Abstract
The aim of this study was to investigate the mechanisms involved in the apoptosis of HeLa cells due to 2,3-dehydrosilybin (DHS) treatment. DHS treatment over 24 h significantly inhibited cell viability and induced apoptosis in a dose-dependent manner. It also triggered the cleavage of caspase-8, caspase-9, caspase-3, and PARP, and significantly increased caspase-3 activity in a dose-dependent manner. Moreover, it triggered the depolarization of the mitochondrial membrane potential (Δψm), the release of cytochrome c into the cytosol, the cleavage of Bid, and the downregulation of Bcl-2 in a dose-dependent manner. Furthermore, z-VAD-fmk (a pan-caspase inhibitor) and z-IETD-fmk (a specific caspase-8 inhibitor) abolished the DHS-induced activation of the caspase-8, -9, and -3, cleavage of PARP, the depolarization of Δψm, the release of cytochrome c, the cleavage of Bid, and the downregulation of Bcl-2. Taken together, these results suggest that DHS-induced apoptosis is mediated by a caspase-dependent pathway in human HeLa cells.
Cancer is a common disease, and one in four deaths in the United States can be attributed to it.Citation1) Currently, the most common cancer therapies are surgery, chemotherapy, and radiotherapy. These therapies utilize endogenous mechanisms to induce apoptosis, or programmed cell death.Citation2) Apoptosis plays an important role in development, aging, and the immune response and is a homeostatic mechanism to maintain cell populations in tissues.Citation3) In general, morphological and biochemical changes during apoptosis include nuclear chromatin condensation, cell shrinkage, plasma membrane blebbing, apoptotic bodies, phosphatidylserine externalization, and caspase activation.Citation2,3) The apoptotic process is typically mediated via two signaling pathways. The extrinsic pathway is mediated by death receptors, such as CD95/Fas, TRAIL R1, and TRAIL R2, on the cell surface that activates caspase-8, which then activates caspase-3. Conversely, the intrinsic pathway is activated by a death stimulus, such as mitochondrial DNA damage, and is regulated by the Bcl-2 family of proteins, including Bid, Bax, Bcl-2, and Bcl-XL. In response to a death stimulus, cytochrome c is released from the mitochondria, and it triggers caspase-9 activation, which activates caspase-3 and induces apoptosis.Citation4)
Recently, natural products derived from plants have attracted the attention of researchers as new sources of potential anticancer lead compounds.Citation5) Several studies have demonstrated that natural compounds, such as asperlin, oleifolioside A, and pristimerin, exhibit anticancer effects by inducing apoptosis in human cervical carcinoma cells.Citation6Citation−Citation8) Silymarin, a flavonolignan from the milk thistle (Silybum marianum), is used in traditional medicine for the treatment of several diseases, including hepatitis and cirrhosis, and has been reported to exhibit anticancer, antiinflammatory, and antioxidant effects.Citation9) An oxidized form of silybin, 2,3-dehydrosilybin (DHS, Fig. ), is a natural flavonoid found in the milk thistle. Previous study suggests that DHS exhibits anticancer activity by suppressing invasion and metastasis and by inducing apoptosis in fibroblast-like FIB cells.Citation10) Recently, Zhan et al.Citation11) reported that silybin and DHS inhibit cellular glucose uptake by interacting directly with GLUT transporters, consequently exhibiting anticancer effects, but its mechanism of action has not yet been completely defined. Hence, the present study aimed to determine the anticancer effects of DHS on HeLa human cervical carcinoma cells and to evaluate the mechanism of DHS-induced apoptosis.
Materials and methods
Materials
DHS was prepared by means of radiation, as described previously.Citation12) Both z-VAD-fmk (a pan-caspase inhibitor) and z-IETD-fmk (a specific caspase-8 inhibitor) were purchased from R&D Systems (Minneapolis, MN). Caspase-8, -9, and -3 antibodies were from Cell Signaling Technology (Danvers, MA). Poly (ADP-ribose) polymerase (PARP) and cytochrome c antibodies were from BD Pharmingen (San Diego, CA) and Clontech (Mountain View, CA) respectively. Bcl-2, Bax, Bid, COX4, and β-tubulin antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). Goat antimouse IgG HRP-conjugated antibody was from SouthernBiotech (Birmingham, AL), and goat antirabbit IgG HRP-conjugated antibody was from Invitrogen (Carlsbad, CA).
Cell culture
HeLa, DLD1, HepG2, and MCF-7 cell lines were from the American Type Culture Collection (Manassas, VA). The cells were cultured in RPMI1640 or DMEM supplemented with 10% fetal bovine serum (Hyclone, Logan, UT), 100 units/mL of penicillin, and 100 μg/mL of streptomycin (Invitrogen, Carlsbad, CA). They were maintained in a humidified incubator at 37°C with 5% CO2.
Cell viability
To measure cell viability, we used an EZ-Cytox Cell Viability Assay Kit (DAEIL lab, Seoul, Korea). Cancer cells were seeded in a 96-well plate at a density of 1 × 105 cells/mL for 24 h. They were then treated with various concentrations of DHS (6.25, 12.5, 25, 50, 75, and 100 μm) for an additional 24 h. Then 10 μL of kit solution was added to each well, and this was incubated for 4 h at 37°C under 5% CO2. Cell viability was determined by measuring formazan production with a microplate reader (Benchmark Plus, Bio-Rad, Hercules, CA) at an absorbance of 480 nm. The reference wavelength was 650 nm. Cell viability was determined relative to untreated control cells. The IC50 value was defined as the concentration of DHS that reduces cell viability by 50%.
Analysis of apoptosis
Apoptotic cell death was measured using a MEBCYTO apoptosis kit (MBL International, Nagoya, Japan) following the manufacturer’s instructions. Briefly, HeLa cells were cultured in a 6-well plate at a density of 2 × 105 cells/mL for 24 h. After incubation, they were treated with various concentrations of DHS (0, 25, 50, and 100 μm) for an additional 24 h. Then they were harvested, washed with PBS, and resuspended in 85 μL of binding buffer. After resuspension, 10 μL of Annexin V-FITC and 5 μL of propidium iodide (PI) were added to each sample and this was incubated at room temperature for 15 min in the dark. Then 400 μL of binding buffer was added and the percentage of apoptotic cells was analyzed with a flow cytometer (Cytomics FC500; Beckman, Miami, FL). At least 1 × 104 cells were analyzed for each sample.
Caspase-3 activity assay
Caspase-3 activity was measured with a Caspase-3 Colorimetric Assay Kit (Sigma-Aldrich, St. Louis, MO) following the manufacturer’s protocol. Briefly, the cells were treated with various concentrations of DHS (0, 12.5, 25, and 50 μm) for 24 h. After incubation, they were harvested and lysed with the kit cell lysis buffer on ice for 20 min, and then centrifuged at 16,000 × g for 15 min at 4°C. The protein concentration was quantified by Bio-Rad Protein Assay (Bio-Rad, Hercules, CA). Equal amounts of protein were mixed with 2 mM Ac-DEVD-pNA in assay buffer and this was incubated at 37°C for 2 h. The amount of pNA generated was measured at 405 nm with a microplate reader (Benchmark Plus, Bio-Rad) and calculated with a calibration curve prepared with defined pNA solutions.
Measurement of mitochondrial membrane potential (Δψm)
Depolarization of the Δψm was detected with a MitoCaptureTM mitochondrial apoptosis detection kit (Biovision, Mountain View, CA) following the manufacturer’s protocol. MitoCapture accumulates and aggregates in the mitochondria of healthy cells, emitting a bright red fluorescence, but it does not aggregate in the mitochondria of apoptotic cells due to altered Δψm, and it remains in the cytoplasm as a fluorescent green monomer. Briefly, HeLa cells were cultured on a 12-mm glass coverslip in a 6-well plate at a density of 2 × 105 cells/mL for 24 h. After incubation, they were treated with various concentrations of DHS (0, 12.5, 25, and 50 μm) for an additional 24 h. Then 1 mL of diluted MitoCapture solution was added and this was incubated for 15 min at 37°C. After washing with PBS, the samples were visualized with a fluorescence microscope (Nikon eclipse TE2000-E; Nikon, Tokyo) and a flow cytometer (Beckman). At least 1 × 104 cells were analyzed for each sample.
Western blotting
Cells were seeded in a 100-mm dish at a density of 2 × 105 cells/mL and then cultured for 24 h. Then they were treated with various concentrations of DHS (0, 12.5, 25, and 50 μm) for an additional 24 h. They were harvested and lysed with cell lysis buffer (50 mM Tris pH 7.4, 250 mM NaCl, 5 mM EDTA, 50 mM NaF, 1 mM Na3VO4, 1% NP40, 0.02% NaN3, and 1 mM PMSF) containing a protease inhibitor cocktail (Sigma-Aldrich) for 30 min on ice, and then the cell extracts were centrifuged. The protein concentration was quantified. Equal amounts of protein were electrophoresed in SDS-polyacrylamide gels and transferred onto nitrocellulose membranes (Hybond ECL Nitrocellulose; Amersham Biosciences, Bucks, UK). The membranes were blocked with 5% skim milk in TBS-T (10 mM Tris-HCl pH 7.4, 150 mM NaCl, and 0.1% Tween 20) and incubated with the appropriate primary antibodies overnight at 4°C. After incubation, they were washed and incubated with an HRP-conjugated secondary antibody for 2 h at room temperature and then washed again. The blotted proteins were detected by enhanced chemiluminescence (GE Healthcare, Bucks, UK).
Preparation of cytosolic and mitochondrial fractions
To investigate the release of cytochrome c from the mitochondria, mitochondrial and cytosolic fractions were prepared from DHS-treated cells with a mitochondria isolation kit (Pierce, Rockford, IL) following the manufacturer’s protocol. After sample extraction, the protein concentration was quantified and the proteins were analyzed by Western blot.
Statistical analysis
All data are presented as mean ± SD. Significant differences between the means of the treated and untreated groups were determined by Student’s t-test. A p < 0.05 value was considered significant.
Results
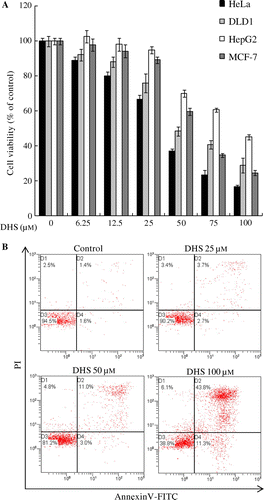
DHS decreased cell viability in HeLa, DLD1, HepG2, and MCF-7 cells and induced apoptosis in the HeLa cells
First, the cytotoxic effects of DHS in HeLa, DLD1, HepG2, and MCF-7 cells were examined with an EZ-Cytox Cell Viability Assay Kit. As shown in Fig. (A), DHS significantly decreased the viability of the HeLa, DLD1, HepG2, and MCF-7 cells. The 50% growth inhibition concentrations (IC50) of those cells were 49.1, 63.0, 75.3, and 64.3 μm, respectively. Based on the results shown in Fig. (A), we selected HeLa cells to evaluate the potential of DHS to induce apoptosis and to investigate the molecular mechanisms underlying DHS-stimulated apoptosis. Furthermore, to determine whether the cytotoxic effect of DHS is associated with the induction of apoptosis, we analyzed early apoptotic marker Annexin V-FITC and late apoptotic marker PI by double-staining flow cytometry. As Fig. (B) shows, DHS induced apoptosis in HeLa cells in a dose-dependent manner.
DHS induced caspase activation and PARP cleavage
To address the mechanism of DHS-triggered apoptosis as to HeLa cells, we investigated the cleavage of caspases and PARP. HeLa cells were treated with 0–50 μm DHS for 24 h, and protein cleavage was determined by Western blot. As shown in Fig. (A), DHS treatment resulted in the cleavage of caspase-8, -9, -3, and PARP in a dose-dependent manner. In addition, we confirmed that the activity of caspase-3 significantly increased in a dose-dependent manner (Fig. (B)). To determine whether the caspase pathway is an essential event in DHS-induced apoptosis we utilized caspase inhibitors. HeLa cells were pretreated with z-VAD-fmk (a pan-caspase inhibitor) or z-IETD-fmk (a specific caspase-8 inhibitor) for 1 h and analyzed by Western blot. We found that z-VAD-fmk and z-IETD-fmk treatment significantly suppressed the cleavage of caspase-8, caspase-9, caspase-3, and PARP (Fig. (A)). Additionally, DHS treatment significantly increased the activity of caspase-3, whereas z-VAD-fmk and z-IETD-fmk treatment significantly decreased this activity (Fig. (B)). These results indicate that DHS-induced apoptosis is involved in the caspase pathway in HeLa cells.
Fig. 3. DHS induced apoptosis in HeLa Cells.
Note: Effects of DHS on the protein expression levels of caspase-8, caspase-9, caspase-3, and PARP (A) and on the activity of caspase-3 in HeLa cells (B). Cells were treated with 0–50 μm DHS for 24 h. Values are expressed as mean ± SD.
*p < 0.05 vs. control, **p < 0.01 vs. control.
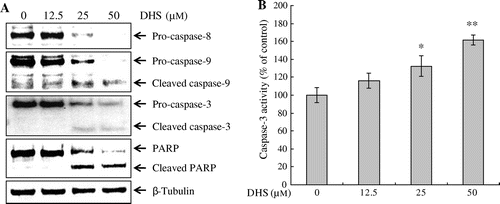
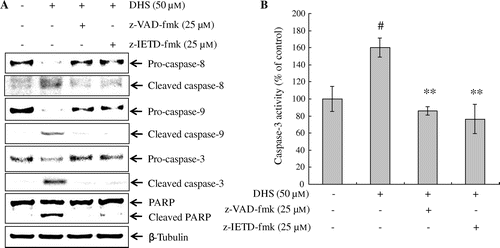
Fig. 4. Effects of caspase inhibitors on DHS-induced caspase activation in HeLa cells. Cells were pretreated with z-VAD-fmk (pan-caspase) or z-IETD-fmk (caspase-8) for 1 h, and then treated with 50 μm DHS for an additional 24 h. (A) Protein expression levels of caspase-8, caspase-9, caspase-3, and PARP. (B) DHS-induced caspase-3 activation. Values are expressed as mean ± SD.
#p < 0.01 vs. control, **p < 0.01 vs. DHS.
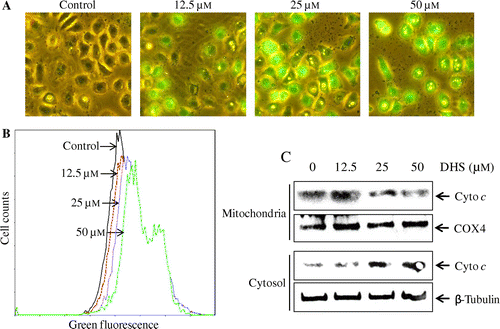
DHS induced depolarization of the mitochondrial membrane potential (Δψm) and the release of cytochrome c
As shown in Fig. (A), DHS treatment resulted in caspase-9 activation, suggesting that the mitochondrial pathway is involved in DHS-induced apoptosis in HeLa cells. To investigate further the potential effects of DHS on the mitochondrial pathway, we measured Δψm with a MitoCaptureTM mitochondrial apoptosis detection kit. The results of fluorescence microscope observation and flow cytometry indicated that DHS increased the intensity of green fluorescence as compared to the untreated cells (Fig. (A) and (B)), suggesting that DHS induced depolarization of the Δψm in the HeLa cells. Next we investigated the release of cytochrome c from the mitochondria into the cytosol during DHS-induced apoptosis. As shown in Fig. (C), the level of mitochondrial cytochrome c was dose-dependently decreased by DHS in the HeLa cells, whereas the level of cytosolic cytochrome c was increased. This suggests that the mitochondrial pathway is important in DHS-induced apoptosis.
Fig. 5. Effects of DHS on the mitochondrial membrane potential (Δψm) and the release of cytochrome c in HeLa cells.
Note: (A and B) Cells were treated with 0–50 μm DHS for 24 h and then stained with MitoCapture for 15 min. They were visualized under a fluorescence microscope (magnification 200 ×) and analyzed by flow cytometry. (C) The release of cytochrome c from the mitochondria into the cytosol was measured by Western blot using a cytochrome c release assay kit.
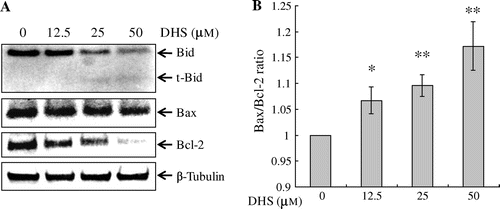
DHS triggered the cleavage of Bid and downregulation of Bcl-2
As shown in Fig. , the mitochondria were involved in DHS-induced apoptosis. The Bcl-2 family of proteins is involved in mitochondria-mediated apoptosis. Hence we determined the expression levels of the following Bcl-2 family proteins: Bid, Bax, and Bcl-2. We observed that DHS treatment resulted in the cleavage of Bid (Fig. (A)), which is triggered by caspase-8 activation. The protein levels of Bcl-2 were downregulated after DHS treatment, whereas those of Bax were unchanged (Fig. (A)). In addition, the ratio of Bax to Bcl-2 expression increased in the DHS-treated HeLa cells (Fig. (B)). These results suggest that DHS-induced apoptosis is mediated by caspase-8, the cleavage of Bid, and the downregulation of Bcl-2.
Fig. 6. Effects of DHS on the protein expression levels of Bid, Bax, and Bcl-2 (A) and the Bax:Bcl-2 in HeLa cells (B).
Note: Cells were treated with 0–50 μm DHS for 24 h. After normalization with β-Tubulin by means of an image analysis program, the ratio of Bax to Bcl-2 was determined. Values are expressed as mean ± SD.
*p < 0.05 vs. control, **p < 0.01 vs. control.
Effects of caspase inhibitors on the DHS-induced depolarization of Δψm, the cleavage of Bid, and the release of cytochrome c
To assess further the potential effects of DHS on the mitochondrial pathway, we used caspase inhibitor z-VAD-fmk and z-IETD-fmk. As evident by the fluorescence microscopy in Fig. (A), DHS treatment significantly increased the depolarization of the Δψm in the HeLa cells, but it was restored in the z-VAD-fmk and z-IETD-fmk-pre-treated HeLa cells.
Fig. 7. Effects of caspase inhibitors on DHS-induced depolarization of mitochondrial membrane potential (Δψm), the cleavage of Bid, and the release of cytochrome c in HeLa cells.
Note: (A) Cells were pretreated with 25 μm z-VAD-fmk or 25 μm z-IETD-fmk for 1 h and then treated with 50 μm DHS for an additional 24 h. They were stained with MitoCapture for 15 min and visualized under a fluorescence microscope (magnification 200 ×). (B) Protein expression of Bid and cytochrome c.
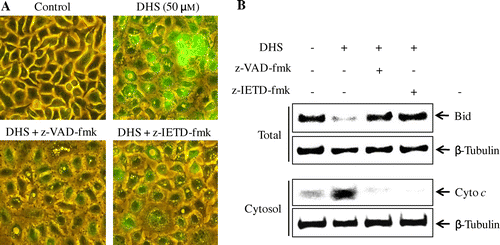
Next, to identify the involvement of caspase-8 in DHS-induced apoptosis via the mitochondria, we evaluated the cleavage of Bid and the release of cytochrome c after treatment with z-VAD-fmk and with z-IETD-fmk. As Fig. (B) shows, z-VAD-fmk and z-IETD-fmk treatment significantly blocked the cleavage of Bid and the release of cytochrome c from the mitochondria into the cytosol.
Discussion
In this study, we investigated the anticancer effects and the underlying apoptotic mechanism of DHS in HeLa human cervical carcinoma cells. Huber et al.Citation10) have reported that DHS induces apoptosis in fibroblast-like FIB cells, but the specific molecular pathway of apoptosis remains unclear.
Apoptotic cell death is characterized by the induction of nuclear chromatin condensation, cell shrinkage, plasma membrane blebbing, apoptotic bodies, phosphatidylserine externalization, and caspase activation.Citation2,3) In this study, we found that DHS exhibited a significant dose-dependent cytotoxic effect on HeLa cells. Our results also indicate that DHS dose-dependently increased apoptotic cell death, which was determined by Annexin V-FITC and PI double-staining in HeLa cells.
The caspase pathway plays an important role in the apoptosis process triggered by death receptors, such as CD95/Fas, TRAIL R1, and TRAIL R2.Citation4) Many studies have found that natural plant compounds induce apoptosis through caspase activation.Citation13Citation−Citation15) Natural compound-induced apoptosis has been found to be blocked by caspase inhibitors such as z-VAD-fmk and z-IETD-fmk.Citation16) In the present study, DHS-induced apoptosis was associated with the activation of caspases-8, -9, and -3. These responses were blocked by z-VAD-fmk and z-IETD-fmk treatment. The cleavage of PARP is a hallmark of apoptosis that is triggered by caspase-3/7.Citation17,18) Our data indicate that PARP was cleaved in DHS-treated HeLa cells, and that treatment with z-VAD-fmk and z-IETD-fmk inhibited DHS-induced PARP cleavage. These results suggest that DHS-induced apoptosis is dependent on the caspase pathway.
Mitochondria are crucially involved in the initiation of apoptosis. Apoptotic stimuli, such as radiation, toxins, and free radicals cause changes in the inner mitochondrial membrane that open the mitochondrial permeability transition pore and loss of Δψm, and release pro-apoptotic proteins, including cytochrome c, from the inter-membrane space into the cytosol. The released cytochrome c binds Apaf-1 and activates caspase-9, which leads to caspase-3 activation.Citation2) Xu et al.Citation19) have reported that a natural compound, corosolic acid, induces apoptosis through the mitochondrial pathway by triggering depolarization of the Δψm, the release of cytochrome c, and caspase activation in HeLa cells. In the present study, we found that DHS significantly induces depolarization of the Δψm, the release of cytochrome c into the cytosol, and the activation of caspase-9. Furthermore, these responses were suppressed by z-VAD-fmk and z-IETD-fmk treatment. Thus, our results indicate that DHS-induced apoptosis is associated with the activation of caspases via a mitochondrial pathway.
Two types of signaling pathways are induced by CD95/Fas. In type I, caspase-8 activates downstream effector caspase-3/7 directly. In type II, caspase-8 generates truncated Bid (tBid), which then induces the release of cytochrome c from the mitochondria into the cytosol. This leads to activation of caspase-9 and caspase-3, and this causes apoptosis.Citation20) In the present study, DHS treatment produced tBid in HeLa cells. Furthermore, we found that Bid cleavage was inhibited by z-VAD-fmk and z-IETD-fmk treatment. These results correspond to the abovementioned results, as indicated by the activation of caspase-8, -9, and -3 and the release of cytochrome c into the cytosol.
The Bcl-2 protein family plays an important role in apoptosis. Bax, a pro-apoptotic protein, is activated by tBid and triggers the mitochondrial apoptotic signal, which is inhibited by antiapoptotic protein Bcl-2.Citation21) The ratio of Bax to Bcl-2 determines the apoptotic fate of the cell.Citation22) In the present study, DHS increased the ratio of Bax to Bcl-2 by downregulating Bcl-2 protein levels, although DHS did not affect Bax protein levels. These results suggest that DHS-induced apoptosis is mediated through caspase-8, Bid cleavage, and Bcl-2 downregulation. On the other hand, Yu et al.Citation7) found that oleifolioside A induces caspase-independent apoptosis involving nuclear relocation of mitochondrial apoptogenic factors AIF and EndoG in HeLa cells. In addition, Zhan et al.Citation11) have reported that silybin and dehydrosilybin indicate anticancer effects via glucose starvation in CHO cells. Hence, we think that the induction of caspase-dependent apoptosis is one of several factors contributing to the antitumor effect of DHS.
In conclusion, our results indicate that DHS has cytotoxic effects and induces apoptosis in HeLa human cervical cancer cells. Moreover, DHS treatment triggered the cleavage of caspase-8, caspase-9, caspase-3, and PARP, and significantly increased caspase-3 activity. Furthermore, it induced depolarization of the Δψm, the release of cytochrome c into the cytosol, the cleavage of Bid, and the downregulation of Bcl-2, but these responses were blocked by z-VAD-fmk and by z-IETD-fmk treatment. To the best of our knowledge, this is the first report of DHS-induced apoptosis in HeLa cells. We suggest that DHS is a potential chemopreventive and therapeutic candidate in the treatment of human cervical carcinoma.
Acknowledgments
This research was supported by the Ministry of Science, ICT & Future Planning (MSIFP) of the Republic of Korea.
References
- Jemal A, Siegel R, Xu J, Ward E. Cancer Statistics. CA: Cancer J. Clin. 2010;60:277–300.10.3322/caac.20073
- Tan ML, Ooi JP, Ismail N, Moad AIH, Muhammad TST. Programmed cell death pathways and current antitumor targets. Pharm. Res. 2009;26:1547–1560.10.1007/s11095-009-9895-1
- Elmore S. Apoptosis: a review of programmed cell death. Toxicol. Pathol. 2007;35:495–516.10.1080/01926230701320337
- Yang SY, Sales KM, Fuller B, Seifalian AM, Winslet MC. Apoptosis and colorectal cancer: implications for therapy. Trends Mol. Med. 2009;15:225–233.10.1016/j.molmed.2009.03.003
- Nobili S, Lippi D, Witort E, Donnini M, Bausi L, Mini E, Capaccioli S. Natural compounds for cancer treatment and prevention. Pharm. Res. 2009;59:365–378.10.1016/j.phrs.2009.01.017
- He L, Nan MH, Oh HC, Kim YH, Jang JH, Erikson RL, Ahn JS, Kim BY. Asperlin induces G2/M arrest through ROS generation and ATM pathway in human cervical carcinoma cells. Biochem. Biophys. Res. Commun. 2011;409:489–493.10.1016/j.bbrc.2011.05.032
- Yu HY, Jin CY, Kim KS, Lee YC, Park SH, Kim GY, Kim WJ, Moon HI, Choi YH, Lee JH. Oleifolioside A mediates caspase-independent human cervical carcinoma HeLa cell apoptosis involving nuclear relocation of mitochondrial apoptogenic factors AIF and EndoG. J. Agric. Food Chem. 2012;60:5400–5406.10.1021/jf3014475
- Byun JY, Kim MJ, Eum DY, Yoon CH, Seo WD, Park KH, Hyun JW, Lee YS, Lee JS, Yoon MY, Lee SJ. Reactive oxygen species-dependent activation of Bax and Poly(ADP-ribose) polymerase-1 is required for mitochondrial cell death induced by triterpenoid pristimerin in human cervical cancer cells. Mol. Pharm. 2009;76:734–744.10.1124/mol.109.056259
- Ramasamy K, Agarwal R. Multitargeted therapy of cancer by silymarin. Cancer Lett. 2008;269:352–362.10.1016/j.canlet.2008.03.053
- Huber A, Thongphasuk P, Erben G, Lehmann WD, Tuma S, Stremmel W, Chamulitrat W. Significantly greater antioxidant anticancer activities of 2,3-dehydrosilybin than silybin. Biochim. Biophys. Acta. 2008;1780:837–847.10.1016/j.bbagen.2007.12.012
- Zhan T, Digel M, Kuch EM, Stremmel W, Fullekrug J. Silybin and dehydrosilybin decrease glucose uptake by inhibiting GLUT proteins. J. Cell. Biochem. 2011;112:849–859.10.1002/jcb.22984
- Cho BO, Ryu HW, So Y, Jin CH, Baek JY, Park KH, Byun EH, Jeong IY. Hepatoprotective effect of 2,3-dehydrosilybin on carbon tetrachloride-induced liver injury in rats. Food Chem. 2013;138:107–115.10.1016/j.foodchem.2012.10.026
- Choi SR, Lee JH, Kim JY, Park KW, Jeong IY, Shim KH, Lee MK, Seo KI. Decursin from Angelica gigas Nakai induces apoptosis in RC-58T/h/SA#4 primary human prostate cancer cells via a mitochondria-related caspase pathway. Food Chem. Toxicol. 2011;49:2517–2523.10.1016/j.fct.2011.06.016
- Mou H, Zheng Y, Zhao P, Bao H, Fang W, Xu N. Celastrol induces apoptosis in non-small-cell lung cancer A549 cells through activation of mitochondria- and Fas/FasL-mediated pathways. Toxicol. Vitro. 2011;25:1027–1032.10.1016/j.tiv.2011.03.023
- Qi F, Li A, Inagaki Y, Xu H, Wang D, Cui X, Zhang L, Kokudo N, Du G, Tang W. Induction of apoptosis by cinobufacini preparation through mitochondria- and Fas-mediated caspase-dependent pathways in human hepatocellular carcinoma cells. Food Chem. Toxicol. 2012;50:295–302.10.1016/j.fct.2011.10.040
- Yamasaki-Miyamoto Y, Yamasaki M, Tachibana H, Yamada K. Fucoidan induces apoptosis through activation of caspase-8 on human breast cancer MCF-7 cells. J. Agric. Food Chem. 2009;57:8677–8682.10.1021/jf9010406
- Hsu CL, Yu YS, Yen GC. Anticancer effects of Alpinia pricei hayata roots. J. Agric. Food Chem. 2010;58:2201–2208.10.1021/jf9038056
- Shim HY, Park JH, Paik HD, Nah SY, Kim DSHL, Han YS. Acacetin-induced apoptosis of human breast cancer MCF-7 cells involves caspase cascade, mitochondria-mediated death signaling and SAPK/JNK1/2-c-Jun activation. Mol. Cells. 2007;24:95–104.
- Xu Y, Ge R, Du J, Xin H, Yi T, Sheng J, Wang Y, Ling C. Corsolic acid induces apoptosis through mitochondrial pathway and caspases activation in human cervix adenocarcinoma HeLa cells. Cancer Lett. 2009;284:229–237.10.1016/j.canlet.2009.04.028
- Mahmood Z, Shukla Y. Death receptors: targets for cancer therapy. Exp. Cell Res. 2010;316:887–899.10.1016/j.yexcr.2009.12.011
- Mellier G, Huang S, Shenoy K, Pervaiz S. TRAILing death in cancer. Mol. Aspects Med. 2010;31:93–112.10.1016/j.mam.2009.12.002
- Kim JE, Chung WY, Chun KS, Lee CK, Park HJ, Kim WB, Park KK. Pleurospermum kamtschaticum extract induces apoptosis via mitochondrial pathway and NAG-1 expression in colon cancer cells. Biosci. Biotechnol. Biochem. 2010;74:788–792.10.1271/bbb.90826