Abstract
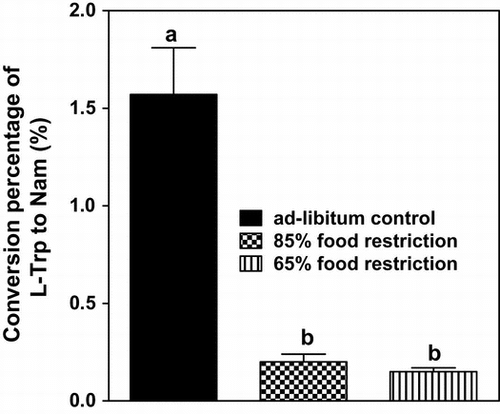
Calorie restriction leads to a change in the metabolism of nutrients. Nicotinamide is biosynthesized from l-tryptophan. We attempted to determine the effects of food restriction on the biosynthesis of nicotinamide from l-tryptophan. Weaning male rats were fed a conventional chemically defined diet without preformed niacin for 63 d. However, the food intake was restricted to 80 and 65% of the intake of the ad libitum-fed control group of rats. The 24-h urine samples were periodically collected, and the urinary excretion of nicotinamide and its catabolites was measured. The conversion percentages were lower in both restricted groups than in the ad libitum-fed control group during the experimental period (control group, 1.37 ± 0.24%; 80%-restricted group, 0.20 ± 0.04%; 65%-restricted group, 0.15 ± 0.02%; control vs. restricted groups, p < 0.01). Food restriction, even at mild level, suppressed the conversion of l-tryptophan to nicotinamide when compared to the ad libitum-fed control group.
Graphical Abstract
Moderate food restriction suppressed the conversion of Trp-Nam in rats. The prevalence of pellagra in the 'hungry' season might be associated with a lower conversion percentage of Trp-Nam.
Calorie restriction, namely food restriction, leads to a change in the metabolism of nutrients. Some reports have revealed preferred phenomena for maintaining health by calorie restriction; for example, longevityCitation1) and preventing the aging of oocytes.Citation2)
The vitamin nicotinamide (Nam) is biosynthesized from the essential amino acid, l-tryptophan (l-Trp), via NAD+ in mammals, and Nam is then distributed to non-hepatic tissues. The de novo synthesis of Nam from l-Trp is therefore very important for supplying the Nam vitamin. In fact, rats and mice do not need preformed niacin when they are ad libitum fed a diet containing 20% casein.Citation3,4) Humans can theoretically produce the necessary amount of niacin by the intake of only l-Trp without preformed niacin.Citation5)
We investigated in the present study how food restriction would affect the conversion percentage of l-Trp to Nam using rats. We found that food restriction, even at a mild level, suppressed the conversion of l-Trp to Nam when compared to ad libitum-fed rats.
Materials and methods
Ethical approval of the study protocol
The care and treatment of experimental animals conformed to the guidelines set by the University of Shiga Prefecture (Shiga, Japan) for the ethical treatment of laboratory animals.
Animals and diets
The animal room was maintained at around 22 °C with 60% humidity. Rats were subjected to a 12 h/12 h light–dark cycle (06:00–18:00/18:00–06:00).
Male 3-week-old Wistar rats were obtained from Clea Japan (Tokyo, Japan) and immediately placed in individual CL-301 metabolic cages (Clea Japan). A control group had ad libitum access to a conventional purified diet for 63 d. This diet comprised 20% vitamin-free milk casein, 0.2% l-methionine, 46.9% gelatinized cornstarch, 23.4% sucrose, 5% corn oil, 3.5% AIN-93-G mineral mixtureCitation6) and a 1% AIN-93 vitamin mixture without nicotinic acidCitation6) containing choline bitartrate.
Calorie restriction was achieved by restricting the food supplied. In the food-restricted groups, the food intake was restricted to 65 or 80% of that eaten by the ad libitum-fed control rats on the previous day. This restriction still resulted in body weight gain, so we have termed it as moderate food restriction.
The 24-h urine samples were periodically collected in amber bottles from 09:00 to 09:00 on the next day and were stored at –20 °C until needed.
Laboratory analyses
N1-Methylnicotinamide (MNA) chloride (C7H9N2O-HCl; 159.61 molecular weight) was purchased from Tokyo Kasei Kogyo (Tokyo, Japan). N1-Methyl-2-pyridone-5-carboxamide (2-Py; C7H8N2O2; 152.15) and N1-methyl-4-pyridone-3-carboxamide (4-Py; C7H8N2O2; 152.15) were respectively synthesized by the methods of Pullman and ColowickCitation7) and Shibata et al.Citation8) All other chemicals used were of the highest purity available from commercial sources.
The contents of Nam, 2-Py, and 4-Py in the urine were simultaneously measured by the high-performance liquid chromatographic (HPLC) method devised by Shibata et al.Citation8) The urine content of MNA was also measured by an HPLC method devised by Shibata et al.Citation9) The conversion percentage was calculated by comparing the l-Trp intake during urine collection and the sum of the urinary excretion amounts of Nam, MNA, 2-Py, and 4-Py.
Statistical analyses
Each value is the mean ± SEM. Statistical significance was determined by ANOVA, followed by Tukey multiple-comparison test; p < 0.05 was considered to be significant. Prism 5.0 (Graph Pad Software, San Diego, CA, USA) was used for all analyses.
Results
Weaning rats of about 40 g in body weight had free or restricted access to the 20% casein diet. The food intake over 63 d was 1,025 ± 19 g for the ad libitum-fed control group, 809 ± 2 g for the 80% restricted group, and 663 ± 2 g for the 65% restricted group. The changes in body weight under these conditions are shown in Fig. . A body weight gain occurred even when they were restricted to 65% of the intake by the ad libitum-fed control group. The final respective body weights for the control, 80% restricted, and 60% restricted groups were 381 ± 9, 297 ± 2, and 237 ± 5 g. The respective body weight ratios among the three groups were 1.00:0.78:0.62 for the ad libitum-fed control, and 80 and 65% restricted groups.
Fig. 1. Change in body weight of the rats.
Note: ●, ad libitum feeding (control group); ○, 80% restriction of food intake; and △, 65% restriction of food intake. Each symbol reflects the mean ± SEM for 5 rats. Values on the last day of the experiment that do not share the same superscripted letters are significantly different by ANOVA and subsequently Tukey multiple-comparison test.
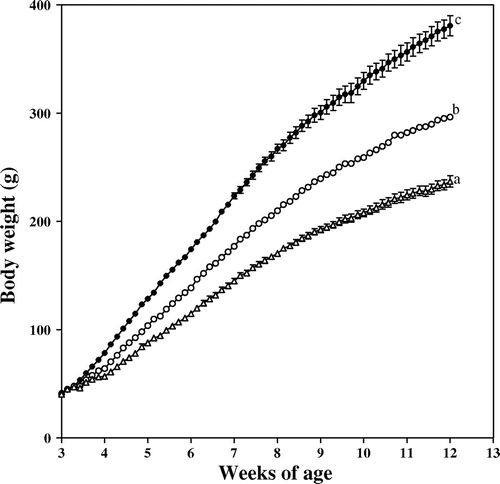
Fig. shows the effect of food restriction on the urinary excretion of Nam and its metabolites, MNA, 2-Py, and 4-Py. The urinary excretion of Nam, MNA, 2-Py, and 4-Py was all higher by the ad libitum-fed control group than by both the restricted groups. There was little difference in the urinary excretion amounts of Nam and its metabolites between the 80 and 65% food-restricted groups.
Fig. 2. Effects of food restriction on the urinary excretion of Nam (A), MNA (B), 2-Py (C), 4-Py (D), and the sum of Nam and its catabolites (E).
Note: ●, ad libitum feeding (control group); ○, 80% restriction of food intake; and △, 65% restriction of food intake. Each symbol denotes the mean ± SEM for 5 rats. Values at the same day of the experiment that do not share the same superscripted letters are significantly different by ANOVA followed by Tukey multiple-comparison test.
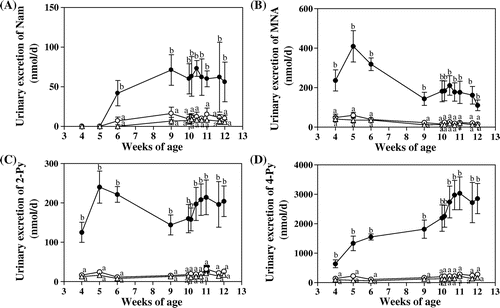
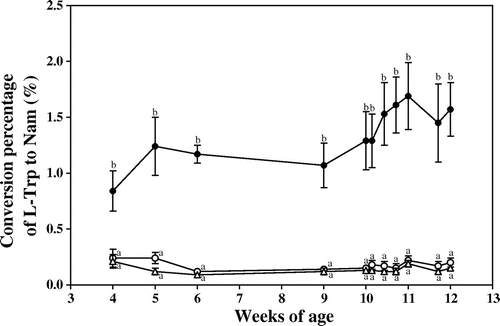
Fig. shows the effect of food restriction on the conversion percentage of l-Trp to Nam. Food restriction markedly decreased the conversion percentage in comparison with the ad libitum-fed control. The conversion percentage was almost the same in the 80 and 65% food-restricted groups.
Fig. 3. Effects of food restriction on the conversion percentage of l-Trp to nam.
Note: ●, ad libitum feeding (control group); ○, 80% restriction of food intake; and △, 65% restriction of food intake. Each symbol denotes the mean ± SEM for 5 rats. Values at the same day of the experiment that do not share the same superscripted letters are significantly different by ANOVA and subsequently Tukey multiple-comparison test.
Discussion
Krehl et al.Citation10) have reported that rats did not need preformed niacin if a sufficient amount of l-Trp was supplied. It is also known that the l-Trp-to-Nam pathway is very weak during the lactating period, because the activities of liver l-Trp 2,3-dioxygenaseCitation11) and liver kynureninaseCitation12) can hardly be detected during lactation. These enzyme proteins would not be fully synthesized during this period; therefore, the increased conversion percentage of l-Trp to Nam suggests that the level of biosynthesis of these enzyme proteins increased according to age. Shibata et al.Citation13) have reported that the conversion percentage increased according to growth up to 8 weeks of age in rats.
The food intake was restricted to 65 and 80% of the amount supplied to the ad libitum-fed control rats. Nam, MNA, 2-Py, and 4-Py originated from l-Trp in the present study because the diet did not contain any preformed niacin. The urinary excretion of Nam, MNA, 2-Py, and 4-Py was much lower in both food-restricted groups than in the ad libitum-fed control group. As anticipated, the excretion of each increased according to growth (from 4 weeks of age to 5 weeks of age) for the control group, but did not increase for the food-restricted groups. Calorie restriction generally facilitates amino acid degradation. Consequently, the food-restricted rats, namely “hungry rats”, could not afford to synthesize the l-Trp 2,3-dioxygenase and kynureninase proteins because of insufficient energy. In the present experiment, the respective l-Trp intake by the control, and 80 and 65% food-restricted groups was around 150, 120, and 100 mg/kg of body weight; these figures were calculated by assuming that the average daily food intake by the control animals was 15 g and that 100 g of the food contained 234 mg of l-Trp (the food contained 20% of casein, the protein content of the casein being 90%, and the l-Trp content of pure casein being 1.3%), and that the average rat body weight was 250 g. Shibata and MurataCitation14) have reported that the minimum requirement for l-Trp in growing rats was around 85 mg/kg of body weight. Therefore, the present finding was not attributable to any insufficiency of dietary l-Trp.
There are three regulation points of l-Trp to Nam; the first point is the initial l-Trp → l-N-formylkynurenine reaction which is catalyzed by TDO; the second is the branching point of kynurenine reactions, l-kynurenine → l-3-hydrokykynurenine which is catalyzed by kynurenine 3-monooxygenase, l-kynurenine → anthranilic acid which is catalyzed by kynureninase, and l-kynurenine → kynurenic acid which is catalyzed by kynurenine aminotransferase; the third is the branching point of α-amino-β-carboxymuconate-ε-semialdehyde (ACMS) reactions, ACMS → α-aminomuconate-ε-semialdehyde (AMS) which is catalyzed by ACMS decarboxylase (ACMSD) and ACMS → QA which is non-enzymatic reaction. The effects of moderate calorie restriction on the regulatory enzymes are not well known.
We have previously studied an outbreak of pellagra based on the hypothesis that a primary factor would be a decrease in the conversion of l-Trp to Nam but not a simple deficiency of niacin and l-Trp. The present finding that the conversion of l-Trp to Nam was significantly reduced by calorie restriction is interesting because it had been considered that the conversion was only affected by the quality of foodCitation15–25) (e.g.,Citation15) it was decreased with increasing dietary proteins and with increasing dietary unsaturated fatty acids, and affected by the dietary sources of carbohydrates.) and not by the quantity of food. However, our hypothesis was not correct: the present findings show for first time that the conversion of l-Trp-Nam was affected by the quantity of food as well as by the quality of food.
Pellagra results from a diet that is deficient in Nam and/or l-Trp. The disease remains a public-health issue in many maize-consuming countries in Africa and Asia, especially in emergency-affected and conflict-affected populations.Citation26–30) The great majority of pellagra suffers have been women.Citation27,28) The time of the year associated with a high prevalence of pellagra is termed the “hungry” season,Citation29) which means people are restricted in their calorie intake by an insufficient supply of food. Although the theory about the cause of pellagra has been elucidated, it still remains a mystery why pellagra incidence is high in women and in the “hungry” season. The high prevalence of pellagra in the “hungry” seasonCitation29) might be associated with a lower conversion percentage of l-Trp to Nam. Furthermore, the high prevalence of pellagra in women may be because men have been given priority for food in the home, thereby resulting in food restriction for the women.
Acknowledgment
This investigation was part of the project “Studies on the nutritional evaluation of amino acids and B-group vitamins” (Katsumi Shibata was the principal investigator), supported by a scientific research from Japan Society for the Promotion of Science.
Notes
Abbreviations: AMS, α-aminomuconate-ε-semialdehyde; ACMS, α-amino-β-carboxymuconate-ε-semialdehyde; ACMSD, α-amino-β-carboxymuconate-ε-semialdehyde decarboxylase; HPLC, high-performance liquid chromatography; MNA, N1-methylnicotinamide; 2-py, N1-methyl-2-pyridone-5-carboxamide; 4-Py, N1-methyl-4-pyridone-3-carboxamide; Nam, Nicotinamide; l-Trp, l-tryptophan.
References
- Park S, Mori R, Shimokawa I. Do sirtuins promote mammalian longevity? A critical review on its relevance to the longevity effect induced by calorie restriction. Mol. Cells. 2013;35:474–480.10.1007/s10059-013-0130-x
- Selesniemi K, Lee H-J, Muhlhauser A, Tilly JL. Prevention of maternal aging-associated oocyte aneuploidy and meiotic spindle defects in mice by dietary and genetic strategies. Proc. Nat. Acad. Sci. 2011;108:12319–12324.10.1073/pnas.1018793108
- Fukuwatari T, Wada H, Shibata K. Age-related alterations of B-group vitamin contents in urine, blood and liver from rats. J. Nutr. Sci. Vitaminol. 2008;54:357–362.10.3177/jnsv.54.357
- Terakata M, Fukuwatari T, Sano M, Nakao N, Sasaki R, Fukuoka S, Shibata K. Establishment of true niacin deficiency in quinolinic acid phosphoribosyltransferase knockout mice. J. Nutr. 2012;142:2148–2153.10.3945/jn.112.167569
- Fukuwatari T, Ohta M, Kimura N, Sasaki R, Shibata K. Conversion ratio of tryptophan to niacin in Japanese women fed a purified diet conforming to the Japanese Dietary Reference Intakes. J. Nutr. Sci. Vitaminol. 2004;50:385–391.10.3177/jnsv.50.385
- Reeves PG. Components of the AIN-93 diets as improvements in the AIN-76A diet. J. Nutr. 1997;127:838S–841S.
- Pullman ME, Colowick SP. Preparation of 2- and 6-pyridones of N1-methylnicotinamide. J. Biol. Chem. 1954;206:121–127.
- Shibata K, Kawada T, Iwai K. Simultaneous micro-determination of nicotinamide and its major metabolites, N1-methyl-2-pyridone-5-carboxamide and N1-methyl-4-pyridone-3-carboxamide, by high-performance liquid chromatography. J. Chromatogr. 1988;424:23–28.10.1016/S0378-4347(00)81072-5
- Shibata K. Ultramicro-determination of N1-methylnicotinamide in urine by high-performance liquid chromatography. Vitamins. 1987;61:599–604 ( in Japanese).
- Krehl WA, Teply LJ, Sarma PS, Elvehjem CA. Growth-retarding effect of corn in nicotinic acid-low rations and its counteraction by tryptophan. Science. 1945;101:489–490.10.1126/science.101.2628.489
- Greengard O, Dewey HK. The prematurely evoked synthesis of liver tryptophan oxygenase. Proc. Nat. Acad. Sci. 1971;68:1698–1701.10.1073/pnas.68.8.1698
- Kawai J, Okuno E, Kido R. Organ distribution of rat kynureninase and changes of its activity during development. Enzyme. 1988;39:181–189.
- Shibata K. Effect of ethanol feeding and growth on the tryptophan-niacin metabolism in rats. Agric. Biol. Chem. 1990;54:2953–2959.10.1271/bbb1961.54.2953
- Shibata K, Murata K. Niacin requirement depending on tryptophan level of diet in rat. Vitamins. 1982;56:469–477.
- Shibata K. Nutritional factors that regulate on the conversion of l-tryptophan to niacin. Adv. Exp. Med. Biol. 1999;467:711–716.10.1007/978-1-4615-4709-9
- Shibata K, Nomamoto R, Iwai K. Effect of dietary protein levels on the urinary excretion of nicotinamide and its metabolites in rats. Agric. Biol. Chem. 1988;53:1765–1769.10.1271/bbb1961.52.1765
- Shibata K, Matsuo H. Effect of supplementing low protein diets with the limiting amino acids on the excretion of N1-methylnicotinamide and its pyridones in rats. J. Nutr. 1989;119:896–901.
- Shibata K, Onodera M. Comparison of tryptophan-niacin conversion in rats fed with a nicotinic acid-free diet containing egg white, egg white proteolysate, or mixtures of amino acids. Agric. Biol. Chem. 1991;55:1291–1298.10.1271/bbb1961.55.1291
- Shibata K, Murotani M, Onodera M. Difference in tryptophan-nicotinamide conversion according to dietary nitrogen sources; casein, casein hydrolysate, and mixtures of amino acids. Biosci. Biotechnol. Biochem. 1992;56:665–669.10.1271/bbb.56.665
- Shibata K. Effect of adding the limiting amino acids to an amino acid diet simulating rice protein on the conversion of tryptophan to nicotinamide in rat. Biosci. Biotechnol. Biochem. 1994;58:442–443.10.1271/bbb.58.442
- Shibata K, Taniguchi I, Onodera M. Effect of adding branched-chain amino acids to a nicotinic acid-free, low-protein diet on the conversion ratio of tryptophan to nicotinamide in rats. Biosci. Biotechnol. Biochem. 1994;58:970–971.10.1271/bbb.58.970
- Shibata K. Conversion ratio of tryptophan to niacin in rats fed with a nicotinic acid-free, tryptophan-limiting diet. Biosci. Biotechnol. Biochem. 1995;59:715–716.10.1271/bbb.59.715
- Shibata K, Mushiage M, Kondo T, Hayakawa T, Tsuge H. Effects of vitamin B6 deficiency on the conversion ratio of tryptophan to niacin. Biosci. Biotechnol. Biochem. 1995;59:2060–2063.10.1271/bbb.59.2060
- Shibata K, Shimada H, Kondo T. Effects of feeding tryptophan-limiting diets on the conversion ratio of tryptophan to niacin in rats. Biosci. Biotechnol. Biochem. 1996;60:1660–1666.10.1271/bbb.60.1660
- Shibata K, Kondo T, Yonezima M. Conversion ratio of tryptophan to niacin in rats fed a vitamin B1-free diet. J. Nutr. Sci. Vitaminol. 1997;43:479–483.10.3177/jnsv.43.479
- Moren A, Lemoult D, Brodel A. Pellagra in Mozambican refugees. Lancet. 1990;335:1403–1404.10.1016/0140-6736(90)91283-G
- Malfait P, Moren A, Dillon JC, Brodel A, Begkoyian G, Etchegorry MG, Malenga G, Hakewill P. An outbreak of pellagra related to changes in dietary niacin among Mozambican refugees in Malawi. Int. J. Epidemiol. 1993;22:504–511.10.1093/ije/22.3.504
- Baquet S, Wuillaume F, Egmond KV, Ibañez F. Pellagra outbreak in Kuito, Angola. Lancet. 2000;355:1829–1830.10.1016/S0140-6736(05)73093-2
- World Health Organization. Pellagra and its prevention and control in major emergencies. Geneva: World Health Organization; 2002.
- Seal AJ, Creeke PI, Dibari F, Cheung E, Kyroussis E, Semedo P, van den Briel T. Low and deficient niacin status and pellagra are endemic in postwar Angola. Am. J. Clin. Nutr. 2007;85:218–224.
