Abstract
We demonstrate the inhibitory effects of κ-casein macropeptide (CMP) on the biofilm formation and virulence of Listeria monocytogenes Scott A. The inhibition of biofilm formation by CMP was initially investigated by using the protocol applied for the 96-well microtiter plate assay. Low concentrations of CMP (0.1, 0.2, 0.3, 0.4, and 0.5 mg/mL) that were tested resulted in a profound inhibitory effect on biofilm formation at a concentration of 0.4 mg/mL. CMP also significantly repressed the transcription of inlA (encoding internalin A) that was responsible for the initial adhesion and invasion event, and prolonged the survival of Caenorhabditis elegans infected by L. monocytogenes. Two-dimensional gel electrophoresis showed that newly identified proteins in the presence of CMP were involved in the stress response and metabolic processes that have important roles in developing listerial biofilms. Our results suggest that CMP from milk protein would be capable of eliminating biofilm formation and virulence by L. monocytogenes in the food industry.
Listeria monocytogenes is a facultative intracellular foodborne pathogen that causes serious illnesses, commonly called listeriosis, including meningitis, septicemia, and stillbirth, with its mortality rate reaching up to 30%.Citation1) Listeriosis generally affects the elderly, neonates, pregnant, and immunocompromised persons. This bacterium can be adsorbed to the inert surfaces found in the food-processing environment. Its biofilms are a potential, chronic source of microbial contamination which may compromise the food quality and then pose a significant health hazard. It is therefore necessary to obtain a greater understanding of the interaction between microorganisms and food-processing surfaces.Citation2)
The adhesion of L. monocytogenes to food-processing surfaces influences the hydrophobic interactions between the surface material and the outer surface components of the bacterium, rather than being a specific ligand-receptor process.Citation3) Pretreatment of surfaces with protein has been shown to reduce the number of adhering microorganisms.Citation4) Over the past decade, several strategies have been proposed to prevent the adhesion of microorganisms and the formation of a biofilm on surfaces. Most of these have focused on surface modification to inhibit adhesion by the adsorption of antimicrobial proteins or compounds such as nisin, lysozyme, benzalkonium chloride, silver, and chlorhexidine.Citation5)
Numerous outbreaks of febrile gastroenteritis have also recently been reported in healthy persons who had ingested L. monocytogenes-contaminated cheese or delicatessen meat.Citation6,7) The symptoms of febrile gastroenteritis are diarrhea, fever, headache, stomach cramps, and vomiting. It remains necessary to clarify the pathophysiological features or the factors affecting the infectious dose and the occurrence and course of infection. Several studies have reported murine listeriosis by intragastric inoculation,Citation8,9) most of which have used a parenteral route of challenge. According to a recent report, intragastric inoculation of L. monocytogenes has caused severe suppurative gastritis that was dependent on the bacterial or mouse strain.Citation10)
The κ-casein macropeptide (CMP) is one of the milk proteins that contain such carbohydrates as galactose, N-acetylgalactosamine, and N-acetylneuraminic acid. Chymosin cleaves the peptide bond between the methionine (105th) and phenylalanine (106th) residues in κ-casein, resulting in the separation of para-κ-casein and κ-casein-macropeptide (CMP, caseinoglycopeptide). Several biological functions have been reported for CMP, such as the bifidobacteria promoting factor, cholera toxin binding, and immune modulating effects.Citation11,12) Current studies have reported that κ-casein CMP inhibited the adhesion of three strains of verotoxigenic Escherichia coli (VTEC), three strains of enteropathogenic Escherichia coli (EPEC), and four strains of Lactobacillus to human HT-29 tissue cell cultures.Citation13) Furthermore, lactic acid bacteria (LAB) can inhibit the growth of L. monocytogenes.Citation14)
We examined in the present study the potential effects of CMP in inhibiting biofilm formation on abiotic and biotic surfaces in human epithelial HT-29 cell lines and AGS cells. We also used a proteome approach to identify the proteins specifically expressed in the infection process.
Materials and methods
CMP
CMP isolated from fresh cheese whey by using a unique ion exchange and membrane technique was purchased from Davisco Foods International (Le Sueur, MN, USA).
Bacterium and cell culture
An L. monocytogenes Scott A culture was maintained at −80 °C in tryptic soy broth (TSB; Difco, Sparks MD, USA) supplemented with 0.6% yeast extract (TSBYE) containing 25% glycerol as a cryoprotectant. Before testing, the strain was sub-cultured in TSBYE at 37 °C for 18 h. The HT-29 human intestinal epithelial cell line and AGS human gastric epithelial cell line were purchased from Korea Cell Line Bank (KCLB; Seoul, Korea). HT-29 cells have been used as a model system to examine the interaction of enteric pathogens with intestinal epithelial cells. All media and fetal bovine serum (FBS) were obtained from Gibco BRL (Life Technologies, Grand Island, NY, USA) and JBI (Jeil Biotech Services, Seoul, Korea). The HT-29 and AGS cells were grown in an RPMI-1640 medium which was supplemented with 10% heat-inactivated FBS. The cells were routinely cultured at 37 °C in a 5% CO2 incubator with 6-well or 12-well tissue culture plates for the adhesion and invasion assays and in 10-mm-diameter tissue culture plates for RNA isolation.
Biofilm formation on abiotic surfaces
The quantification of biofilm production on plastic microtiter plates and glass tubes was based on the previously described methods.Citation15) As abiotic surfaces for biofilm formation, 96-well polyvinyl chloride (PVC) microplates (BD Biosciences, San Jose, CA, USA) and Pyrex glass tube (10 mm by 20 mm) were used. Overnight cultures of L. monocytogenes Scott A were twice washed with PBS and inoculated at 106 CFU/mL into a modified Welshimer’s broth (MWB) minimal mediumCitation16) supplemented with different concentrations of CMP (0.1, 0.2, 0.3, 0.4, and 0.5 mg/mL). After incubating at 30 °C without agitation for 24 and 48 h, the microplates were twice rinsed thoroughly with double-distilled water, and a 0.1% (w/v) solution of crystal violet (CV) was added to stain the attached cells. After staining at room temperature for 30 min, CV was removed, and the wells were rinsed with double-distilled water. The microplates were then air dried, and the dye was rendered soluble with a 95% ethanol solution. The absorbance at 595 nm of the soluble dye was subsequently determined by using an enzyme-linked immunosorbant assay (ELISA) plate reader (Molecular Devices, Sunnyvale, CA, USA). An equal volume of 95% ethanol was added to the control wells.
Invasion assays
The invasion of epithelial cells by L. monocytogenes Scott A was determined by using the gentamicin-based assayCitation17) with modifications. Confluent monolayers of HT-29 and AGS cells (1 × 106 cells/well) in 24-well tissue culture plates were washed twice with PBS and incubated at 37 °C in 5% CO2 with 100 μL of a L. monocytogenes Scott A suspension in RPMI-1640 at 1 × 106 and 1 × 108 CFU/mL to achieve a respective multiplicity of infection (MOI; the ratio of L. monocytogenes Scott A organisms to cells) of 1:1 and 100:1, and incubated for 1.5 h at 37 °C in the absence or presence of 0.4 mg/mL of CMP. The monolayers were washed with PBS, 250 μL of 1.0 mg/mL of gentamicin (Sigma) was added, and the solution incubated for an additional 1.5 h. The monolayers were then washed with PBS and lysed with 250 μL of 0.1% (v/v) Triton X-100 (Sigma) on ice. Appropriate dilutions were plated on TSBYE and incubated for 48 h at 37 °C. The inhibition of invasion is expressed as a percentage, using the relative decrease in invasion by Listeria in the presence of CMP, as determined by the formula, inhibition of invasion = (1 − T1/T2) × 100, where T1 and T2 are the numbers of invading Listeria cells (CFU/mL) in the respective presence and absence of CMP.
Semi-quantitative RT-PCR analysis
The regulation of virulence-associated genes was investigated by using the semi-quantitative RT-PCR technique. Total RNA was isolated from each treated sample by using an RNeasyTM total RNA kit (Qiagen) according to the manufacturer’s instructions. One microgram of total RNA was reverse transcribed in a 20-μL reaction mixture with SuperScriptTM III first-strand cDNA synthesis kit (Invitrogen, Carlsbad, CA, USA). The cDNA was then amplified by using Taq DNA polymerase (Takara, Japan). Denaturation for 5 min at 94 °C was followed by 35 cycles composed of 1 min of denaturation at 94 °C, 2 min of annealing at 60 °C, and 1 min of elongation at 72 °C. The last cycle was followed by 10 min of final elongation at 72 °C. The oligonucleotide (Bioneer, Daejun, Korea) sequences used for the amplification of each gene fragment including the expression of proteins (HT-29) are shown in Table . The intensity of each band was determined by a densitometric analysis of the gel, using a DC290 zoom digital camera and 1D image analysis software (Eastman Kodak Company, Rochester, NY, USA).
Table 1. Primer pairs used for amplification of virulence genes in L. monocytogenes.
Caenorhabditis elegans killing assay
The C. elegans killing assay was performed as describedCitation18) with some modifications. Briefly, L. monocytogenes Scott A was grown overnight in 10 mL of a TSBYE culture. The culture samples (10 μL each) were then spread on NGM agar plates (30 mm diameter for each). Instead of wild-type Bristol N2 worms, we tested the killing of C. elegans glp-4 mutant worms in the present study. C. elegans glp-4 mutant animals have a normal morphology and brood size at 15 °C, but do not make gonads and are unable to produce eggs at 25 °C. For each strain, healthy nematodes (n = 40) at the L4 stage were transferred from NGM plates seeded with Escherichia coli OP50 to the plates seeded with L. monocytogenes Scott A in the absence or presence of 0.4 mg/mL of CMP at 25 °C. The number of living worms per plate was determined by a CH30 optical microscope (Olympus, Tokyo, Japan) at various time intervals for 8 d. Nematodes were considered dead when they failed to respond to tapping of the plate. Each experimental condition was tested in duplicate or triplicate. E. coli OP50 was used as the control in analogous experiments.
Protein extraction for two-dimensional gel electrophoresis
This biofilm formation protocol was adapted from that used for the microtiter plate assay in this study, and was used to obtain a sufficient quantity of biofilm cells for two-dimensional gel electrophoresis (2-DE). Biofilms were prepared by using a slightly modified version of a previously described method.Citation19) L. monocytogenes was grown overnight in TSBYE, and a 2.5-mL overnight culture was washed twice with PBS. The cells were then inoculated into a 250-mL shake flask containing 100 mL of fresh MWB with or without the effective concentration of CMP on 10 g of sterilized glass wool (Pyrex fiber glass; Corning, NY, USA). The cells were incubated while shaking (250 rpm in a shaking incubator; Vision Scientific Co., Korea) at 30 °C to form a biofilm on the glass wool. The glass wool was retrieved from the culture 24 h after inoculation and then quickly (within 30 s) and gently washed twice in 100 mL of a 0.85% sodium chloride buffer at 4 °C. The biofilm cells were removed from the glass wool by sonication in 200 mL of a 0.85% sodium chloride buffer at 4 °C for 2 min. The buffer was then centrifuged (8000 × g in a VS-21S MT centrifuge; Vision Scientific Co.) for 10 min at 4 °C to precipitate the biofilm cells. These cells were washed twice by centrifugation (8000 × g, 10 min, 4 °C) with a 0.85% sodium chloride buffer; the cell pellets were then flash-frozen in liquid nitrogen. While sonicating, the suspended biofilm cells were harvested by centrifugation (8000 × g) at room temperature for 10 min. Each cell pellet was then flash-frozen in liquid nitrogen and kept at −80 °C until needed for protein extraction.
The biofilm cells harvested from the glass wool were suspended in the same lysis buffer (TE buffer; 40 mM Tris, 1 mM EDTA, pH 8.0) and lysed 20 times for 30 s on ice by using a UP200S Ultraschallprozessor (70 amplification, 0.5 cycle; Dr Hielscher GmbH). Following sonication, the unlysed cells, cell debris, and glass fiber were removed by centrifugation (8000 × g, 4 °C), and the supernatant was recovered. Protein samples were precipitated by using the phenol protocol.Citation20) The protein concentration was determined by using the modified Bradford assay method.Citation21) The protein samples were stored at −80 °C until needed for a proteomic analysis.
Two-dimensional gel electrophoresis
In the first dimension of isoelectric focusing (IEF), precast immobilized pH gradient (IPG) strips, with a nonlinear gradient from pH 3 to 10 (Bio-Rad Laboratories, Richmond, CA, USA), were rehydrated for 12–18 h with 500 μg of protein in 300 μL of a rehydration buffer (8 M urea, 2% CHAPS, 50 mM DTT, 10% isopropanol, 5% glycerol, 0.002% bromophenol blue, and 0.2% ampholytes). In accordance with the manufacturer’s instructions, focusing was performed by using a Protean IEF cell system (Bio-Rad Laboratories) at 20 °C under 60,000 Vh. After equilibration, the strips were transferred to 12.5% sodium dodecyl sulfate (SDS)-polyacrylamide gels (20 × 22 cm) for running in the second dimension. The proteins were then separated by using a Protean II xi system (Bio-Rad Laboratories) with 20 mA per gel at 4 °C. The protein spots were visualized by blue-silver staining.Citation22) The stained gels were then scanned with a UTA 2100XL densitometric scanner (UMAX, Dallas, TX, USA), and the spots were analyzed by using PDQuest software (Bio-Rad Laboratories) in accordance with the manufacturer’s instructions. Four gels resulting from two independent experiments were obtained, and two gels of good quality were used for further analysis. Only significant spot intensity changes (at least 3.0-fold) were considered and selected for mass spectrometric and sequencing analyses.
Protein identification
The resulting tryptic peptides were separated and then analyzed by reversed-phase capillary high-performance liquid chromatography (HPLC) directly coupled to Finnigan LCQ ion trap mass spectrometry (LC-MS/MS). Both the 0.1 × 20 mm trapping and 0.075 × 130 mm resolving columns were packed with Vydac 218MS low-trifluoroactic acid C18 beads (5 μL in size, 300 Å in pore size; Vydac, Hesperia, CA, USA) and placed in line. After the peptides had been bound to the trapping column for 10 min with 5% (v/v) aqueous acetonitrile containing 0.1% (v/v) formic acid, they were eluted with a 50-min gradient of 5–80% (v/v) acetonitrile containing 0.1% (v/v) formic acid at a flow rate of 0.2 μL/min. The full-mass scan range mode was used for MS/MS (m/z = 450–2000). After determining the charge states of ions on the zoom scans, product ion spectra were acquired in the MS/MS mode with a relative collision energy of 55%.
The individual spectra from MS/MS were processed by using TurboSEQUEST software (ThermoQuest, San Jose, CA, USA). The generated peak list files were used to query either the MSDB or National Center for Biotechnology Information (NCBI) database with the MASCOT program (http://www.matrixscience.com). Modifications of methionine and cysteine, peptide mass tolerance at 2 Da, MS/MS ion mass tolerance at 0.8 Da, allowance for missed cleavage at 2, and charge states (+1, +2 and +3) were considered. Only significant hits defined by the MASCOT probability analysis were initially considered.
Statistical analyses
All experiments were carried out in triplicate. All data were analyzed by ANOVA, and differences among means were tested for significance (p < 0.05) by Duncan’s multiple-range test with the SAS software package (version 9.1; SAS, Cary, NC, USA).
Results and discussion
Effects of CMP on biofilm formation on the abiotic surfaces
Biofilm formation on PVC and glass tube by L. monocytogenes was measured according to the procedures described in the Materials and Methods section. In the preliminary experiments, L. monocytogenes had formed a clear ring in the well just below the interface of air and liquid (Fig. (C)).
Fig. 1. Effects on biofilm formation of the active components in CMP.
Note: Biofilm formation by L. monocytogenes Scott A in the presence of a high (A; 1, 2, 3, 4, and 5 mg/mL) or low (B; 0.1, 0.2, 0.3, 0.4, and 0.5 mg/mL) concentration of CMP on a 96-well plate. Biofilm formation on the abiotic surfaces was quantified by the experimental procedure in the Materials and Methods section under static aerobic growth conditions in MWB for 24 h. Mean values for three independent experiments are shown with the standard deviation. Microscopic analyses of the inhibition by CMP of biofilm formation by L. monocytogenes Scott A on a glass surface (C). Cells of L. monocytogenes Scott A were incubated on a glass surface in an MWB medium in the presence (0.4 mg/mL) or absence of CMP and analyzed by optical microscopy for biofilm formation after 24 h. The cells were stained with 0.1% CV for optical microscopy. The area of the biofilm ring, as typically observed in the microtiter assay, was visualized over the full width.
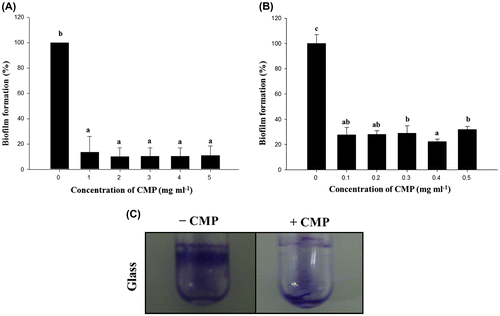
We initially determined whether CMP isolated from milk protein could influence the biofilm formation of L. monocytogenes. The PVC microplates that contained 1, 2, 3, 4, or 5 mg/mL of CMP showed the L. monocytogenes biofilms to be significantly decreased by <90% compared to the control (Fig. (A)). However, no significant differences were apparent among the tested concentrations of CMP. Next, when a low concentration of CMP (0.1, 0.2, 0.3, 0.4, or 0.5 mg/mL) was used, a profound inhibitory effect on biofilm formation was apparent at the concentration of 0.4 mg/mL (Fig. (B)). We also confirmed that the inhibitory effect of CMP on biofilm formation occurred in glass capillary tubes under continuous-flow culture conditions (data not shown). In addition, as we expected, a low concentration of CMP did not significantly affect the growth yield of L. monocytogenes in MWB at 30 °C (data not shown). Therefore, the biofilm inhibition in this study did not result from a growth defect due to the bactericidal activity of CMP.
It is important to note that it has been well established that milk proteins or peptides have strong health-promoting functions. In particular, CMP present in a whey protein fraction can be attributed to its various biological activities, and their potential functionality has grown to a great extent.Citation23) However, a few reports indicated that CMP could influence the virulence of foodborne pathogens including biofilm formation. Recent studies have indicated that several milk peptides, including osteopontinCitation24) and lactoferrin,Citation25) strongly reduced the amount of dental biofilm. We indicated here that CMP would be potentially useful in preventing biofilm formation on PVC and glass surfaces. This is the first report to our knowledge on the impact of CMP on the biofilm formation of L. monocytogenes.
Inhibitory effects of CMP on the invasion of epithelial cells
Some virulence features of L. monocytogenes, including invasion, have been critically associated with biofilm formation.Citation26) CMP in this study was therefore examined for its ability to inhibit the invasion by L. monocytogenes Scott A of HT-29 and AGS cells. The inhibitory effectiveness of CMP on the invasion by L. monocytogenes of HT-29 and AGS is shown in Table . Importantly, under both MOI conditions, CMP significantly repressed the invasion ability by L. monocytogenes Scott A of both HT-29 and AGS cells (over 40% reduction compared to L. monocytogenes alone). Many researchers have previously reported protective effects against the adhesion or invasion of a variety of enteric pathogens, including L. monocytogenes, as a result of acidification,Citation27) secreted non-acidic productsCitation28) and interference with the attachment to receptors or spaces which could occur both directly and indirectlyCitation29); hence, the surface features of L. monocytogenes are important for determining the success of an invasion event into the host cells. To date, specific milk components have resulted in a lower invasion capability of L. monocytogenes when compared to casein and lactose.Citation30) Similarly, some researchers have reported that milk components inhibited the adhesion to or invasion of mammalian cells.Citation31) CMP possesses specific glycosidic residues made up of fucose, galactose, galactosamine, sialic acid, and other sugars.Citation32) Importantly, such carbohydrate-containing CMP successfully prevented binding of Streptococcus sobrinus and S. sangiusCitation33) to the surface as well as the human influenza virus.Citation34) We have also shown that the glycosylated fraction of CMP was able to inhibit the cytotoxicity of cholera toxin that bound to the globotriaosyl ceramide (Gb3) receptor on host cells.Citation35) Taken together, we suggest that CMP can affect the characteristics of a bacterial surface (e.g. invasion-related factors) directly or indirectly.
Table 2. Invasion of L. monocytogenes Scott A to HT-29 and AGS cells in the presence or absence of 0.4 mg/mL of CMP.
Virulence gene mRNA expression mediated by CMP in HT-29 cells
We next investigated the mRNA levels of such virulence-associated genes of L. monocytogenes as inlA (encoding internalin A), plcA (encoding encoding phosphatidylinositol-specific phospholipase C) and hlyA (encoding a hemolysin) in the presence of 0.4 mg/mL of CMP. It has been well established that inlA mainly involved in invasion event of L. monocytogenes in all cell types, whereas plcA and hlyA contributed to lyse phagosome in mammalian cells and invaded other tissues.Citation1) Importantly, we have shown that inlA was significantly repressed by 5.2 ± 0.1-fold in the presence of 0.4 mg/mL of CMP in L. monocytogenes Scott A-infected HT-29 cells. However, the transcription of plcA and hlyA was not changed under the same concentration of CMP (respective reductions of 1.5 ± 0.9- and 1.8 ± 0.5-fold when compared with L. monocytogenes alone). Park and Kroll have reported that the presence in a growth medium of the plant-derived disaccharide, cellobiose, strongly repressed the expression of both plcA and hlyA in L. monocytogenes NCTC 7973.Citation36) Unfortunately, they did not determine the impact of cellobiose on a listerial biofilm, nor the enhanced transcription of the inlA gene.
Internalin A (encoded by inlA), is a cell wall-bound protein of L. monocytogenes that is responsible for adhesion to and invasion of host cells expressing specific forms of E-cadherin.Citation1) However, there are a few reports on the specific interaction between internalin A and biofilm formation for L. monocytogenes. The biofilm-forming ability of L. monocytogenes strains has recently been reported to be significantly influenced by the modification of internalin A.Citation37) We have indicated here that the mRNA level of inlA in HT-29 cells was specifically repressed in the presence of 0.4 mg/mL. These findings are consistent with our results that CMP significantly inhibited the invasion of L. monocytogenes in mammalian cells (Table ) as well as the biofilm formation (Fig. ). Importantly, CMP had the ability for adhesion to pathogens including Salmonella and EHEC O157:H7, this activity being dependent on carbohydrate moieties like sialic acid in GMP.Citation38) CMP has been separated by column chromatography into seven distinct fractions with up to five sialic acid groups containing one or two of the di-, tri-, and tetra-saccharides.Citation32) One possibility is that specific carbohydrate moieties in GMP could influence the initial adhesion and invasion by repressing the transcription of inlA, and consequently repressing biofilm development of L. monocytogenes. We are currently purifying the active fractions of CMP for interacting with inlA-associated factors by using column chromatography and LC-MS/MS.
Nematode killing assay
This study was designed to determine whether CMP could affect the inhibition of pathogenicity from L. monocytogenes Scott A. We employed C. elegans as the in vivo host for determining the impact of CMP against listerial infection as previously described.Citation39) Survival assays showed that there was a significant decrease in the survival percentage in the group treated with L. monocytogenes Scott A. There were significant differences in the survival rates of C. elegans after the first day between the control and treatment group. Compared to E. coli OP50 (the normally fed strain) as a negative control, C. elegans placed on a mat of L. monocytogenes Scott A resulted in only 33.3% of the nematodes surviving for 8 days (Fig. ). In contrast, 45.8% of the worms survived on an agar medium supplemented with CMP (0.4 mg/mL) under the same conditions.
Fig. 2. Effects of CMP (0.4 mg/mL) on the survival of C. elegans nematodes living on a Lawn of L. monocytogenes Scott A for 8 days.
Note: Errors bars indicate the standard deviation (SD) between independent experiments.
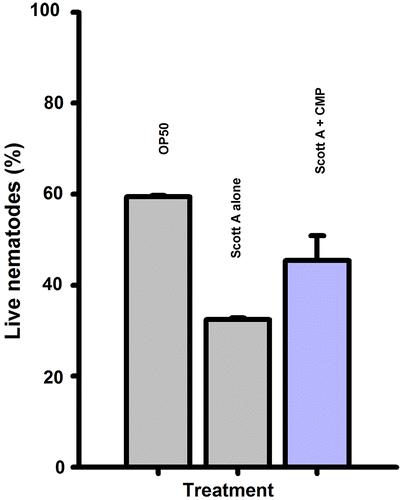
Dietary supplements of functional foods have previously been reported to have a positive effect on longevity in C. elegans.Citation40) Our groups have also demonstrated that the functional fractions of LAB significantly prolonged the survival of C. elegans when exposed to pathogenic bacteria.Citation18) We similarly showed that L. monocytogenes in the presence of CMP exhibited decreased virulence by slowly killing the C. elegans nematodes (Fig. ). Invasion and biofilm formation are major virulence phenotypes of L. monocytogenes. We had already confirmed that CMP not only decreased invasion but also critically inhibited biofilm development; this combination of data suggests that the low pathogenicity of L. monocytogenes toward the host may have been mediated by inhibiting the invasion and biofilm formation by CMP.
Identification of CMP activity regulating proteins in biofilm cells
A proteomics approach was conducted to determine the proteins involved in inhibiting biofilm formation by CMP. Total proteins of L. monocytogenes Scott A cultured either in biofilm cells in the absence or in the presence of 0.4 mg/mL of CMP for 24 h at 30 °C were analyzed by 2-DE. A total of approximately 320 protein spots could be visualized by CBB G-250 staining (Fig. ). It is interesting that a total of nine protein spots with an increase or decrease in amount were detected from L. monocytogenes Scott A in the presence of CMP when compared with the control (two and seven spots were, respectively, up- and down-regulated). The proteins identified by LC-MS/MS are summarized in Table .
Fig. 3. Two-dimensional gel electrophoretic images of protein extracts of the L. monocytogenes Scott A biofilm cells in the absence (A) and presence of 0.4 mg/mL of CMP (B).
Note: The crude protein extracts (500 μg) were separated on pH 3.0–10.0 non-linear IPG strips and followed by 12.5% SDS-PAGE. The proteins were separated, detected by blue silver staining, and the data were analyzed as described in the experimental procedure.
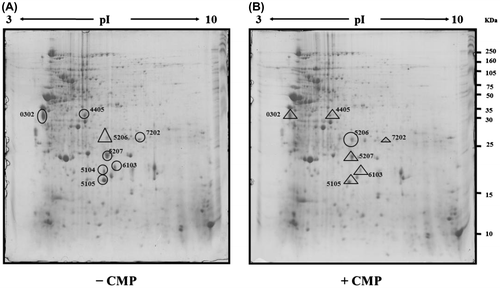
Table 3. Differentially regulated proteins of L. monocytogenes Scott A in the presence of CMP (0.4 mg/mL) and identification of selected spots by LC-MS/MS.
Only four spots were similar to known proteins related to the metabolism and virulence factors (Table ). Comparative proteomes obtained with the CMP treatment showed that the intensity of one protein spot increased, while that of seven spots decreased. Spot 0302 was identified as enolase which might be one of the key enzymes in the glycolytic cycle of cytoplasm. Enolase binds to plasminogen and subsequently facilitates penetration through the host’s cell membrane by activating plasminogen during an invasive infection. This has also recently been shown to be surface-exposed and able to bind plasminogen in L. monocytogenes, a Gram-positive facultative intracellular pathogen.Citation41) Spot 4405 was identified as 6-phosphofructokinase that has been reported as important to the glycolytic pathway for invasion.Citation42) This previous study demonstrated that the regulation of carbon metabolism and the expression of glycolysis gene pfkA were critical for synthesis of the virulence gene regulators, VirF and VirB, and that both the glycolytic and gluconeogenic pathways influenced steps in intracellular pathogen Shigella flexneri invasion and plaque formation. CMP may therefore play an essential role in cell defense against invasion by L. monocytogenes Scott A. Moreover, spot 6103 appeared to be the glutamine synthase which mediates virulence in many bacterial pathogens, including Mycobacterium bovisCitation43) and Streptococcus suis serotype 2.Citation44) The presence of glutamine synthetase in Lactobacillus crispatus has also been proposed for adhesion to epithelial cells.Citation45) It has been suggested that this moonlighting protein contributed to some probiotic traits.Citation46) In this respect, we suggest that CMP was involved in the glutamine sythetase down-regulation that inhibited bacterial adhesion on abiotic surfaces. Spot 5206 was a glyceraldehyde-3-phosphate dehydrogenase which is one of the key components of the Krebs cycle. A previous study has reported that the synthesis of this enzyme increased in B. subtilis during cold-shock stress.Citation47)
These results indicate that the newly identified proteins were associated with the control of listerial biofilms and that these proteins can be potential targets for the development of anti-biofilm or anti-virulence agents for L. monocytogenes.
Conclusions
CMP that naturally occurs in substances from milk critically inhibited biofilm formation by repressing invasion by L. monocytogenes. In addition, significant reduction in the pathogenicity of L. monocytogenes was apparent in the in vivo model of C. elegans nematodes by adding CMP. Our results suggest that CMP would be capable of eliminating biofilm formation by L. monocytogenes in the food industry.
Acknowledgments
We are grateful to Dr Joseph F. Frank (University of Georgia) for providing us with L. monocytogenes Scott A.
Funding
This work was supported by the Cooperative Research Program for Agriculture Science & Technology Development [project number PJ009769] of the Rural Development Administration in Republic of Korea (Y.K.).
References
- Schlech WF. Foodborne listeriosis. Clin. Infect. Dis. 2000;31:770–775.10.1086/314008
- Bryers JD. Biofilms and the technological implications of microbial cell adhesion. Colloids Surf., B. 1994;2:9–23.10.1016/0927-7765(94)80013-8
- Møretrø T, Langsrud S. Listeria monocytogenes: biofilm formation and persistence in food-processing environments. Biofilms. 2004;1:107–121.10.1017/S1479050504001322
- Barnes LM, Lo MF, Adams MR, Chamberlain AHL. Effect of milk proteins on adhesion of bacteria to stainless steel surfaces. Appl. Environ. Microbiol. 1999;65:4543–4548.
- Donelli G, Francolini I. Efficacy of antiadhesive, antibiotic and antiseptic coatings in preventing catheter-related infections: Review. J. Chemother. 2001;13:595–606.10.1179/joc.2001.13.6.595
- Carrique-Mas JJ, Hokeberg I, Andersson Y, Arneborn M, Tham W, Danielsson-Tham ML, Osterman B, Leffler M, Steen M, Eriksson E, Hedin G, Giesecke J. Febrile gastroenteritis after eating on-farm manufactured fresh cheese-an outbreak of listeriosis? Epidemiol. Infect. 2003;130:79–86.10.1017/S0950268802008014
- Frye DM, Zweig R, Sturgeon J, Tormey M, LeCavalier M, Lee I, Lawani L, Mascola L. An outbreak of febrile gastroenteritis associated with delicatessen meat contaminated with Listeria monocytogenes. Clin. Infect. Dis. 2002;35:943–949.10.1086/cid.2002.35.issue-8
- Marco AJ, Prats N, Ramos JA, Briones V, Blanco M, Dominguez L, Domingo M. A microbiological, histopathological and immunohistological study of the intragastric inoculation of Listeria monocytogenes in mice. J. Comp. Pathol. 1992;107:1–9.10.1016/0021-9975(92)90090-H
- Czuprynski CJ, Faith NG, Steinberg H. Ability of the Listeria monocytogenes strain Scott A to cause systemic infection in mice infected by the intragastric route. Appl. Environ. Microbiol. 2002;68:2893–2900.10.1128/AEM.68.6.2893-2900.2002
- Park JH, Park YH, Seok SH, Cho SA, Kim DJ, Lee HY, Kim SH, Park JH. Suppurative gastritis in BALB/c mice infected with Listeria monocytogenes via the intragastric route. J. Comp. Pathol. 2004;130:130–136.10.1016/j.jcpa.2003.10.001
- El-Salam MHA, El-Shibiny S, Buchheim W. Characteristics and potential uses of the casein macropeptide. Int. Dairy J. 1996;6:327–341.10.1016/0958-6946(95)00026-7
- Kawasaki Y, Isoda H, Tanimoto M, Dosako S, Idota T, Ahiko K. Inhibition by lactoferrin and kappa-casein glycomacropeptide of binding of Cholera toxin to its receptor. Biosci. Biotechnol. Biochem. 1992;56:195–198.10.1271/bbb.56.195
- Rhoades JR, Gibson GR, Formentin K, Beer M, Greenberg N, Rastall RA. Caseinoglycomacropeptide inhibits adhesion of pathogenic Escherichia coli strains to human cells in culture. J. Dairy Sci. 2005;88:3455–3459.10.3168/jds.S0022-0302(05)73029-0
- Katla T, Moretro T, Sveen I, Aasen IM, Axelsson L, Rorvik LM, Naterstad K. Inhibition of Listeria monocytogenes in chicken cold cuts by addition of sakacin P and sakacin P-producing Lactobacillus sakei. J. Appl. Microbiol. 2002;93:191–196.10.1046/j.1365-2672.2002.01675.x
- Djordjevic D, Wiedmann M, McLandsborough LA. Microtiter plate assay for assessment of Listeria monocytogenes biofilm formation. Appl. Environ. Microbiol. 2002;68:2950–2958.10.1128/AEM.68.6.2950-2958.2002
- Premaratne RJ, Lin WJ, Johnson EA. Development of an improved chemically defined minimal medium for Listeria monocytogenes. Appl. Environ. Microbiol. 1991;57:3046–3048.
- Bunduki MC, Beliveau CM, Donnelly CW. Examination of attachment and phagocytic uptake of Listeria species by mammalian intestinal cells. Food Microbiol. 1993;10:507–516.10.1006/fmic.1993.1056
- Kim Y, Oh S, Park S, Seo JB, Kim SH. Lactobacillus acidophilus reduces expression of enterohemorrhagic Escherichia coli O157:H7 virulence factors by inhibiting autoinducer-2-like activity. Food Control. 2008;19:1042–1050.10.1016/j.foodcont.2007.10.014
- Ren D, Bedzyk LA, Thomas SM, Ye RW, Wood TK. Gene expression in Escherichia coli biofilms. Appl. Microbiol. Biotechnol. 2004;64:515–524.10.1007/s00253-003-1517-y
- Kim YH, Lee Y, Kim S, Yeom J, Yeom S, Seok Kim B, Oh S, Park S, Jeon CO, Park W. The role of periplasmic antioxidant enzymes (superoxide dismutase and thiol peroxidase) of the Shiga toxin-producing Escherichia coli O157:H7 in the formation of biofilms. Proteomics. 2006;6:6181–6193.
- Ramagli L. Quantifying Protein in 2-D PAGE Solubilization Buffers In: Link A, editor. 2-D Proteome analysis protocols. Totowa: Humana Press; 1999. p. 99–103.
- Candiano G, Bruschi M, Musante L, Santucci L, Ghiggeri GM, Carnemolla B, Orecchia P, Zardi L, Righetti PG. Blue silver: a very sensitive colloidal Coomassie G-250 staining for proteome analysis. Electrophoresis. 2004;25:1327–1333.10.1002/(ISSN)1522-2683
- El-Sayed MH, Chase H. Trends in whey protein fractionation. Biotechnol. Lett. 2011;33:1501–1511.10.1007/s10529-011-0594-8
- Schlafer S, Raarup MK, Wejse PL, Nyvad B, Städler BM, Sutherland DS, Birkedal H, Meyer RL. Osteopontin reduces biofilm formation in a multi-species model of dental biofilm. PLoS ONE. 2012;7:e41534.10.1371/journal.pone.0041534
- Dashper SG, Pan Y, Veith PD, Chen Y-Y, Toh ECY, Liu SW, Cross KJ, Reynolds EC. Lactoferrin inhibits porphyromonas gingivalis proteinases and has sustained biofilm inhibitory activity. Antimicrob. Agents Chemother. 2012;56:1548–1556.10.1128/AAC.05100-11
- Riedel CU, Monk IR, Casey PG, Waidmann MS, Gahan CGM, Hill C. AgrD-dependent quorum sensing affects biofilm formation, invasion, virulence and global gene expression profiles in Listeria monocytogenes. Mol. Microbiol. 2009;71:1177–1189.10.1111/mmi.2009.71.issue-5
- Oh DH, Marshall DL. Monolaurin and acetic acid inactivation of Listeria monocytogenes attached to stainless steel. J. Food Prot. 1996;59:249–252.
- Coconnier MH, Bernet MF, Kerneis S, Chauviere G, Fourniat J, Servin AL. Inhibition of adhesion of enteroinvasive pathogens to human intestinal Caco-2 cells by Lactobacillus acidophilus strain LB decreases bacterial invasion. FEMS Microbiol. Lett. 1993;110:299–305.10.1111/fml.1993.110.issue-3
- Hirano J, Yoshida T, Sugiyama T, Koide N, Mori I, Yokochi T. The effect of Lactobacillus rhamnosus on enterohemorrhagic Escherichia coli infection of human intestinal cells in vitro. Microbiol. Immunol. 2003;47:405–409.10.1111/mim.2003.47.issue-6
- Pricope-Ciolacu L, Nicolau AI, Wagner M, Rychli K. The effect of milk components and storage conditions on the virulence of Listeria monocytogenes as determined by a Caco-2 cell assay. Int. J. Food Microbiol. 2013;166:59–64.10.1016/j.ijfoodmicro.2013.05.027
- Petrone G, Conte MP, Longhi C, di Santo S, Superti F, Ammendolia MG, Valenti P, Seganti L. Natural milk fatty acids affect survival and invasiveness of Listeria monocytogenes. Lett. Appl. Microbiol. 1998;27:362–368.10.1046/j.1472-765X.1998.00441.x
- Brody EP. Biological activities of bovine glycomacropeptide. Br. J. Nutr. 2000;84:S39–46.
- Neeser JR, Golliard M, Woltz A, Rouvet M, Dillmann ML, Guggenheim B. In vitro modulation of oral bacterial adhesion to saliva-coated hydroxyapatite beads by milk casein derivatives. Oral Microbiol. Immunol. 1994;9:193–201.10.1111/omi.1994.9.issue-4
- Kawasaki Y, Isoda H, Shinmoto H, Tanimoto M, Dosako S, Idota T, Nakajima I. Inhibition by kafppa-casein glycomacropeptide and lactoferrin of influenza virus hemagglutination. Biosci. Biotechnol. Biochem. 1993;57:1214–1215.10.1271/bbb.57.1214
- Oh S, Worobo RW, Kim B, Rheem S, Kim S. Detection of the cholera toxin-binding activity of kappa-casein macropeptide and optimization of its production by the response surface methodology. Biosci. Biotechnol. Biochem. 2000;64:516–522.10.1271/bbb.64.516
- Park SF, Kroll RG. Expression of listeriolysin and phosphatidylinositol-specific phospholipase C is repressed by the plant-derived molecule cellobiose in Listeria monocytogenes. Mol. Microbiol. 1993;8:653–661.10.1111/mmi.1993.8.issue-4
- Franciosa G, Maugliani A, Scalfaro C, Floridi F, Aureli P. Expression of internalin A and biofilm formation among Listeria monocytogenes clinical isolates. Int. J. Immunopathol. Pharmacol. 2009;22:183–193.
- Nakajima K, Tamura N, Kobayashi-Hattori K, Yoshida T, Hara-Kudo Y, Ikedo M, Sugita-Konishi Y, Hattori M. Prevention of intestinal infection by glycomacropeptide. Biosci. Biotechnol. Biochem. 2005;69:2294–2301.
- Thomsen LE, Slutz SS, Tan M-W, Ingmer H. Caenorhabditis elegans is a model host for Listeria monocytogenes. Appl. Environ. Microbiol. 2006;72:1700–1701.10.1128/AEM.72.2.1700-1701.2006
- Shibamura A, Ikeda T, Nishikawa Y. A method for oral administration of hydrophilic substances to Caenorhabditis elegans: Effects of oral supplementation with antioxidants on the nematode lifespan. Mech. Ageing Dev. 2009;130:652–655.10.1016/j.mad.2009.06.008
- Schaumburg J, Diekmann O, Hagendorff P, Bergmann S, Rohde M, Hammerschmidt S, Jansch L, Wehland J, Karst U. The cell wall subproteome of Listeria monocytogenes. Proteomics. 2004;4:2991–3006.10.1002/(ISSN)1615-9861
- Gore AL, Payne SM. CsrA and Cra influence Shigella flexneri pathogenesis. Infect. Immun. 2010;78:4674–4682.10.1128/IAI.00589-10
- Chandra H, Basir SF, Gupta M, Banerjee N. Glutamine synthetase encoded by glnA-1 is necessary for cell wall resistance and pathogenicity of Mycobacterium bovis. Microbiology. 2010;156:3669–3677.10.1099/mic.0.043828-0
- Si Y, Yuan F, Chang H, Liu X, Li H, Cai K, Xu Z, Huang Q, Bei W, Chen H. Contribution of glutamine synthetase to the virulence of Streptococcus suis serotype 2. Vet. Microbiol. 2009;139:80–88.10.1016/j.vetmic.2009.04.024
- Kainulainen V, Loimaranta V, Pekkala A, Edelman S, Antikainen J, Kylvaja R, Laaksonen M, Laakkonen L, Finne J, Korhonen TK. Glutamine synthetase and glucose-6-phosphate isomerase are adhesive moonlighting proteins of Lactobacillus crispatus released by epithelial cathelicidin LL-37. J. Bacteriol. 2012;194:2509–2519.10.1128/JB.06704-11
- Henderson B, Martin A. Bacterial moonlighting proteins and bacterial virulence. Curr. Top Microbiol. Immunol. 2013;358:155–213.
- Graumann P, Schroder K, Schmid R, Marahiel MA. Cold shock stress-induced proteins in Bacillus subtilis. J. Bacteriol. 1996;178:4611–4619.
