Abstract
We demonstrate that 0.78 mm glyceric acid activated the proliferation of human dermal fibroblasts by about 45%, whereas 34 mm α-glucosylglyceric acid (GGA) increased collagen synthesis by the fibroblasts by 1.4-fold compared to that in the absence of GGA. The two substances also exerted protective effects on both DNA scission by the hydroxyl radical and protein aggregation by heat in vitro.
Glyceric acid (2,3-dihydroxypropionic acid; GA, Fig. (A)) is a natural organic acid and a minor constituent of some plants.Citation1) GA and its derivatives have such biological activities as accelerating ethanol and acetaldehyde metabolism in rats,Citation2) antitrypsin activity,Citation3) and activating ethanol-dosed gastric cells.Citation4) However, few studies on the application of GA have been conducted because GA production is limited by the high cost of catalytic synthesis. Our recent research has shown that GA can be mass produced from glycerol by acetic acid-bacterial fermentationCitation5–7); the yield reached 136 g/L after a 6-d fermentation. Further applications of GA as well as its derivatives can therefore be developed to expand its commercial production and uses.
Of the promising GA derivatives, α-glucosylglycerate (GGA, see Fig. (B)) is an anionic compatible solute comprising α-glucose and d-glycerate, and is found in cyanobacteria, archaea, and some γ-proteobacteria.Citation8,9) Compatible solutes are small organic molecules that accumulate in micro-organisms and plants to balance osmotic pressure and to protect the functions of such biological molecules as DNA and proteins.Citation10,11) Some of these solutes, e.g. polyols, carbohydrates, amino acids, and phosphates, have been identified, and their physiological properties as well as biosynthetic mechanisms have been investigated.Citation12,13) GGA is a rare solute compared to such others as trehalose, sucrose, glycinebetaine, and glucosylglycerol, and is preferentially accumulated under high-salt and nitrogen-limited conditions in GGA-accumulating micro-organisms. In respect to cyanobacteria, a nitrogen-limited condition was favorable for GGA accumulation which can be used in place of glutamate to counteract the effects of inorganic ions.Citation14)
The in vitro functions of compatible solutes in terms of protecting biological molecules are another important aspect.Citation15,16) Ectoine, a commercially available compound produced by bacterial fermentation, exhibits broad-spectrum protection of biological molecules, cells, and skin, such as enzyme-stabilizing effects against heat and freeze-thawing,Citation17) inhibition of insulin amyloid formation,Citation18) and prevention of UVA-induced photoaging.Citation19) Mannosylglycerate is a commercial product that protects proteins.Citation20) Therefore, other compatible solutes also have strong application potential in the food, cosmetic, toiletry, and pharmaceutical industries. However, their complex structures are barriers to their economical synthesis, resulting in few evaluations of these solutes having been conducted.Citation21)
The enzymatic synthesis of glucosylglycerol and GGA has recently been achieved via transglycosylation, using sucrose phosphorylase as a catalyst.Citation22,23) This conventional method for region- and enantio-selective synthesis of GGA prompted us to evaluate a novel application of GA. As the first step for applying GGA as a cosmetic ingredient to protect cells and biological molecules from some stress, we therefore investigated the effects of GA and GGA on the cellular proliferation and protection of DNA and protein in vitro.
Sodium d-glycerate (GA-Na) was prepared from calcium d-glycerate dihydrate (Sigma–Aldrich, St. Louis, MO, USA) by passing through Cl− anion-exchange resin (Dowex, Dow Chemicals, Germany), before eluting with 0.5 M sodium chloride. Sodium glucosylglycerate (GGA-Na) was synthesized from sucrose (Wako Pure Chemicals, Osaka, Japan) and calcium d-glycerate dihydrate by sucrose phosphorylase (Oriental Yeast, Osaka, Japan), as previously reported.Citation23) The purity and structure were confirmed by thin-layer chromatography (TLC), nuclear magnetic resonance (NMR), and electro-spray ionization-mass spectrometry (ESI-MS). TLC was performed by using Silica gel 60 F254 (Merck, Darmstadt, Germany) with an ethyl acetate/methanol/acetic acid/water (4/3/3/1, by vol.) solvent system to develop the chromatogram. The developed substances were visualized by dipping the plate in a 5% H2SO4-methanol solution and then heating at 120 °C for 5 min to visualize the spots of sugar derivatives. The 400 MHz 1H- and 100 MHz 13C-NMR data of synthesized GGA dissolved in deuterium oxide were recorded by using an AV-400 NMR spectrometer (Bruker, Karlsruhe, Germany). ESI-MS data were recorded by a Shimadzu LC/MS 2020 instrument without column separation. A 90:10 mixture of 0.1% formic acid: acetonitrile was used as the mobile phase at 0.2 mL/min for sample injection.
The proliferation of normal human dermal fibroblasts (NHDF) by GA-Na and GGA-Na was evaluated. NHDF was an original cell line provided by Adjusted Cell Experiment Laboratory (Sagamihara, Japan). NHDF was grown in Dulbecco’s modified Eagle’s medium (DMEM; Nacalai Tesque, Kyoto, Japan) containing 1% fetal bovine serum (FBS; Invitrogen, Carlsbad, CA, USA). Cells (5 × 103/100 μL per well) in a 96-well plate were cultivated for 24 h at 37 °C in a 5% CO2 atmosphere to reach 50% confluence. The culture was then transferred to the growth medium (DMEM + 1% FBS) containing a test substance and incubated for an additional 24 h. After incubation, the viable cells were enumerated using a viability test reagent (Cell Count Reagent SF; Nacalai Tesque) as previously reported.Citation24) Fig. (A) shows the presence of 0.078–7.8 mm GA-Na proliferated NHDF cells, with 0.78 mm GA-Na showing the greatest proliferation of NHDF by 45% compared to the no-addition control. A further increase in the GA-Na concentration (to 156 mM) induced severe toxicity to the cells. GGA-Na did not significantly proliferate NHDF at the concentrations tested (Fig. (C)). Such α-hydroxy acids as glycolic acid and lactic acid have had little or no effect on the proliferation of human dermal fibroblasts,Citation25) suggesting that the multihydroxy groups in GA were involved in the fibroblast activation. β-Glucosides like cellobiose activated fibroblasts at about 2.5%,Citation26) whereas around 1% trehalose had no effect on the growth of fibroblasts.Citation27) Despite GGA having a GA moiety, no significant effect of GGA on the proliferation of fibroblasts was therefore apparent, because GA binds to glucose by the α-glucoside bond.
Fig. 2. Effects of GA-Na (A and B) and GGA-Na (C and D) on NHDF proliferation and the amount of type I collagen by NHDF.
Notes: A and C, NHDF proliferation was evaluated by incubating with appropriate concentrations of GA-Na or GGA-Na for 24 h at 37 °C in a 5% CO2 atmosphere. The cell viability of the control (with no addition of a test substance) is expressed as 100%. B and D, The amount of type I collagen was examined by incubating GA-Na or GGA-Na at appropriate concentrations for 72 h at 37 °C in a 5% CO2 atmosphere. The amount of collagen produced by NHDF in the absence of a test substance (6.58 μg/mL in the culture supernatant) is expressed as 100%. ND, not determined. All values are expressed by the mean and standard error (n = 5). The statistical significance of the data was calculated by Dunnet’s multiple comparison test, and a level of p < 0.05 vs. the no-addition control was considered statistically significant, as shown by *.
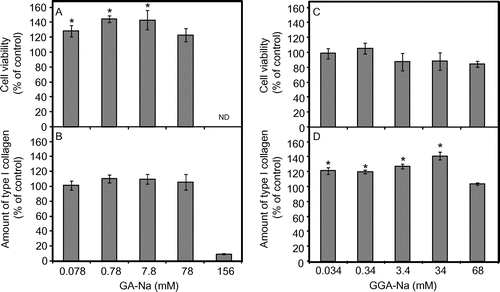
We next examined the response of NHDF in terms of collagen synthesis. The amount of type I collagen was assayed by incubating NHDF with a test substance at an appropriate concentration in the growth medium (DMEM + 1% FBS) for 72 h. After incubation, the collagen level in the culture was determined by using a Human Collagen Type I ELISA kit (Adjusted Cell Experiment Laboratory) according to the supplier’s instructions. Fig. (B) shows that NHDF proliferated by 0.078–7.8 mm GA-Na exhibited no increase in the amount of type I collagen when compared to the no-addition control. Interestingly, 0.034–34 mm GGA-Na increased the amount of type I collagen by 19–41% when compared to the no-addition control (Fig. (D)), although GGA-Na did not affect the viability of NHDF. Harada et al. have reported that a topical application of α-glucosylglycerol, a structural analog of GGA, enhanced collagen synthesis in mice via an increase in IGF-I production.Citation28) Due to the structural similarity, the GGA stimulation of collagen synthesis might have been induced by the same mechanism as that of α-glucosylglycerol’s. GGA-Na thus has potential as a skin conditioner and ingredient in cosmetic and toiletry products.
We then evaluated GA-Na and GGA-Na as protective solutes in vitro. The protective effect against DNA scission by the hydroxyl radical was determined in water with a hydroxyl radical-generation system that uses H2O2, FeCl3, and ascorbic acid.Citation15) pUC118 (Takara, Otsu, Shiga) was used as the DNA source. The reaction was incubated at 37 °C for 30 min and then subjected to 1% agarose electrophoresis. A dose-dependent decrease in the number of open circular plasmids was apparent in the presence of GGA-Na (data not shown). At 200 mm GGA-Na, most of the plasmid DNA remained in closed circular form after a 30-min incubation (Fig. (A)). In addition, both GA-Na and GGA-Na protected DNA from scission by hydroxyl radicals to a greater degree than did such sugars as glucose, sucrose, and trehalose. This suggests that the GA moiety of GGA mediated the protection of DNA from hydroxyl radical scission.
Fig. 3. Protective effects of GA-Na and GGA-Na on DNA scission (A) and protein aggregation (B).
Notes: (A) DNA scission by the hydroxyl radical was visualized by 1% agarose electrophoresis. The pUC118 plasmid was incubated with 200 mm of a test substance at 37 °C for 30 min. M, molecular ladder (λHindIII digests); 1, pUC118 incubated without hydroxyl radical generation; 2, no addition of a test substance (negative control); 3, GGA-Na; 4, glucose; 5, sucrose; 6, trehalose; 7, GA-Na. (B) Protein aggregation was examined in 50 mm sodium phosphate at pH 8.0 by incubating at 70 °C with 1% of a test substance. The turbidity of the protein solution at 720 nm was monitored. Unfilled circles, GA-Na (78 mm); filled circles, GGA-Na (34 mm); unfilled triangles, glucose (56 mm); filled triangles, sucrose (29 mm); unfilled squares, trehalose (29 mm); filled squares, sorbitol (55 mm); crosses, no-addition control.
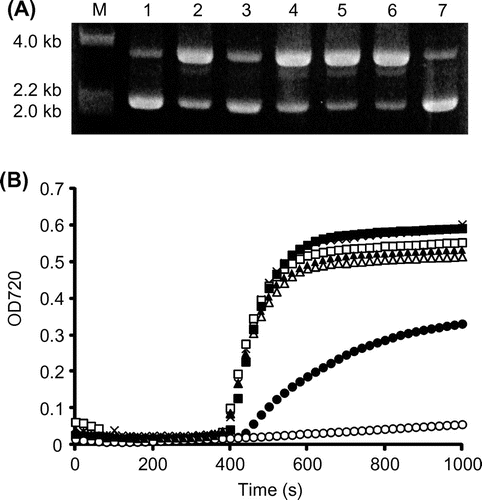
The protective effect of GA-Na and GGA-Na against heat-induced protein aggregation was also evaluated. A test substance at 1% was added to the reaction mixture which was composed of a 50 mm sodium–phosphate buffer and 2% egg white; the mixture was then heated at 70 °C. The optical density at 720 nm was monitored with a UV–vis spectrophotometer to evaluate the turbidity due to protein aggregation. Fig. (B) shows that GA-Na (78 mm) and GGA-Na (34 mm) inhibited heat aggregation of the egg white to a greater degree than such other sugars as glucose (56 mm), sucrose (29 mm), trehalose (29 mm), and sorbitol (55 mm). We preliminarily confirmed that sorbitol, a practical agent for protecting of protein that is used in the food industry at 4–5%,Citation29) inhibited heat aggregation of egg white at a concentration of 5% (275 mm), whereas 5% GGA-Na (170 mm) had a stronger inhibitory effect on the heat aggregation of egg white than that of 5% sorbitol’s (data not shown). The GA structure may therefore contribute a protein-protective property of GGA as an enzyme stabilizer,Citation30) and GA itself represents a superior protein-protective agent. Such activity will play an important role in vivo, such as protecting biological molecules during adaptation to environmental changes.
In summary, we have demonstrated that GA-Na activated the proliferation of human dermal cells and that GGA-Na promoted collagen synthesis by fibroblasts. Both GA-Na and GGA-Na also protected biological molecules in vitro. These substances are thus candidates for ingredients in such biocompatible chemical products as those for skin care and toiletry.
References
- Habe H, Fukuoka T, Kitamoto D, Sakaki K. Biotechnological production of D-glyceric acid and its application. Appl. Microbiol. Biotechnol. 2009;84:445–452.10.1007/s00253-009-2124-3
- Eriksson CJP, Saarenmaa TPS, Bykov IL, Heino PU. Acceleration of ethanol and acetaldehyde oxidation by D-glycerate in rats. Metabolism. 2007;56:895–898.10.1016/j.metabol.2007.01.019
- Habe H, Fukuoka T, Sato S, Kitamoto D, Sakaki K. Synthesis and evaluation of dioleoyl glyceric acids showing antitrypsin activity. J. Oleo Sci. 2011;60:327–331.10.5650/jos.60.327
- Habe H, Sato S, Fukuoka T, Kitamoto D, Sakaki K. Effect of glyceric acid calcium salt on the viability of ethanol-dosed gastric cells. J. Oleo Sci. 2011;60:585–590.10.5650/jos.60.585
- Habe H, Shimada Y, Yakushi T, Hattori H, Ano Y, Fukuoka T, Kitamoto D, Itagaki M, Watanabe K, Yanagishita H, Matsushita K, Sakaki K. Microbial production of glyceric acid, an organic acid that can be mass produced from glycerol. Appl. Environ. Microbiol. 2009;75:7760–7766.10.1128/AEM.01535-09
- Habe H, Shimada Y, Fukuoka T, Kitamoto D, Itagaki M, Watanabe K, Yanagishita H, Sakaki K. Production of glyceric acid by Gluconobacter sp. NBRC3259 using raw glycerol. Biosci. Biotechnol. Biochem. 2009;73:1799–1805.10.1271/bbb.90163
- Sato S, Morita N, Kitamoto D, Yakushi T, Matsushita K, Habe H. Change in product selectivity during the production of glyceric acid from glycerol by Gluconobacter strains in the presence of methanol. AMB Express. 2013;3:20.10.1186/2191-0855-3-20
- Kollman VH, Hanners JL, London RE, Adame EG, Walker TE. Photosynthetic preparation and characterization of 13C-labeled carbohydrates in agmenellum quadruplicatum. Carbohydr. Res. 1979;73:193–202.10.1016/S0008-6215(00)85489-0
- Robertson DE, Lai MC, Gunsalus RP, Roberts MF. Composition, variation, and dynamics of major osmotic solutes in Methanohalophilus strain FDF1. Appl. Environ. Microbiol. 1992;58:2438–2443.
- Santos H, da Costa MS. Compatible solutes of organisms that live in hot saline environments. Environ. Microbiol. 2002;4:501–509.10.1046/j.1462-2920.2002.00335.x
- Müller V, Spanheimer R, Santos H. Stress response by solute accumulation in archaea. Curr. Opin Microbiol. 2005;8:729–736.10.1016/j.mib.2005.10.011
- Empadinhas N, da Costa MS. Diversity, distribution and biosynthesis of compatible solutes in prokaryotes. Contrib. Sci. 2009;5:95–105.
- Klähn S, Hagemann M. Compatible solute biosynthesis in cyanobacteria. Environ. Microbiol. 2011;13:551–562.10.1111/emi.2011.13.issue-3
- Klähn S, Steglich C, Hess WR, Hagemann M. Glucosylglycerate: a secondary compatible solute common to marine cyanobacteria from nitrogen-poor environments. Environ. Microbiol. 2010;12:83–94.10.1111/emi.2010.12.issue-1
- Lentzen G, Schwarz T. Extremolytes: Natural compounds from extremophiles for versatile applications. Appl. Microbiol. Biotechnol. 2006;72:623–634.10.1007/s00253-006-0553-9
- Hincha DK, Hagemann M. Stabilization of model membranes during drying by compatible solutes involved in the stress tolerance of plants and microorganisms. Biochem. J. 2004;383:277–283.
- Göller K, Galinski EA. Protection of a model enzyme (lactate dehydrogenase) against heat, urea and freeze-thaw treatment by compatible solute additives. J. Mol. Catal. B Enzym. 1999;7:37–45.10.1016/S1381-1177(99)00043-0
- Arora A, Ha C, Park CB. Inhibition of insulin amyloid formation by small stress molecules. FEBS Lett. 2004;564:121–125.10.1016/S0014-5793(04)00326-6
- Buenger J, Driller H. Ectoin: an effective natural substance to prevent UVA-induced premature photoaging. Skin Pharmacol. Physiol. 2004;17:232–237.10.1159/000080216
- Ramos A, Raven N, Sharp RJ, Bartolucci S, Rossi M, Cannio R, Lebbink J, Oost Jvd, Vos WMd, Santos H. Stabilization of Enzymes against Thermal Stress and Freeze-Drying by Mannosylglycerate. Appl. Environ. Microbiol. 1997;63:4020–4025.
- Lourenço EC, Maycock CD, Rita Ventura M. Synthesis of potassium (2R)-2-O-α-d-glucopyranosyl-(1->6)-alpha-d-glucopyranosyl-2,3-dihydroxypropanoate a natural compatible solute. Carbohydr. Res. 2009;344:2073–2078.10.1016/j.carres.2009.06.037
- Goedl C, Sawangwan T, Mueller M, Schwarz A, Nidetzky B. A high-yielding biocatalytic process for the production of 2-O-(alpha-D-glucopyranosyl)-sn-glycerol, a natural osmolyte and useful moisturizing ingredient. Angew. Chem. Int. Ed. 2008;47:10086–10089.10.1002/anie.v47:52
- Sawangwan T, Goedl C, Nidetzky B. Single-step enzymatic synthesis of (R)-2-O-alpha-D-glucopyranosyl glycerate, a compatible solute from micro-organisms that functions as a protein stabiliser. Org. Biomol. Chem. 2009;7:4267–4270.10.1039/b912621j
- Sato S, Habe H, Fukuoka T, Kitamoto D, Sakaki K. Stepwise synthesis of 2,3-O-dipalmitoyl-D-glyceric acid and an in vitro evaluation of its cytotoxicity. J. Oleo Sci. 2012;61:337–341.10.5650/jos.61.337
- Garric X, Molès JP, Garreau H, Braud C, Guilhou JJ, Vert M. Growth of various cell types in the presence of lactic and glycolic acids: the adverse effect of glycolic acid released from PLAGA copolymer on keratinocyte proliferation. J. Biomater. Sci. Polym. Ed. 2002;13:1189–1201.10.1163/156856202320892957
- Yamasaki N, Japanese patent, P5192762. 2013 May 8.
- Takeuchi K, Nakazawa M, Ebina Y, Sato K, Metoki T, Miyagawa Y, Ito T. Inhibitory effects of trehalose on fibroblast proliferation and implications for ocular surgery. Exp. Eye Res. 2010;91:567–577.10.1016/j.exer.2010.07.002
- Harada N, Zhao J, Kurihara H, Nakagata N, Okajima K. Effects of topical application of alpha-D-glucosylglycerol on dermal levels of insulin-like growth factor-I in mice and on facial skin elasticity in humans. Biosci. Biotechnol. Biochem. 2010;74:759–765.10.1271/bbb.90797
- Watanabe H, Aramaki H, Iwase Y. Report of food and agricultural materials inspection center. 1986;11:62–75. Japanese.
- Sawangwan T, Goedl C, Nidetzky B. Glucosylglycerol and glucosylglycerate as enzyme stabilizers. Biotechnol. J. 2010;5:187–191.10.1002/biot.200900197