19–25.10.7861/clinmedicine.6-1-19
- Cusi K. Clin. Liver Dis. 2009;13:545–563.10.1016/j.cld.2009.07.009
- Fabbrini E, Sullivan S, Klein S. Hepatology. 2010;51:679–689.10.1002/hep.23280
- Kamari Y, Shaish A, Vax E, Shemesh S, Kandel-Kfir M, et al. J. Hepatology. 2011;55:1086–1094.10.1016/j.jhep.2011.01.048
- Li L, Chen L, Hu L, Liu Y, Sun HY, et al. Hepatology. 2011;54:1620–1630.10.1002/hep.24552
- Wu J, Meng Z, Jiang M, Zhang E, Trippler M, et al. Immunology. 2010;129:363–374.10.1111/imm.2010.129.issue-3
- Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. J. Clin. Invest. 2006;116:3015–3025.10.1172/JCI28898
- Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, Tuncman G, Görgün C, Glimcher LH, Hotamisligil GS. Science. 2004;306:457–461.10.1126/science.1103160
- Boden G, Duan X, Homko C, Molina EJ, Song W, Perez O, Cheung P, Merali S. Diabetes. 2008;57:2438–2444.10.2337/db08-0604
- Malhi H, Kaufman RJ. J. Hepatol. 2011;54:795–809.10.1016/j.jhep.2010.11.005
- Hai T, Hartman MG. Gene. 2001;273:1–11.10.1016/S0378-1119(01)00551-0
- Galehdar Z, Swan P, Fuerth B, Callaghan SM, Park DS, Cregan SP. J. Neurosci. 2010;30:16938–16948.10.1523/JNEUROSCI.1598-10.2010
- Solanki YB, Jain SM. Pharm. Biol. 2010;48:915–923.10.3109/13880200903406147
- Itoh T, Furuichi Y. Nutrition. 2009;25:318–321.10.1016/j.nut.2008.08.011
- Yeo JY, Ha TJ, Nam JS, Jung MH. Biosci. Biotechnol. Biochem. 2011;75:2223–2228.10.1271/bbb.110538
- Son Y, Nam JS, Jang MK, Jung IA, Cho SI, Jung MH. Biosci. Biotechnol. Biochem. 2013;77:332–338.
- Lee MR, Chen CM, Hwang BH, Hsu LM. J. Mass Spectrom. 1999;34:804–812.10.1002/(ISSN)1096-9888
- Zheng T, Shu G, Yang Z, Mo S, Zhao Y, Mei Z. J. Ethnopharmacol. 2012;139:814–821.10.1016/j.jep.2011.12.025
- Sakurai M, Takamura T, Ota T, Ando H, Akahori H, et al. J. Gastroenterol. 2007;42:312–317.10.1007/s00535-006-1948-
- Krawczyk M, Bonfrate L, Portincasa P. Best Pract. Res. Clin. Gastroenterol. 2010;24:695–708.10.1016/j.bpg.2010.08.005
- Sanyal AJ, Campbell-Sargent C, Mirshahi F, Rizzo WB, Contos MJ, Sterling RK, Luketic VA, Shiffman ML, Clore JN. Gastroenterology. 2001;120:1183–1192.
- Franks PW, Brage S, Luan J, et al. Obes. Res. 2005;13:1476–1484.10.1038/oby.2005.178
- Matsuzawa N, Takamura T, Kurita S, Misu H, Ota T, et al. Hepatology. 2007;46:1392–1403.10.1002/hep.v46:5
- Wouters K, van Bilsen M, van Gorp PJ, Bieghs V, Lütjohann D, Kerksiek A, Staels B, Hofker MH, Shiri-Sverdlov R. FEBS Lett. 2010;584:1001–1005.10.1016/j.febslet.2010.01.046
- Das SK, Balakrishnan V, Indian J. Indian J. Clin. Biochem. 2011;26:202–209.10.1007/s12291-011-0121-7
- Hotamisligil GS. Int. J. Obes. Relat. Metab. Disord. 2003;27:S53–S55.10.1038/sj.ijo.0802502
- Senn JJ, Klover PJ, Nowak IA, Mooney RA. Diabetes. 2002;51:3391–3399.10.2337/diabetes.51.12.3391
- Pardina E, Ferrer R, Baena-Fustegueras JA, et al. Obes. Surg. 2010;20:623–632.10.1007/s11695-010-0103-5
- Ogborne RM, Rushworth SA, O’Connell MA. Arterioscler. Thromb. Vasc. Biol. 2005;25:2100–2105.10.1161/01.ATV.0000183745.37161.6e
- Klune JR, Dhupar R, Cardinal J, Billiar TR, Tsung A. Mol. Med. 2008;14:476–484.
- Rivera CA, Adegboyega P, van Rooijen N, Tagalicud A, Allman M, Wallace M. J. Hepatol. 2007;47:571–579.10.1016/j.jhep.2007.04.019
- Flyvbjerg A, Denner L, Schrijvers BF, Tilton RG, Mogensen TH, Paludan SR, Rasch R. Diabetes. 2004;53:166–172.10.2337/diabetes.53.1.166
- Gao X, Zhang H, Schmidt AM, Zhang C. Am. J. Physiol. Heart Circ. Physiol. 2008;295:H491–H498.10.1152/ajpheart.00464.2008
- Valencia JV, Mone M, Koehne C, Rediske J, Hughes TE. Diabetologia. 2004;47:844–852.
- Jung TS, Kim SK, Shin HJ, Jeon BT, Hahm JR, Roh GS. Liver Int. 2012;32:1565–1573.10.1111/liv.2012.32.issue-10
- Zisman A, Peroni OD, Abel ED, Michael MD, Mauvais-Jarvis F, et al. Nat. Med. 2000;6:924–928.
- Park SY, Cho YR, Kim HJ, Higashimori T, Danton C, et al. Diabetes. 2005;54:3530–3540.10.2337/diabetes.54.12.3530
- Anderson CD, Upadhya G, Conzen KD, Jia J, Brunt EM, et al. Liver Transpl. 2011;17:189–200.10.1002/lt.v17.2
- Back SH, Kaufman RJ. Annu. Rev. Biochem. 2012;81:767–793.10.1146/annurev-biochem-072909-095555
- Oyadomari S, Harding HP, Zhang Y, Oyadomari M, Ron D. Cell Metab. 2008;7:520–532.10.1016/j.cmet.2008.04.011
- Cao J, Dai DL, Yao L, Yu HH, Ning B, et al. Mol. Cell Biochem. 2012;364:115–129.10.1007/s11010-011-1211-9
- Puri P, Mirshahi F, Cheung O, Natarajan R, Maher JW, Kellum JM, Sanyal AJ. Gastroenterology. 2008;134:568–576.10.1053/j.gastro.2007.10.039
