Abstract
Some snakes have several anti-toxic proteins in their sera that neutralize their own venom. Five new small serum proteins (SSPs) were isolated from Japanese mamushi (Gloydius blomhoffii) serum by gel-filtration and RP-HPLC, and their N-Terminal sequences were determined. The amino acid sequences of the precursor proteins were deduced from the nucleotide sequences of cDNAs encoding them. Due to the sequence similarity to those of SSPs in habu snake (Protobothrops flavoviridis) serum (>75% identity), these proteins were designated mSSP-1 to mSSP-5 as the homologs of habu proteins. mSSP-1 was stable at 100 °C and in the pH range of 1–10, and inhibited the proteolytic activity of a certain snake venom metalloproteinase. The inhibitory activity was extinguished by modifying the amino groups of mSSP-1. mSSP-1 is the first prostate secretory protein of the 94 amino acid-family protein with a carbohydrate chain in the Asn37 residue.
Graphical Abstract
Sequence Alignment of PSP94-Family Proteins. G. blomhoffii and P. flavoviridis SSPs are respectively designated by mSSPs and hSSPs. A glycosylation site in mSSP-1 is underlined.
Many snake venom metalloproteinases (SVMPs) that induce serious hemorrhage have been isolated from the venom of Viperidae snakes.Citation1) An innate immunity to snake venoms is apparent not only in certain warm-blooded animals, but also in a few snakes.Citation2) This natural resistance has been explained by the presence of some endogenous factors in sera of the resistant animals which may neutralize several toxic factors in snake venom.Citation3) Many anti-hemorrhagic factors have been purified from snake sera, e.g. HSF from Japanese habu (Protobothrops flavoviridis),Citation4) BJ46a from Bothrops jararaca,Citation5) and mamushi serum factor (MSF) from Japanese (Gloydius blomhoffii) and Chinese mamushi (Gloydius blomhoffii brevicaudus).Citation6) HSF and MSF are highly stable to temperature and extreme pH levels and inhibit several SVMPs as well as some non-hemorrhagic metalloproteinases.Citation6,7) Although these proteins may play a role in self-defense against their own venom, the inhibition mechanism of natural SVMP inhibitors remains unknown.
The human prostate secretory protein of 94 amino acids (PSP94), also called β-microseminoprotein, is a non-glycosylated, cysteine-rich protein (molecular mass of about 10 kDa) and one of the major constituents in human seminal plasma.Citation8) Homologous proteins have been identified in several organisms.Citation9–12) Although the 10 cysteine residues forming five disulfide bonds were conserved, the overall amino acid similarity is only 40–50% among mammalian proteins, suggesting that they evolved at a somewhat rapid rate.Citation13) The three-dimensional structures of human and porcine PSP94 have already been elucidated.Citation14) Both have a very similar 3D structure and are β-sheet-rich proteins with two distinct domains. Although the biological function of PSP94 has not been unequivocally established, it has the ability to bind a protein in human blood (the PSP94-binding protein) and cysteine-rich secretory protein-3 (CRISP-3) from human leukocytes.Citation15,16) Anklesaria et al.Citation17) have recently shown prostatic acid phosphatase to be another binding protein for PSP94 and proved the presence of the acid phosphatase-PSP94 complex in human seminal plasma.
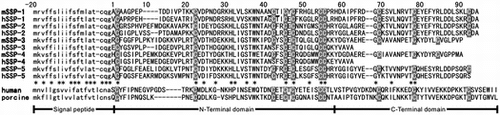
The small serum protein (SSP) is a low-molecular-mass protein isolated from the serum of P. flavoviridis and belongs to the PSP94 protein family.Citation18) Thereafter, four similar proteins (hSSP-2, hSSP-3, hSSP-4, and hSSP-5)Footnote1 have been found in the same serum; thus, the first SSP has been designated as hSSP-1. hSSPs are composed of approximately 90 amino acids, except for hSSP-3 and hSSP-4. C-Terminal 30 residues are absent in hSSP-3 and hSSP-4. Each of the full-length cDNAs encode a mature protein and a highly conserved signal peptide.Citation19)
hSSP-1 and hSSP-3 have shown an inhibitory effect on brevilysin H6, a weakly hemorrhagic SVMP isolated from Chinese mamushi venom.Citation18,20) hSSP-2 and hSSP-5 have exhibited strong affinity for triflin, a CRISP family protein in the P. flavoviridis venom which is responsible for blocking smooth muscle contraction.Citation21) A gel filtration analysis has revealed all the hSSPs in the serum to be eluted at a molecular mass of more than 60 kDa, suggesting the presence of an SSP-binding protein. A novel protein, termed serotriflin, with sequence homology to triflin has recently been purified from P. flavoviridis serum as a candidate for the SSP-binding protein.Citation22) Among the five hSSPs, only hSSP-2 and hSSP-5 formed a noncovalent complex with serotriflin. We have also found HSF to be the carrier protein for all SSPs.Citation23) Interestingly, the hSSP-1–HSF complex effectively suppressed the apoptosis of vascular endothelial cells and caspase 3 activation induced by HV1, an SVMP isolated from the venom of P. flavoviridis.Citation24,25)
During the isolation of MSF,Citation6) we observed the presence of a novel protein with SVMP inhibitory activity during reverse phase-high performance liquid chromatography (RP-HPLC) used to purify the serum of Chinese mamushi. This protein seemed to be a homolog of hSSP because of the similarity of its N-Terminal sequence. We hypothesized that a set of mamushi SSPs (mSSPs) may be present in the serum. We investigated in this study the identification and characterization of mSSPs in the serum of Japanese mamushi (G. blomhoffii). We could isolate five homologous proteins (mSSP-1 to mSSP-5) and determine the nucleotide sequences of cDNAs encoding them. Interestingly, 13 kDa mSSP-1 was glycosylated, and a non-glycosylated form was also found, although both had the same activity.
Materials and methods
Materials and animals
Japanese (G. blomhoffii) and Chinese mamushis (G. blomhoffii brevicaudus) were purchased from the Japan Snake Institute (Ohta, Gunma, Japan). The sera were obtained and stored at –20 °C until needed. Several SVMPs, HSF, and 5 hSSPs were prepared as previously described.Citation18,23,26–28) The serum was fractionated by cold ethanol precipitationCitation7) with 0.5, 0.75, 1.25, and 2.0 volumes of ethanol, respectively, yielding E0.5, E0.75, E1.25, and E2.0. N-Bromosuccinimide (NBS), thermolysin, 2,4,6-trinitrobenzenesulphonic acid (TNBS), and trypsin were purchased from Sigma Chemical Co. (St. Louis, MO, USA). All other reagents used were of analytical grade of the highest purity. Fluorescein thiocarbamoyl casein (FTC-casein) was prepared according to the method of Twining.Citation29) All the other chemicals were purchased from Wako Pure Chemical Industries (Osaka, Japan).
Electrophoresis
Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) was done in the presence of 2-mercaptoethanol on 16.5% polyacrylamide slab gel.Citation30) A Rainbow marker kit (GE Healthcare, Bucknghamshire, UK) containing ovalbumin (46,000), carbonic anhydrase (30,000), soybean trypsin inhibitor (20,500), lysozyme (14,300), aprotinin (6500), and insulin B-chain (3400) was used to provide molecular weight markers. Proteins were detected by staining with 0.1% Coomassie brilliant blue R-250.
Measurement of the SVMP-inhibitory activity
Inhibition of the proteolytic activity of SVMP was measured at 37 °C for 15 min in 5 mM CaCl2 and 50 mM Tris–HCl (pH 8.5), using 0.2% FTC-casein as previously described.Citation27) The enzyme concentrations were 20–40 μg/mL. The increase in fluorescence was analyzed at 520 nm with excitation at 490 nm by an FP-550A spectrofluorometer (Jasco, Tokyo, Japan). Unless otherwise noted, brevilysin H6 (20 μg/mL) was used as the enzyme.Citation20)
RP-HPLC of the Chinese mamushi serum
Prior to RP-HPLC, the serum of G. blomhoffii brevicaudus was fractionated by cold ethanol to remove such high-molecular-mass proteins as immunoglobulin and lipoproteins that could seriously damage the column used for RP-HPLC. Serum fraction E2.0 was dissolved in a small amount of 0.1% trifluoroacetic acid (TFA) and loaded into a μBondasphere 5μ-C8–300Å column (1.9 × 15 cm; Nihon Waters, Tokyo, Japan). Elution was programmed as a linear gradient of 20–50% of acetonitrile containing 0.1% TFA over 50 min at a flow rate of 5.0 mL/min, and monitored at 230 nm by a flow cell with a 1-mm light path. Each peak was collected and lyophilized, and the inhibitory activity to brevilysin H6 was measured as already described.
Mass spectrometry by matrix-assisted laser desorption ionization time-of-flight (MALDI–TOF)
Molecular mass was analyzed with a Voyager DE-STR matrix-assisted laser desorption ionization time-of-flight (MALDI–TOF) mass spectrometer (Applied Biosystems Japan, Tokyo, Japan). A sample was dissolved in 0.1% TFA-50% acetonitrile containing α-cyano-4-hydroxy-cinnamic acid (10 mg/mL) as the matrix, and 2-μL aliquots were analyzed. The spectrum was calibrated by the apomyoglobin. The analysis was performed by following the manufacturer’s instructions.
Enzymatic digestion of S-pyridylethylated proteins and sequence analysis
Protein was reduced and S-pyridylethylated (Pe) according to the method of Friedman et al.Citation31) Pe-protein (100 μg each) was digested with bovine trypsin with an enzyme-to-substrate concentration of 1:50 (w/w) at 37 °C for 24 h in 20 mM Tris–HCl (pH 8.0). The digested peptides were isolated by RP-HPLC in a YMC-Pack ODS column (0.46 × 25 cm; YMC, Kyoto, Japan) with a linear gradient of 0–40% of acetonitrile containing 0.1% TFA over 50 min at a flow rate of 1.0 mL/min. N-Terminal Edman sequencing of the modified protein and digested peptides was performed by a PPSQ 21 automatic protein sequencer (Shimadzu, Kyoto, Japan) according to the manufacturer’s instructions.
cDNA cloning of Chinese mSSP-1
Total RNA was prepared from 0.5 g of G. blomhoffii brevicaudus liver, using Sepasol®-RNA I Super (Nacalai Tesque, Kyoto, Japan) according to the manufacturer’s instructions, and this RNA was then reverse-transcribed to prepare the first cDNA strand by using an adaptor-linked oligo(dT) primer [5′-GGCCACGCGTCGACTAGTAC-(dT)17-3′]. The resulting cDNAs were further amplified by PCR by using an ssp-sigN specific primer [5′-CCCTACCAAGAGTTTCCTGGGTCTTCN-3′, corresponding to the 5′-untranslated region (UTR) of hSSP] and a 3′-Adp adaptor primer (5′-GGCCACGCGTCGACTAGTAC-3′, corresponding to the adaptor sequence). The PCR products were subcloned into the pBluescript II SK + cloning plasmid. Nucleotide sequences were analyzed by using the ABI PRISM BigDye Terminator Cycle Sequencing ready reaction kit and the ABI PRISM 377 DNA Sequencer (Applied Biosystems, Foster City, CA, USA) according to the manufacturer’s instructions.
Thermal and pH stability of mSSP-1
mSSP-1 was dissolved in 50 mM Tris–HCl (pH 7.4), and the solution (250 μg/mL) was heated at 40–100 °C for 0.5 h and then cooled in ice. Each reaction mixture was diluted with an equal volume of 50 mM Tris–HCl (pH 8.5), and the proteolytic activity of brevilysin H6 was measured as already described to evaluate the residual SVMP-inhibitory activity. hSSP-1 was also treated as a comparison. The pH stability was examined in various buffers (20 mM each): KCl–HCl (pH 1–3), acetate (pH 4–5), phosphate (pH 6–7), Tris–HCl (pH 8–9), and Gly–NaOH (pH 10–12). The solutions (250 μg/mL) in these buffers were kept at 37 °C for 1 h, and then an equal volume of 0.5 M Tris–HCl, pH 8.5, was added to each. The residual inhibitory activity was measured as already described.
Chemical modifications of mSSP-1
All the reactions were conducted at room temperature with 5 × 10−7 M mSSP-1, and terminated with an excess of the quenching reagent by adding the amino acid that can react with the reagent. The reaction mixtures were directly used to measure the residual inhibitory activity as already described. (i) Oxidation of the Trp residue: mSSP-1 was treated with various amounts of NBS in a 50 mM acetate buffer (pH 4.0) for 5 min,Citation32) and l-tryptophan was added at a final concentration of 0.5 mM. (ii) Trinitrophenylation of the Lys residues: mSSP-1 was treated with TNBS in a 50 mM borate buffer (pH 9.5).Citation33) The reaction was quenched by adding l-lysine at a final concentration of 1.8 mM.
Purification of Japanese mamushi SSPs
The serum (2.2 mL) of G. blomhoffii was loaded into a Sephacryl S-300HR column (2.5 × 86 cm) that had been equilibrated with 50 mM Tris–HCl (pH 7.4) containing 50 mM NaCl. Elution was carried out at 4 °C with the same buffer, and 5-mL fractions were collected. Three protein peaks termed A to C were observed. The proteins in peak C were collected, desalted by dialysis, and lyophilized. Several mSSPs in peak C were separated by RP-HPLC in a SepaxBio-C8 column (0.46 × 25 cm, Sepax Technologies, Delaware, USA). Elution was programmed as a three-step gradient of acetonitrile containing 0.1% TFA, using a Waters automated gradient controller (GE Healthcare) at a flow rate of 1.0 mL/min and monitored at 230 nm: (i) a non-linear gradient (gradient curve profile no. 5 of the controller) of 25–45% of acetonitrile for 30 min, (ii) a linear gradient of 45–60% for 25 min, and (iii) a linear gradient of 60–80% for 15 min. Each peak was collected and lyophilized, and the purity was analyzed by SDS–PAGE. Some protein samples were further purified with re-chromatography by RP-HPLC under appropriate conditions: linear gradients of 25 to 40–60% of acetonitrile over 30–40 min depending on the sample.
Cloning of cDNAs encoding Japanese mamushi SSPs
Crude cDNA samples were prepared from 0.5 g of G. blomhoffii liver by the same method as that described in the section for cDNA cloning of Chinese mSSP-1. cDNA samples encoding Japanese mSSPs were then cloned by using the same set of primers (ssp-sigN and 3′-Adp) as those already described. mSSP-5 was cloned in two steps. First, the cDNA clones were amplified by PCR using the mhssp5_Ns primer (5′-GGCTGCAGGCCAAGGAGCATGTTTTCAGGG-3′, corresponding to 5′-UTR and the deduced ACFQG amino acid sequence) and the 3′-Adp adaptor primer. The resulting cDNAs were then screened by PCR with the second set of primers, mSSPs5UTs (5′-CCCTACCAAGAGTTTCCTGCAGCTTCC-3′, designed from the consensus 5′-UTR sequence of other mSSPs), and mSSP5Cas2 (5′-GATGGATCCAGGTTCATTTACACACGAGAGATTA-3′, corresponding to 3′-UTR sequence of mSSP-5).
Phylogenetic analysis
DNASIS DNA sequence analysis software (Hitachi Software Engineering, Tokyo, Japan) was used for the nucleic acid and amino acid alignment. The phylogenetic analysis was conducted by the neighbor-joining method,Citation34) using CLUSTALW on the DNA Database Japan web page, and a tree was drawn by using TreeView phylogenetic tree-drawing software developed by R. Page (http://taxonomy.zoology.gla.ac.uk/rod/rod.html).
Results
Identification of the SSP in G. blomhoffii brevicaudus serum
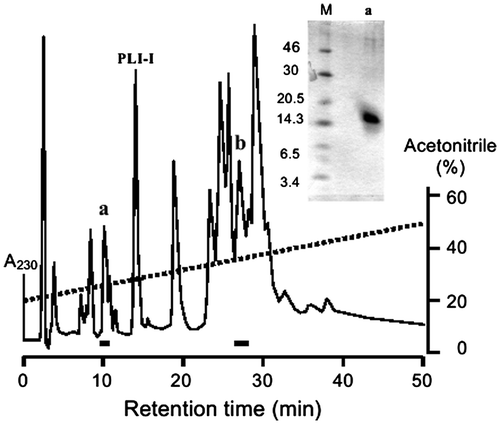
Ethanol fractionation of the Chinese viper serum yielded approximately 70% of SVMP-inhibitory activity in fraction E2.0 that contained mainly serum albumin and many low-molecular-mass proteins (data not shown). This fraction was subjected to RP-HPLC, and the SVMP-inhibitory activity of each peak was examined. Two active peaks a and b were detected as marked by bars in Fig. . Peak b was attributed to an anti-hemorrhagic factor (MSF) as previously reported.Citation6) The peak of phospholipase A2 inhibitor-I (PLI-I)Citation35) is indicated in Fig. as the internal marker, because the sera of mamushi and P. flavoviridis contained similar proteins with high sequence similarity, and the retention times by RP-HPLC were almost identical to each other. Peak a eluted before PLI-I contained a low-molecular-mass protein of approximately 14 kDa by SDS–PAGE (Fig. , the inset). The measured mass of the protein in peak a was 12,262.3 by MALDI-TOF mass spectroscopy. The protein in peak a was converted by S-pyridylethylation, and subjected to an N-Terminal sequence analysis, yielding the following 50 residues: ACAAGPEPTDDIVPTKKCVDPNNGRKHLVLSKWNAAXCTICYCFRHGLRC. No amino acid was detected at residue 37. Since the closest similarity was apparent between this sequence and that of hSSP-1,Citation18) this protein was named mamushi SSP-1 (mSSP-1).
Fig. 1. Detection of SVMP-inhibitory activity in the serum of G. blomhoffii brevicaudus.
Notes: The ethanol fraction (E2.0) prepared from the serum was injected into a μBondasphere 5μ-C8–300Å column (1.9 × 15 cm, Waters) for an RP-HPLC analysis. The SVMP-inhibitory proteins are marked by bars. The peak of PLI-I is indicated as the internal marker of the serum proteins. The SDS–PAGE result for peak a is shown in the inset. M, marker proteins.
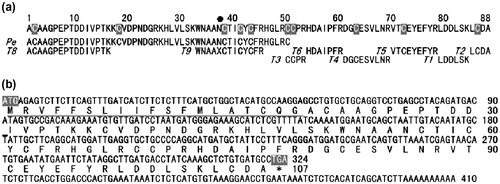
The entire amino acid sequence of mSSP-1 was determined by a peptide analysis of trypsinized peptides (T1–T9) by the conventional Edman degradation method. The result is shown in Fig. (a). The primary structure of hSSP-1 was utilized for the arrangement of these peptides. mSSP-1 consisted of 88 amino acids, including 10 cysteine residues. The fifth residue of peptide T9 could not be sequenced. The presence of the Cys-Thr sequence indicates that an N-linked sugar chain was attached to residue 5 (Asn37). The molecular weight was calculated to be 9922.3 based on the amino acid sequence. The discrepancy between this value and the exact molecular mass suggests that Asn37 was glycosylated.
Fig. 2. Determination of the primary structure of G. blomhoffii brevicaudus mSSP-1.
Notes: The N-Terminal sequence determined by a direct analysis of the Pe-protein is designated as Pe. Peptides obtained by tryptic digestion are designated as T and aligned according to the known sequence of hSSP-1. Cysteine residues are shown in white on gray. The filled circle (●) below the sequence of mSSP-1 is estimated to be a glycosylated Asn residue.
To confirm the entire amino acid sequence of mSSP-1 as well as that of its precursor, we cloned cDNA encoding the mSSP-1 precursor from the liver of G. blomhoffii brevicaudus. The entire cDNA sequence is shown in Fig. (b). Chinese mSSP-1 contained an open reading frame (ORF) of 324 bp. The amino acid sequence deduced from the nucleotide sequence is cited together with cDNA. It encoded 107 amino acids. The N-Terminal 19-amino acid stretch was estimated to be a signal sequence. As was expected, residue 37 was Asn. Details of the nucleotide sequence analysis will be described later.
Characterization of mSSP-1
The SVMP-inhibitory activity was examined by using brevilysin H6 as the target enzyme. mSSP-1 as well as hSSP-1 inhibited the enzyme (Fig. (a)). The proteolytic activity of brevilysin L4,Citation28) a P-I class SVMP isolated from G. blomhoffii brevicaudus venom, was also inhibited by mSSP-1 (Fig. (b)). However, brevilysins H2 and H3 from the same venom,Citation27) HR1A, HR1B, HR2a, HR2b, and H2-proteinase from P. flavoviridis venom, and bacterial thermolysin were not inhibited at all (Fig. (b)). This inhibitory specificity is consistent with that of hSSP-1.
Fig. 3. SVMP-inhibitory activity of SSP-1.
Notes: (a) SVMP inhibitory activity of G. blomhoffii brevicaudus and P. flavoviridis SSPs. ○, mSSP-1; ●, hSSP-1. Brevilysin H6 (15 μg/mL) was used as the target enzyme. (b) Effect of mSSP-1 on the proteolytic activity of several metalloproteinases. Target enzymes are brevilysin L4 (Δ, 40 μg/mL), brevilysin H2 (◇, 20 μg/mL), brevilysin H3 (▽, 40 μg/mL), HR1A (Δ, 10 μg/mL), HR1B (■, 40 μg/mL), HR2a (▲, 40 μg/mL), HR2b (▼, 40 μg/mL), H2 (◆, 40 μg/mL), and thermolysin (□, 2 μg/mL). (c) Thermal stability. An SSP-1 (6.4 μg/mL) solution was left at 0–100 °C for 0.5 h in 50 mM Tris–HCl (pH 7.4), and then the SVMP-inhibitory activity was measured at pH 8.5. These values are displayed as the activity relative to a control value of 100% at room temperature. G. blomhoffii brevicaudus and P. flavoviridis proteins are, respectively, shown by unfilled and filled bars. (d) Stability at various pH values. The SVMP-inhibitory activity was measured after pre-incubating SSP-1 in one of various buffers (20 mM each) for 1 h at 37 °C: KCl–HCl (pH 1–4), Tris–HCl (pH 6–9) and glycine–NaOH (pH 11 and 12). Each measurement was carried out at least twice (n = 2). A pH value of 7.0 was used as the control. The residual SVMP inhibitory activity was determined by using brevilysin H6 (20 μg/mL).
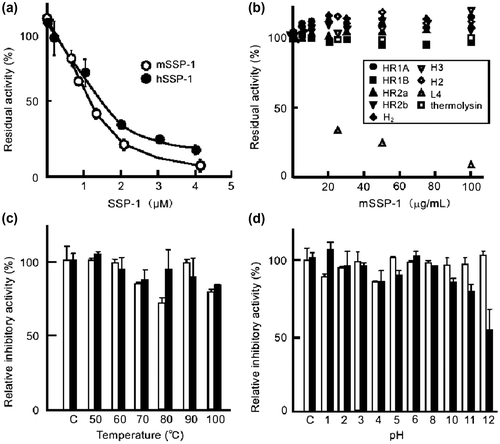
The thermal and pH stabilities of the SVMP-inhibitory activity of mSSP-1 were measured and compared with those of hSSP-1. Both proteins were stable in the 50–100 °C range in 50 mM Tris–HCl (pH 7.4) (Fig. (c)). Likewise, when the inhibitory activity was measured after a short treatment at various pH values, both proteins were stable up to pH 10, although the activity of mSSP-1 slightly decreased at pH 11 and 12 (Fig. (d)). Both proteins thus showed resistance to inactivation at high temperature and extreme pH values.
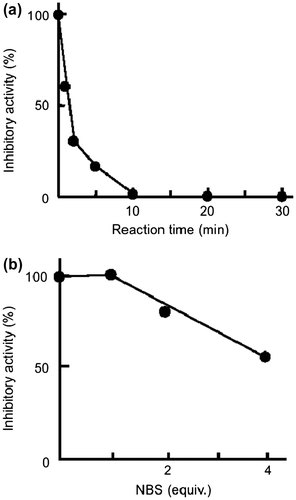
The SVMP-inhibitory activity of mSSP-1 was greatly reduced within 10 min by trinitrophenylation of the α- or ε-amino group with an excess amount of TNBS (Fig. (a)). In contrast, oxidation of a Trp residue by NBS had little effect on the activity of mSSP-1 (Fig. (b)). The same phenomena were apparent in the modification of hSSP-1 with TNBS and NBS (data not shown). These results suggest that the α- or ε-amino group of SSP-1 played an important role in the interaction with brevilysin H6.
Purification of G. blomhoffii mSSPs
A chromatographic analysis was carried out in order to investigate whether a similar protein to Chinese viper mSSP-1 was present in the serum of Japanese mamushi. G. blomhoffii serum was first subjected to gel filtration in a Sephacryl S-300HR column to remove the high-molecular-mass proteins (Fig. (a)). Peak C containing the low-molecular-mass proteins was then applied to RP-HPLC by using an analytical SepaxBio-C8 column. Gradient elution with acetonitrile yielded 14 separate peaks (Fig. (b)). The proteins recovered from these peaks were identified by MALDI–TOF mass spectrometry and an N-Terminal peptide sequence analysis, the results being summarized in Table . Both peaks 6 and 8 were assigned to PLIs, and peak 13 was MSFCitation6) by the amino acid sequences. Novel low-molecular-mass proteins were found in peaks 1–5, 7, and 9. The inhibitory activity to brevilysin H6 was detected in peaks 4, 5, and 13 (data not shown). The N-Terminal sequence of the protein in peak 4 was the same as that of Chinese viper mSSP-1. The protein in peak 5 also gave the identical sequence to mSSP-1. This protein was assigned to be a non-glycosylated form of mSSP-1, because the apparent respective molecular masses of peaks 4 and 5 were 13 kDa and 10 kDa (Fig. (b), the inset). The molecular weights of peaks 4 and 5 were, respectively, determined to be 12,633.9 and 9922.7 (Table ). Both glycosylated and non-glycosylated mSSP-1 showed identical inhibitory activity to brevilysin H6, indicating that the sugar chain had no influence on the inhibitory activity (data not shown).
Fig. 5. Purification of mSSPs from the Serum of G. blomhoffii.
Notes: (a) Gel filtration of Japanese mamushi serum in a Sephacryl S-300HR column. Three fractions indicated by a bar were collected. (b) Chromatographic analysis of fraction C isolated by gel filtration using an RP-HPLC SepaxBio-C8 column (0.46 × 25 cm). Acetonitrile (%) in 0.1% TFA is shown by a broken line. SDS–PAGE analyses of peaks 4 and 5 of the RP-HPLC-purified proteins are shown in the inset. M, molecular weight markers.
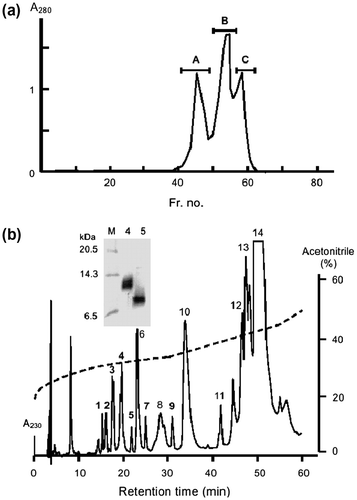
Table 1. Identification of G. blomhoffii serum proteins separated by RP-HPLC.
Other proteins in peaks 1, 2, 3, 7, and 9 had molecular weights of approximately 9800–10,000, and may have been related to mSSP-1. The proteins in these peaks were tentatively assigned as mSSP-2 to mSSP-5 based on the sequence homology to hSSP paralogs as listed in Table . Although all P. flavoviridis SSPs have always eluted before the peak of PLI-I,Citation18) some mSSPs emerged after PLI-I (peak 6 in Fig. (b)). Therefore, the elution profile by RP-HPLC could not be a definite criterion for the assignment of SSP paralogs.
The serum of G. blomhoffii contained five SSPs, namely mSSP-1 to mSSP-5. Thus, G. blomhoffii has developed the same repertoire of SSP paralogs as that of P. flavoviridis. Identical numbers of SSPs were also present in Chinese mamushi serum as shown in Fig. .
Fig. 6. Sequence alignment of PSP94-family proteins.
Notes: G. blomhoffii and P. flavoviridis SSPs are, respectively, designated by mSSPs and hSSPs. Human PSP94 (MSMB_HUM, GenBank accession no. AJ133356) and porcine PSP94 (MSMB_PIG, GenBank accession no. S80724) are included for comparison. Asterisks indicate residues conserved in all SSPs. White characters indicate Cysteine residues. A glycosylation site in mSSP-1 is underlined.

Cloning of cDNAs encoding G. blomhoffii SSPs
In order to obtain comprehensive information concerning the entire structure of five mSSP paralogs in the serum of Japanese mamushi, cDNAs encoding for all mSSPs were cloned. Full-length cDNA for mSSP-1 to mSSP-4 was obtained by PCR, using a pair of oligonucleotides ssp-sigN that had originally been designed based on the cDNA nucleotide sequence for 5′-UTR, highly conserved between hSSPs (5′-UTR at 12 bp upstream of the start codonCitation19)) and an adaptor-linked oligo(dT) primer. Several positive clones of the expected size (approx. 0.55 kb) were identified as the corresponding DNAs encoding mSSPs-1 to mSSP-4. However, no clone related to mSSP-5 could be obtained by the same method. An ORF search and comparison with the amino acid sequences of SSP proteins revealed that their deduced N-Terminal sequences completely agreed with those of the purified proteins.
mSSP-5 cDNA was cloned in two steps. The first PCR was carried out by using specific primer mhssp5_Ns corresponding to the N-Terminal sequence of mSSP-5, because the N-Terminal 5 residues of mSSP-5 were identical to those of hSSP-5. The nucleotide sequence of the 5′-UTR and N-Terminal region of mSSP-5 was then determined with the second set of primers corresponding to part of the 5′- and 3′-UTR sequences.
The nucleotide sequences of cDNAs for mSSP-1, mSSP-3, and mSSP-4 consisted of 324-bp ORF encoding 107 amino acids and 86-bp (mssp-1) or 126-bp (mssp-3 and mssp-4) 3′-UTR (data not shown). The sequence for Japanese mSSP-1 was identical to that for Chinese snake mSSP-1 (Fig. (b)). On the other hand, the nucleotide sequence for mSSP-2 consisted of 336-bp ORF encoding 111 amino acids and 86-bp 3′-UTR. The nucleotide sequence for mSSP-5 consisted of 330-bp ORF encoding 109 amino acids and 86-bp 3′-UTR. These sequences have been deposited in the DDBJ data bank with accession numbers AB576135 – AB576139 for mSSP-1 – mSSP5.
Comparison of the amino acid sequences of the PSP94-family proteins
The primary structures of five mSSP precursors deduced from their nucleotide sequences are summarized in Fig. , together with P. flavoviridis homologs and some mammalian PSP94 proteins. cDNAs encoded a mature protein of 88–92 residues and a highly conserved 19-residue signal peptide for the secretory pathway. Unlike hSSP-3 and hSSP-4 consisting of 60 residues, the corresponding G. blomhoffii proteins had longer C-Terminal domains. The respective lengths of the mature proteins were 88, 92, 88, 88, and 90 residues for mSSP-1, mSSP-2, mSSP-3, mSSP-4, and mSSP-5. It is notable that the signal sequences of the G. blomhoffii proteins were highly homologous with those of hSSPs and that most of the Cys residues were well conserved. Table shows the percentage similarity between hSSPs and mSSPs which were calculated in pairwise comparison from their amino acid and nucleotide sequences in the ORF regions. The SSP paralogs were definitively assigned as listed in Table . The combinations in Table show the highest sequence similarity. The nucleotide sequences of mssp-1 – mssp-5 exhibit high similarity to those of the corresponding P. flavoviridis hSSPs (78.6% on average in ORF).
Table 2. Degree of similarity among five SSPs isolated from the sera of P. flavoviridis and G. blomhoffii. The amino acids and nucleotide sequences of the protein-coding region were compared, and the percent similarity is persented.
Discussion
Identification of the G. blomhoffii SSP family proteins
mSSP-1 is the first PSP94-family protein having a carbohydrate chain. An N-glycosylation site was found at Asn37, and two forms of mSSP-1s could be identified in the serum: the glycosylated 13-kDa protein and the non-glycosylated 10-kDa protein (Fig. (b)). Like hSSP-1, mSSP-1 was stable to heating and extreme pH. Such stability may be attributable to the rich presence of disulfide bonds. All the SSPs therefore be purified in their native forms by RP-HPLC under acidic conditions.
It is noteworthy that the C-Terminal deletions in hSSP-3 and hSSP-4 were absent in the G. blomhoffii homologs. In addition, mSSP-3 and mSSP-4 had a similar extension of the C-Terminal residues to mammalian homologs after the last Cys residues. Interestingly, when the nucleotide sequences of hssp-3 and hssp-4 following the stop codon were translated into amino acids, their C-Terminal sequences were similar to those of mSSP-3 and mSSP-4, and three cysteine residues in the C-Terminal regions were also conserved. This suggests that the appearance of stop codons in the hssp-3 and hssp-4 sequences occurred only in the progenitor gene of P. flavoviridis.
Phylogenetic analysis of the evolution of SSP genes
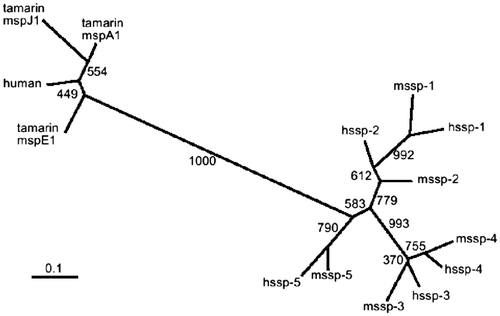
To better understand the evolutionary profiles of the SSP genes, a phylogenetic tree was constructed based on the nucleotide sequences of cDNAs encoding the mature protein regions of 10 SSPs. The result is shown in Fig. . For comparison, human PSP94 and three isoforms of tamarin genes are included. The snake SSP genes could be grouped into three branches: ssp-1/ssp-2, ssp-3/ssp-4, and ssp-5. Four pairs, mssp-1 vs. hssp-1, mssp-3 vs. hssp-3, mssp-4 vs. hssp-4, and mssp-5 vs. hssp-5, were apparent. The presence of such counterparts in P. flavoviridis and G. blomhoffii strongly suggests that the SSP genes diverged before the evolutionary separation of these snakes. Fig. shows ssp-3 and ssp-4 located in the same branch, but only the P. flavoviridis proteins had the deletion at their C-Terminal.Citation19) Therefore, the diversification of a common progenitor gene of ssp-3/ssp-4 into mssp-3/hssp-3 and mssp-4/hssp-4 appears to have occurred after the divergent evolution of the two snakes. This implies that the common ancestor of P. flavoviridis and G. blomhoffii may have had ssp-1, ssp-2, ssp-5, and ssp-3/4 genes.
Fig. 7. Phylogenetic tree of snake SSPs from P. flavoviridis and G. blomhoffii and mammal PSP94 proteins.
Notes: G. blomhoffii and P. flavoviridis SSPs are designated by mSSPs and hSSPs, the GenBank accession nos. of these proteins being AB360906 (hSSP-1), AB360907 (SSP-2), AB360908 (hSSP-3), AB360909 (SSP-4), AB360910 (hSSP-5), AB576135(mSSP-1), AB576136(mSSP-2), AB576137(mSSP-3), AB576138(mSSP-4), and AB576139 (mSSP-5). The respective GenBank accession numbers of Human PSP94 and three cotton-top tamarin proteins J1, E1, and A1 are AJ133356, AJ010155, AJ010154, and AJ010158. The tree for protein-coding regions of these proteins was constructed by the neighbor-joining method.Citation34) The boot-strap probability is shown on the branches.Citation40)
Physiological functions of mSSPs
It is necessary to focus on the physiological functions of mSSPs in order to understand their roles in the serum. Although the carrier protein of all the hSSPs in the serum of P. flavoviridis is HSF,Citation23) the target protein of each hSSP was different: (i) Despite the inhibitory activity of hSSP-1 to brevilysin H6, the true physiological target of hSSP-1 is thought to be HV1Citation24) isolated from P. flavoviridis venom, because the SSP-1–HSF complex could suppress the apoptosis of vascular endothelial cells induced by HV1.Citation25) (ii) hSSP-2 and hSSP-5 exhibited potent affinity to triflin, which belongs to the CRISP family and is known as a smooth muscle contraction blocker.Citation22) (iii) Previous investigation has revealed that hSSP-3 weakly inhibited the proteolytic activity of brevilysin H6.Citation18) The hSSPs therefore show different physiological activities for their own venom.
If five SSP genes may have diverged before the divergence of the snakes as just proposed, the function of mSSPs as well as the carrier protein in the serum would be the same or similar to the case of hSSPs. In fact, mSSP-1 showed the same SVMP-inhibitory specificity as hSSP-1 (Fig. (a) and (b)). All the mSSPs may exist in G. blomhoffii serum as the high-molecular-mass complex because they were eluted with albumin by gel filtration. As a preliminary experiment, we identified a carrier protein of all mSSPs, capable of binding to MSF, probably a homolog of HSF.
According to Ghasriani et al.Citation14) mammalian PSP94 is a β-sheet-rich, rod-like protein with two distinct domains, N- and C-Terminal domains, as shown in Fig. . Since most of the long-chain SSPs had 10 well-conserved cysteines forming five disulfide bonds, they probably had similar folding of the polypeptide backbones. This may enable all the SSPs to bind to the same molecule, and the differences in their primary sequences are a reflection of functional change to new biological roles using the same scaffold. With regard to the binding properties of SSPs to the serum protein, the C-Terminal domain does not seem to be involved, because of the low sequence homology and the different chain length between the 10 SSPs in this domain. The central region ranging from Cys22 to Trp37 in the N-Terminal domain shows relatively high similarity among the 10 SSPs (Fig. ), and may participate in binding to their carrier protein in the serum.
Snake venoms are a complex mixtures of proteins, including enzymes and other biologically active components.Citation37) These components are responsible for the effect caused by snake bites and display mostly neurotoxic or proteolytic activities.Citation38,39) Interestingly, some venomous vipers as well as non-venomous snakes are armed with a self-defense system against their own venom components by several anti-toxic proteins in the sera. Two categories of serum proteins have been reported: anti-hemorrhagic factorsCitation4–7) and inhibitors of myotoxic or neurotoxic phospholipase A2-like proteins.Citation35) We can add SSPs as the third category of self-defensive proteins. Venomous snakes have developed two different types of inhibitor based on the PSP94-family protein as the scaffold. One is such SVMP-inhibitors as SSP-1, SSP-3, and SSP-4, and the others are inhibitors of such CRISP-family ion-channel blockers as SSP-2 and SSP-5. However, the precise physiological function of these serum proteins has not been completely elucidated. Further investigation of the SSPs would provide a better understanding of the true role in the self-defense and inhibitory mechanism.
Acknowledgments
The authors express thanks to the staff in the Radioisotope Center of Fukuoka University for their assistance in the protein sequencing analysis. This work was supported in part by MEXT (Ministry of Education, Culture, Sports, Science and Technology of Japan) and by grant-aid for young scientists (B) 22770134.
Notes
Abbreviations: CRISP, cysteine-rich secretory protein; FTC, fluorescein thiocarbamoyl; MALDI-TOF, matrix-assisted laser desorption ionization time-of-flight; MSF, mamushi serum factor; NBS, N-bromosuccinimide; ORF, open reading frame; PAGE, polyacrylamide gel electrophoresis; PCR, polymerase chain reaction; Pe, S-pyridylethylated; PLI, phospholipase A2 inhibitor; PSP94, prostatic prostate secretory protein of 94 amino acids; RP-HPLC, reverse-phase high-performance liquid chromatography; SSP, small serum protein; SVMP, snake venom metalloproteinase; TNBS, 2,4,6-trinitrobenzenesulphonic acid; UTR, untranslated region.
The nucleotide sequence data reported here have been deposited in the DDBJ sequence data bank: Japanese mamushi SSP-1, AB576135; SSP-2, AB576136; SSP-3, AB576137; SSP-4, AB576138; SSP-5, AB576139.
1. The suffices “h” and “m” are used in this paper to respectively indicate habu (P. flavoviridis) and mamushi (G. blomhoffii) proteins.
References
- Bjarnason JB, Fox JW. Hemorrhagic metalloproteinases from snake venoms. Pharmacol. Ther. 1994;62:325–372.10.1016/0163-7258(94)90049-3
- Perales J, Neves-Ferreira AG, Valente RH, Domont GB. Natural inhibitors of snake venom hemorrhagic metalloproteinases. Toxicon. 2005;45:1013–1020.10.1016/j.toxicon.2005.02.028
- Thwin MM, Gopalakrishnakone P. Snake envenomation and protective natural endogenous proteins: a mini review of the recent developments. Toxicon. 1998;36:1471–1482.10.1016/S0041-0101(98)00137-8
- Yamakawa Y, Omori-Satoh T. Primary structure of the antihemorrhagic factor in serum of the Japanese Habu: a snake venom metalloproteinase inhibitor with a double-headed cystatin domain. J. Biochem. 1992;112:583–589.
- Valente RH, Dragulev B, Perales J, Fox JW, Domont GB. BJ46a, a snake venom metalloproteinase inhibitor. Isolation, characterization, cloning and insights into its mechanism of action. Eur. J. Biochem. 2001;268:3042–3052.10.1046/j.1432-1327.2001.02199.x
- Aoki N, Tsutsumi K, Deshimaru M, Terada S. Properties and cDNA cloning of antihemorrhagic factors in sera of Chinese and Japanese Mamushi (Gloydius blomhoffii). Toxicon. 2008;51:251–261.10.1016/j.toxicon.2007.09.007
- Deshimaru M, Tanaka C, Fujino K, Aoki N, Terada S, Hattori S, Ohno M. Properties and cDNA cloning of an antihemorrhagic factor (HSF) purified from the serum of Trimeresurus flavoviridis. Toxicon. 2005;46:937–945.10.1016/j.toxicon.2005.09.003
- Dubé JY, Pelletier G, Gagnon P, Tremblay RR. Immunohistochemical localization of a prostatic secretory protein of 94 amino acids in normal prostatic tissue, in primary prostatic tumors and in their metastases. J. Urol. 1987;138:883–887.
- Fernlund P, Granberg LB, Roepstorff P. Amino acid sequence of β-microseminoprotein from porcine seminal plasma. Arch. Biochem. Biophys. 1994;309:70–76.10.1006/abbi.1994.1086
- Xuan JW, Kwong J, Chan FL, Ricci M, Imasato Y, Sakai H, Fong GH, Panchal C, Chin JL. cDNA, genomic cloning, and gene expression analysis of mouse PSP94 (prostate secretory protein of 94 amino acids). DNA Cell Biol. 1999;18:11–26.10.1089/104454999315583
- Mäkinen M, Valtonen-Andre C, Lundwall A. New world, but not Old World, monkeys carry several genes encoding β-microseminoprotein. Eur. J. Biochem. 1999;264:407–414.10.1046/j.1432-1327.1999.00614.x
- Lazure C, Villemure M, Gauthier D, Naudé RJ, Mbikay M. Characterization of ostrich (Struthio camelus) β-microseminoprotein (MSP): identification of homologous sequences in EST databases and analysis of their evolution during speciation. Protein Sci. 2001;10:2207–2218.
- Nolet S, St-Louis D, Mbikay M, Chrétien M. Rapid evolution of prostatic protein PSP94 suggested by sequence divergence between rhesus monkey and human cDNAs. Genomics. 1991;9:775–777.10.1016/0888-7543(91)90375-O
- Ghasriani H, Teilum K, Johnsson Y, Fernlund P, Drakenberg T. Solution structures of human and porcine β-microseminoprotein. J. Mol. Biol. 2006;362:502–515.10.1016/j.jmb.2006.07.029
- Reeves JR, Xuan JW, Arfanis K, Morin C, Garde SV, Ruiz MT, Wisniewski J, Panchal C, Tanner JE. Identification, purification and characterization of a novel human blood protein with binding affinity for prostate secretory protein of 94 amino acids. Biochem. J. 2005;385:105–114.
- Udby L, Lundwall Å, Johnsen AH, Fernlund P, Valtonen-André C, Blom AM, Lilja H, Borregaard N, Kjeldsen L, Bjartell A. beta-Microseminoprotein binds CRISP-3 in human seminal plasma. Biochem. Biophys. Res. Commun. 2005;333:555–561.10.1016/j.bbrc.2005.05.139
- Anklesaria JH, Jagtap DD, Pathak BR, Kadam KM, Joseph S, Mahale SD. Prostate secretory protein of 94 amino acids (PSP94) binds to prostatic acid phosphatase (PAP) in human seminal plasma. PLoS One. 2013;8:1–10.
- Aoki N, Sakiyama A, Deshimaru M, Terada S. Identification of novel serum proteins in a Japanese viper: Homologs of mammalian PSP94. Biochem. Biophys. Res. Commun. 2007;359:330–334.10.1016/j.bbrc.2007.05.091
- Aoki N, Matsuo H, Deshimaru M, Terada S. Accelerated evolution of small serum proteins (SSPs)—The PSP94 family proteins in a Japanese viper. Gene. 2008;426:7–14.10.1016/j.gene.2008.08.021
- Fujimura S, Oshikawa K, Terada S, Kimoto E. Primary structure and autoproteolysis of brevilysin H6 from the venom of Gloydius halys brevicaudus. J. Biochem. 2000;128:167–173.10.1093/oxfordjournals.jbchem.a022737
- Yamazaki Y, Koike H, Sugiyama Y, Motoyoshi K, Wada T, Hishinuma S, Mita M, Morita T. Cloning and characterization of novel snake venom proteins that block smooth muscle contraction. Eur. J. Biochem. 2002;269:2708–2715.10.1046/j.1432-1033.2002.02940.x
- Aoki N, Sakiyama A, Kuroki K, Maenaka K, Kohda D, Deshimaru M, Terada S. Serotriflin, a CRISP family protein with binding affinity for small serum protein-2 in snake serum. Biochim. Biophys. Acta. 2008;1784:621–628.10.1016/j.bbapap.2007.12.010
- Shioi N, Narazaki M, Terada S. Novel function of antihemorrhagic factor HSF as an SSP-binding protein in Habu (Trimeresurus flavoviridis) serum. Fukuoka Univ. Sci. Rep. 2011;41:177–184.
- Masuda S, Hayashi H, Atoda H, Morita T, Araki S. Purification, cDNA cloning and characterization of the vascular apoptosis-inducing protein, HV1, from Trimeresurus flavoviridis. Eur. J. Biochem. 2001;268:3339–3345.10.1046/j.1432-1327.2001.02246.x
- Shioi N, Ogawa E, Mizukami Y, Abe S, Hayashi R, Terada S. Small serum protein-1 changes the susceptibility of an apoptosis-inducing metalloproteinase HV1 to a metalloproteinase inhibitor in habu snake (Trimeresurus flavoviridis). J. Biochem. 2013;153:121–129.10.1093/jb/mvs127
- Omori-Satoh T, Sadahiro S. Resolution of the major hemorrhagic component of Trimeresurus flavoviridis venom into two parts. Biochim. Biophys. Acta. 1979;580:392–404.10.1016/0005-2795(79)90151-X
- Deshimaru M, Terada S. Chromatographic separation of brevilysins, metalloproteinases in Chinese Mamushi (Gloydius halys brevicaudus) venom. Fukuoka Univ. Sci. Rep. 2003;33:37–43.
- Fujimura S, Rikimaru T, Baba S, Hori J, Hao XQ, Terada S, Kimoto E. Purification and characterization of a non-hemorrhagic metalloprotease from Agkistrodon halys brevicaudus venom. Biochim. Biophys. Acta. 1995;1243:94–100.10.1016/0304-4165(94)00115-E
- Twining SS. Fluorescein isothiocyanate-labeled casein assay for proteolytic enzymes. Anal. Biochem. 1984;143:30–34.10.1016/0003-2697(84)90553-0
- Schägger H, von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal. Biochem. 1987;166:368–379.10.1016/0003-2697(87)90587-2
- Friedman M, Krull LH, Cavins JF. The chromatographic determination of cystine and cysteine residues in proteins as S-β-(4-pyridylethyl)cysteine. J. Biol. Chem. 1970;245:3868–3871.
- Spande T, Witkop B. Determination of the tryptophan content of proteins with N-bromosuccinimide. In: Hirs CHW, editor. Methods in enzymolog. Vol. 11. New York (NY): Academic Press; 1967. p. 498–506.
- Okuyama T, Satake K. On the preparation and properties of 2,4,6-trinitrophenyl-L-amino acids and peptides. J. Biochem. 1960;47:454–466.
- Saitou N, Nei M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987;4:406–425.
- Ohkura N, Okuhara H, Inoue S, Ikeda K, Hayashi K. Purification and characterization of three distinct types of phospholipase A2 inhibitors from the blood plasma of the Chinese mamushi, Agkistrodon blomhoffii siniticus. Biochem. J. 1997;325:527–531.
- Aoki N, Deshimaru M, Kihara K, Terada S. Snake fetuin: Isolation and structural analysis of new fetuin family proteins from the sera of venomous snakes. Toxicon. 2009;54:481–490.10.1016/j.toxicon.2009.05.018
- Aird SD. Ophidian envenomation strategies and the role of purines. Toxicon. 2002;40:335–393.10.1016/S0041-0101(01)00232-X
- Harvey AL. Twenty years of dendrotoxins. Toxicon. 2001;39:15–26.10.1016/S0041-0101(00)00162-8
- Fox JW, Bjarnason JB. Atrolysins: metalloproteinases from Crotalus atrox venom. Methods Enzymol. 1995;248:368–387.10.1016/0076-6879(95)48024-2
- Felsenstein J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution. 1985;39:783–791.10.2307/2408678