Abstract
The ginsenosides in Panax ginseng have vast structural and pharmacological efficacies. We covalently conjugated polyethylene glycol on the surface of CK (PEG-CK) through an acid-labile ester-linkage that showed increased solubility of CK. HPLC analysis showed that the release of CK was enhanced at acidic pH 5, whereas it was dramatically decreased at physiological pH 7.4. This might enhance the efficacy of CK.
Korean ginseng (Panax ginseng) is known as a medicinal herb, and ginsenosides are triterpenoid steroidal saponins that are regarded as the main active components of ginseng. Ginsenosides are classified as protopanaxadiols (PPD) and protopanaxatriols (PPT), and further into major and minor ginsenosides. Ginseng has been reported to have various pharmacological activities, including anti-tumor activity,Citation1,2) but orally administered ginseng is poorly absorbedCitation3) and has very low bioavailability.Citation4) Various PPD-type ginsenosides and crude extracts administered orally are metabolized primarily into 20-O-β-(d-glucopyranosyl)-20(S)-protopanaxadiol (also called compound K or CK) via stepwise cleavage of sugar moieties by intestinal gut microbiota. CK is one of the major metabolites to reach systemic circulation,Citation5) and has been reported to have for various anticancer activities.Citation6)
It has been reported that esterification increases pharmacokinetic properties. Fatty acid esterification of CK increases its absorption and bioavailability,Citation7) and has a considerable effect on its cytotoxicity against various human cancer cell lines. Although improved efficacies of various ginsenosides has been reported,Citation8) difficulties in target-based delivery of ginsenosides to tumor sites as well as their poor bioavailability, absorption, and solubility remain major drawbacks to the use of ginsenosides in clinical trials.
Polyethylene glycol (PEG) is a water-soluble, non-ionic, and nontoxic polymer widely used as a carrier in the field of polymer-based drug delivery.Citation9) Surface modification of drugs by means of PEG enhances water solubility, protects from proteolytic digestion, prolongs circulation time in the blood, and reduces systemic toxicity.Citation10) Owing to these unique features, it is classified as Generally Regarded as Safe (GRAS) by the US FDA. In particular, pH-responsive drug conjugates have garnered increased attention due to the differences in pH between normal tissues and tumor tissues. Specifically, the intracellular pH of tumor cells (pH 5.0–6.5) is usually much lower than physiological pH (pH 7.4), and has encouraged researchers to develop pH-responsive pro-drug formulations to improve the therapeutic efficacy of drugs.Citation11–15) Here, we report a simple method of enhancing the solubility of CK. CK was chemically modified with hydrophilic PEG through esterification (PEG-CK). The physicochemical characteristics of the prepared PEG-CK were determined by 1H NMR, TEM, and HPLC, and the cytotoxicity of PEG-CK was determined in HT-29 cells.
In an attempt to enhance the solubility of CK, PEGylation was performed on the surface of the ginsenoside through ester linkages. The details of the synthetic procedure and the methods used in the preparation of PEG-CK are presented in the Supplemental Information. For the synthesis of PEG-CK, primary carboxylated PEG was synthesized and conjugated onto the surface of CK (Fig. (A)). Fig. (B) shows the 1H NMR spectra of PEG-COOH. These indicated that a characteristic succinic acid peak was present at 2.6 ppm. At the next step, PEG-COOH was conjugated to CK via the formation of ester bonds. As expected, the 1H NMR spectra of PEG-CK showed the characteristic peaks of PEG and CK. The extent of PEG conjugation was calculated based on the integration ratio of the proton peak appearing in the angular methyl group of CK at 0.86 ppm and that PEG at 3.60 ppm. From the 1H NMR results, we determined that the C-3 hydroxyl and C-6 sugar hydroxyl groups underwent esterification. TEM images indicated that the morphology of the conjugates was spherical in shape, as shown in Fig. (C).
Fig. 1. Preparation and characterization of conjugates.
Notes: (A) Synthetic route for preparation of PEG-CK conjugates; (B) 1H NMR spectra of PEG-CK conjugates and intermediates; (C) TEM image of PEG-CK conjugate; (D) Solubility of free CK and PEG-CK conjugates in water; (E) pH-dependent release of CK from conjugates as determined by HPLC; (F) In vitro cytotoxicity of PEG, PEG-COOH; and (G) In vitro cytotoxicity of CK and PEG-CK. Error bars represent standard deviation (n = 3). **p< 0.01 vs. free CK.
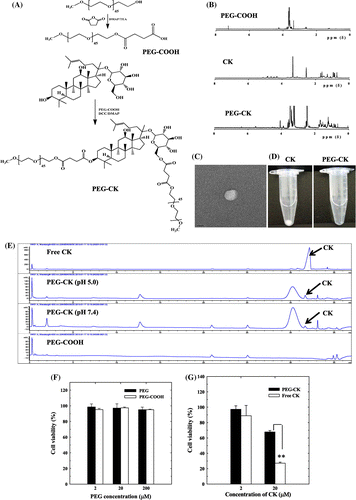
Aqueous solubility is an important physicochemical factor in drug absorption. PEG conjugation enhances the solubility, bioavailability, and anti-tumor activity of various anticancer drugs, including camptothecin and curcumin.Citation16,17) Owing to the hydrophilic nature of PEG, the prepared PEG-CK conjugate (0.7 mg) was readily soluble in 450 μL of water, whereas, due to the hydrophobic nature of free CK, it was insoluble even at much lower concentrations (Fig. (D)).
The hydrolysis of PEG-CK was monitored by incubating samples under different pH conditions (pH 7.4 and 5.0) as a function of time by HPLC. After 12 h, the PEG-CK conjugates that were incubated under acidic conditions exhibited enhanced CK release as compared with samples incubated under physiological conditions. As shown in Fig. (E), the CK peak appeared under both pH conditions, but was significantly larger for the acidic pH condition. Since the conjugate was synthesized via an acid-labile ester linkage, the hydrolysis that occurred in the slightly acidic buffer was similar to that under intracellular tumor pH conditions.
The in vitro cytotoxicity of PEG-CK and PEG was examined with HT-29 cancer cells by MTT assay. As shown in Fig. (F), most of the cells were viable, and did not exhibit any significant cytotoxicity even at 200 μM PEG and PEG-COOH, which indicates the biocompatibility of the hydrophilic polymer. On the other hand, free CK and PEG-CK exhibited dose-dependent toxicity (Fig. (G)). The slightly lower toxicity of PEG-CK was probably due to slow release of CK from the conjugates after the hydrolysis of ester linkages within the acidic compartments of the endosomes and lysosomes.
In conclusion, we successfully conjugated PEG and ginsenoside CK to form PEG-CK, yielding enhanced solubility. The release of CK from the PEG-CK conjugates was greater under acidic pH (pathophysiological) conditions than under normal (physiological) conditions. This very high drug solubility, pH-selectivity, and enhanced accumulation of PEG-CK conjugates at pathophysiological sites might increase the overall efficacy of CK, which might thus be considered a potential drug.
Supplemental material
The supplemental material for this paper is available at http://dx.doi.org/10.1080/09168451.2014.885827
Materials and Methods
Download PDF (196.1 KB)Funding
This research was fully supported by the Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries [KIPET No. 309019-3], and also by a grant from the Next-Generation BioGreen 21 Program [SSAC, Grant No. PJ00952903] of the Rural Development Administration of the Republic of Korea.
References
- Choi KT. Botanical characteristics, pharmacological effects and medicinal components of Korean Panax ginseng C. A. Meyer. Acta Pharmacol. Sin. 2008;29:1109–1118.10.1111/aphs.2008.29.issue-9
- Sathiyamoorthy S, In JG, Gayathri S, Kim YJ, Yang DC. Generation and gene ontology based analysis of expressed sequence tags (EST) from a Panax ginseng C. A. Meyer roots. Mol. Biol. Rep. 2010;37:3465–3472.10.1007/s11033-009-9938-z
- Yang L, Liu Y, Liu CX. Metabolism and pharmacokinetics of ginsenosides. J. Pharmacokinet. Pharmacodyn. 2006;6:103–120.
- Akao T, Kida H, Kanaoka M, Hattori M, Kobashi K. Intestinal bacterial hydrolysis is required for the appearance of compound K in rat plasma after oral administration of ginsenoside Rb1 from Panax ginseng. J. Pharm. Pharmacol. 1998;50:1155–1160.10.1111/jphp.1998.50.issue-10
- Hasegawa H, Sung JH, Matsumiya S, Uchiyama M. Main ginseng saponin metabolites formed by intestinal bacteria. Planta Med. 1996;62:453–457.10.1055/s-2006-957938
- Kim DY, Park MW, Yuan HD, Lee HJ, Kim SH, Chung SH. Compound K induces apoptosis via CAMK-IV/AMPK pathways in HT-29 colon cancer cells. J. Agric. Food Chem. 2009;57:10573–10578.10.1021/jf902700h
- Zhang B, Zhu XM, Hu JN, Ye H, Luo T, Liu XR., Li HY, Li W, Zheng YN, Deng ZY. Absorption mechanism of ginsenoside compound K and its butyl and octyl ester prodrugs in Caco-2 cells. J. Agric. Food Chem. 2012;60:10278–10284.10.1021/jf303160y
- Sathishkumar N, Sathiyamoorthy S, Ramya M, Yang DU, Lee HN, Yang DC. Molecular docking studies of anti-apoptotic BCL-2, BCL-XL, and MCL-1 proteins with ginsenosides from Panax ginseng. J. Enzyme Inhib. Med. Chem. 2012;27:685–692.10.3109/14756366.2011.608663
- Knop K, Hoogenboom R, Fischer D, Schubert US. Poly (ethylene glycol) in drug delivery: pros and cons as well as potential alternatives. Angew. Chem. Int. Ed. Engl. 2010;49:6288–6308.10.1002/anie.200902672
- Sun Y, Yan X, Yuan T, Liang J, Fan Y, Gu Z, Zhang X. Disassemblable micelles based on reduction-degradable amphiphilic graft copolymers for intracellular delivery of doxorubicin. Biomaterials. 2010;31:7124–7131.10.1016/j.biomaterials.2010.06.011
- Lee H, Lee K, Park TG. Hyaluronic acid-paclitaxel conjugate micelles: synthesis, characterization, and antitumor activity. Bioconjug. Chem. 2008;19:1319–1325.10.1021/bc8000485
- Min KH, Lee HJ, Kim K, Kwon IC, Jeong SY, Lee SC. The tumor accumulation and therapeutic efficacy of doxorubicin carried in calcium phosphate-reinforced polymer nanoparticles. Biomaterials. 2012;33:5788–5797.10.1016/j.biomaterials.2012.04.057
- Thambi T, Yoon HY, Kim K, Kwon IC, Yoo CK, Park JH. Bioreducible block copolymers based on poly(ethylene glycol) and poly(gamma-benzyl L-glutamate) for intracellular delivery of camptothecin. Bioconjug. Chem. 2011;22:1924–1931.10.1021/bc2000963
- Thambi T, Deepagan VG, Yoo CK, Park JH. Synthesis and physicochemical characterization of amphiphilic block copolymers bearing acid-sensitive orthoester linkage as the drug carrier. Polymer. 2011;52:4753–4759.10.1016/j.polymer.2011.08.024
- Thambi T, Deepagan VG, Ko H, Lee DS, Park JH. Bioreducible polymersomes for intracellular dual-drug delivery. J. Mater. Chem. 2012;22:22028–22036.10.1039/c2jm34546c
- Li J, Wang Y, Yang C, Wang P, Oelschlager DK, Zheng Y, Tian DA, Grizzle WE, Buchsbaum DJ, Wan M. Polyethylene glycosylated curcumin conjugate inhibits pancreatic cancer cell growth through inactivation of Jab1. Mol. Pharmacol. 2009;76:81–90.10.1124/mol.109.054551
- Scott LC, Yao JC, Benson AB 3rd, Thomas AL, Falk S, Mena RR, Picus J, Wright J, Mulcahy MF, Ajani JA, Evans TR. A phase II study of pegylated-camptothecin (pegamotecan) in the treatment of locally advanced and metastatic gastric and gastro-oesophageal junction adenocarcinoma. Cancer Chemother. Pharmacol. 2009;63:363–370.10.1007/s00280-008-0746-2
- Park SY, Han DK, Kim SC. Synthesis and Characterization of Star-Shaped PLLA-PEO Block Copolymers with Temperature-Sensitive Sol-Gel Transition Behavior. Macromolecules. 2001;34:8821–8824.10.1021/ma010789d
