Abstract
One new (1) and three known (2–4) sesquiterpenes and four known diterpenes (5–8) were isolated from the root bark of Tripterygium hypoglaucum. Their structures were elucidated on the basis of extensive spectroscopic analyses (IR, ESI-MS, HR-ESI-MS, 1D-NMR, and 2D-NMR). The inhibitory activity toward LPS-induced NO production of these terpenoids was evaluated, all the compounds showing inhibitory effects.
Tripterygium hypoglaucum (Lévl.) Hutch, belonging to the family Celastraceae, is a shrub distributed mainly in Yunnan, Sichuan, and Guizhou Provinces of China.Citation1) Its root bark has been used as a traditional medicine for its anti-inflammatory, immunosuppressive, and anticancer effects.Citation1) Previous phytochemical investigations of T. hypoglaucum have revealed the main presence of terpenoids,Citation2−8) especially sesquiterpenoids.Citation3−8) Although the chemical constituents of T. hypoglaucum have been investigated, there have been no reports on the bioactive constituents responsible for the anti-inflammatory activities. In the course of our survey on biologically active substances in traditional medicines, strong attention has been given to the presence of compounds with an inhibitory effect on NO production, since these substances were expected to be useful as potential anti-inflammatory agents. As a continuation of our work on the search for bioactive natural products,Citation9−13) the root bark of T. hypoglaucum, a traditional medicine, evoked our interest, whose bioactive constituents responsible for anti-inflammation had not been investigated. Our chemical investigation isolated one new (1) and three known (2–4) sesquiterpenes and four known diterpenes (5–8) from the root bark of T. hypoglaucum. Their structures were elucidated as 9α-cinnamoyloxy-1β-furoyloxy-4-hydroxy-6α-nicotinoyloxy-β-dihydroagarofuran (1), 1β,9α-dibenzoyloxy-4-hydroxy-6α-nicotinoyloxy-β-dihydroagarofuran (2), 1β-benzoyloxy-9α-cinnamoyloxy-4-hydroxy-6α-nicotinoyloxy-β-dihydroagarofuran (3), 1β-acetoxy-9α-benzoyloxy-4-hydroxy-6α-nicotinoyloxy-β-dihydroagarofuran (4), hypolide (5), neotriptophenolide (6), triptobenzene A (7), and triptonediol (8) (Fig. ) by spectroscopic data analyses (IR, ESI-MS, HR-ESI-MS, 1D-NMR, and 2D-NMR). This report describes their isolation, structural elucidation, and inhibitory effects on LPS-induced NO production in murine microglial BV-2 cells.
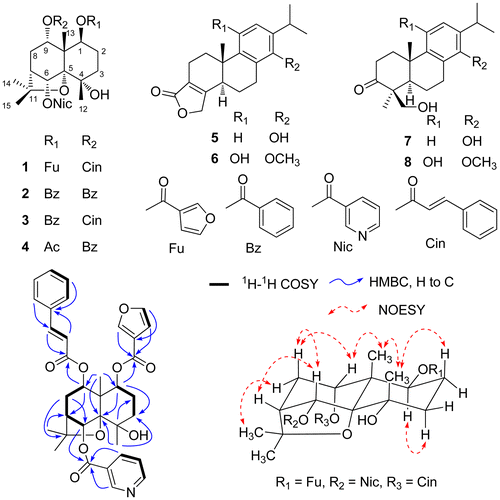
Fig. 1. Structures of compounds 1–8, and key 1H-1H COSY, HMBC, and NOESY correlations for compound 1.
The ethyl acetate-soluble part of the methanol extract of the root bark of T. hypoglaucum was fractionated over silica gel to give five fractions (F1-F5) based on TLC analyses. F4 was fractionated by medium-pressure liquid chromatography (MPLC) over octadecylsilyl (ODS), eluting with a stepwise gradient from 55 to 85% MeOH in H2O to give five subfractions (F4-1-F4-5). F4-4 was further purified by preparative HPLC (YMC-pack ODS-AM, 20 × 250 mm, 79% MeOH in H2O) to afford compounds 1 (tR = 18 min, 11.0 mg), 2 (tR = 21 min, 14.1 mg), and 3 (tR = 26 min, 12.5 mg). Compounds 4 (tR = 26 min, 15.0 mg) and 7 (tR = 23 min, 16.2 mg) were obtained from subfraction F4-3 by using the same HPLC system (YMC-pack ODS-AM, 20 × 250 mm, 75% MeOH in H2O). Fraction F3 was subjected to the same MPLC fractionation (60–85% MeOH in H2O) to obtain three subfractions F3-1-F3-3, and subsequent purification of F3-2 by the same HPLC system (69% MeOH in H2O) yielded compounds 5 (tR = 31 min, 9.3 mg), 6 (tR = 27 min, 11.2 mg), and 8 (tR = 24 min, 10.7 mg).
Compound 1 was isolated as white powder. Its HR-ESI-MS data provided the molecular formula, C35H37NO9, by the presence of a peak at m/z 616.2549 [M + H]+ (calcd. for C35H38NO9 616.2547), which was compatible with the NMR data. The 1H-NMR data (Table ) for 1 exhibited four methyl groups at δH 1.55 (6H, s, H3-14 and H3-15), 1.50 (s, H3-13), and 1.39 (s, H3-12), three oxygenated methine protons at δH 5.59 (br. d, J = 10.8 Hz, H-1), 5.69 (1H, s, H-6), and 4.95 (d, J = 6.5 Hz, H-9), and a pair of olefinic protons of a trans-double bond at δH 6.31 and 7.48 (each 1H, d, J = 16.0 Hz). In addition, 12 aromatic protons were also revealed in the 1H-NMR spectrum of 1. The 13C-NMR spectrum of 1 showed 35 carbon resonances, including three carbonyl carbons, 17 olefinic carbons, and residual 15 carbons in the mid- and upfield regions. Analyses of the 13C, DEPT, and HMQC NMR spectra enabled the residual 15 carbons in the mid- and upfield regions to be classified into four methyl groups [δC 23.9 (C-12), 19.9 (C-13), 25.8 (C-14), and 29.8 (C-15)], three methylene groups [δC 23.7 (C-2), 38.8 (C-3), and 31.9 (C-8)], four methine groups [δC 72.7 (C-1), 80.9 (C-6), 49.0 (C-7), and 72.5 (C-9)], and four quaternary carbons [δC 70.8 (C-4), 91.5 (C-5), 51.5 (C-10), and 84.5 (C-11)]. A comparison of the chemical shifts for the residual 15 carbons with those of skeletal carbons of dihydroagarofuran sesquiterpenes suggested compound 1 to be a sesquiterpene with the dihydroagarofuran skeleton.Citation14−16) Subsequent interpretation of the HMQC and HMBC spectra confirmed this dihydroagarofuran skeleton for 1. After defining the skeleton, the chemical shifts for carbonyl and olefinic carbons in the 13C-NMR spectrum suggested the presence of one cinnamoyloxy, one furoyloxy, and one nicotinoyloxy group based on the corresponding aromatic proton signals and the substituents of dihydroagarofuran sesquiterpenes reported in the literature.Citation14,16,17) The positions of the three acyloxy groups were determined by the interpretation of the HMBC spectrum. The correlation of H-1 at δH 5.59 with the carbonyl carbon signal at δC 162.3 (CO of the furoyloxy moiety) indicated the presence of the furoyloxy group at C-1. Similarly, respective long-range coupling of the protons at δH 5.69 (H-6) and 4.95 (H-9) with the carbons at δC 164.8 and 165.9 demonstrated that the nicotinoyloxy group was attached at C-6 and the cinnamoyloxy group at C-9. By further analyzing the HMQC, HMBC, and 1H-1H COSY spectra (Fig. ), all the proton and carbon signals were unambiguously assigned, resulting in establishment of the planar structure for 1.
Table 1. 1H- and 13C-NMR data for the sesquiterpene skeleton of compound 1 (δ ppm in CDCl3 and J in Hz).*
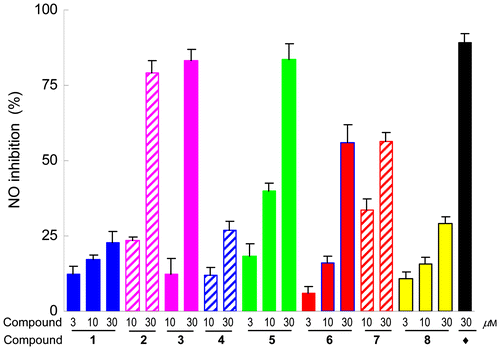
Fig. 2. Inhibitory effects of compounds 1–8 on LPS-induced NO production in BV-2 cells.
Notes: BV-2 cells were treated with LPS alone or together with each compound at the concentrations indicated. After 20 h of incubation, each supernatant was tested by a Griess assay, and the NO inhibitory rate calculated. The experiment was performed three times, and data are expressed as mean ± SD values. The inhibitory effect on NO production was calculated as follows: inhibitory rate (%) = (1 − (LPS/sample − untreated)/(LPS - untreated)) × 100. ♦ Indicated positive control, SMT.
The relative configuration of 1 was next elucidated. In all of the natural dihydroagarofuran-type sesquiterpenes reported, two six-membered rings consisting of C-1-C-10 have been transfused with each other, H3-12 and H3-13 both being biogenetically β-oriented with an axial orientation, and H-7 being biogenetically in a β-position with an equatorial orientation.Citation14−17) NOESY correlations observed for H-9/H3-13, H3-12/H3-13, H-1/H-3α, H3-12/H-2β, H-6/H-8β, H-6/H-7, and H-8α/H3-14(15), but not for H-1/H3-13, suggested that the C-1 furoyloxy group was in a β-position, and the C-6 nicotinoyloxy group, and C-9 cinnamoyloxy group were both in α-positions. The structure of compound 1 was therefore elucidated as 9α-cinnamoyloxy-1β-furoyloxy-4-hydroxy- 6α-nicotinoyloxy-β-dihydroagarofuran (1).Footnote1
Based on spectroscopic analyses and a comparison with the literature, the known compounds were identified as 1β,9α-dibenzoyloxy-4-hydroxy-6α-nicotinoyloxy-β-dihydroagarofuran (2),Citation16) 1β-benzoyloxy-9α-cinnamoyloxy-4-hydroxy-6α-nicotinoyloxy-β-dihydroagarofuran (3),Citation17) 1β-acetoxy-9α-benzoyloxy-4-hydroxy-6α-nicotinoyloxy-β-dihydroagarofuran (4),Citation8) hypolide (5),Citation2) neotriptophenolide (6),Citation3) triptobenzene A (7),Citation18) and triptonediol (8).Citation19)
In order to characterize the bioactive constituents responsible for the anti-inflammatory effects of the root bark of T. hypoglaucum, compounds 1–8 were evaluated for their inhibitory activity toward LPS-induced NO production in murine microglial BV-2 cells by the Griess reaction as described.Citation13,20) 2-Methyl-2-thiopseudourea sulfate (SMT) was used as a positive control (IC50 of 4.8 μM). All the compounds exhibited inhibitory effects on LPS-induced NO production, the NO inhibition rates at various concentrations being shown in Fig. . Among these terpenoids, 2, 3, 5, 6, and 7 showed strong inhibitory effects with respective IC50 values of 18.6, 19.0, 37.6, 28.1, and 20.9 μM, and compounds 1, 4, and 8 showed weak activities with IC50 values >100 μM. An MTT assay indicated that all of the compounds had no significant cytotoxicity toward BV-2 cells at their effective concentration for inhibiting NO production (data not shown).
In summary, one new (1) and three known (2–4) sesquiterpenes and four diterpenes (5–8) were successfully isolated from the root bark of T. hypoglaucum. Their structures were elucidated by 1D- and 2D- NMR spectra. Biological studies disclosed that all of the isolates exhibited inhibitory effects on LPS-induced NO production, with compounds 2, 3, and 5 exerting promising inhibition against NO production. The results of our chemical investigation further revealed the chemical composition of T. hypoglaucum, and biological screening of these isolates identified the bioactive constituents responsible for the anti-inflammatory effects of T. hypoglaucum. The current biological data suggest that these terpenes from T. hypoglaucum, especially compounds 2, 3, and 5 with strong NO inhibitory activities, may have the potential to be developed as anti-inflammatory agents for various inflammatory diseases. Further biological studies on these compounds are still underway by our group.
Notes
1. Compound 1: White powder; = +17.2 (c = 0.11, MeOH); IR (KBr) νmax cm–1: 3440, 2931, 1722, 1634, 1591, 1281, 1071. ESI-MS m/z 616 [M + H]+; HR-ESI-MS m/z 616.2549 [M + H]+; calcd. for C35H38NO6, 616.2547; 1H-NMR (400 MHz, CDCl3): 1-furoyloxy group [δH 7.80 (1H, s), 7.32 (1H, br. s), 6.50 (1H, br. s)], 6-nicotinoyloxy group [δH 9.40 (1H, s), 8.84 (1H, s), 8.56 (1H, d, J = 7.8 Hz), 7.47 (1H, overlapped)], 9-cinnamoyloxy group [δH 6.31, 7.48 (each 1H, d, J = 16.0 Hz), 7.41 (2H, overlapped), 7.50 (2H, overlapped), 7.41 (1H, overlapped)], see Table for the other signals; 13C-NMR (100 MHz, CDCl3): 1-furoyloxy group (δC 147.5, 119.4, 109.5, 143.5, 162.3), 6-nicotinoyloxy group (δC 125.9, 137.9, 123.8, 153.5, 151.3, 164.8), 9-cinnamoyloxy group (δC 117.7, 145.5, 134.4, 128.8 × 2, 128.2 × 2, 130.3, 165.9), see Table for the other signals.
References
- Nanjing University of Chinese Medicine. Dictionary of Chinese herb medicines. 2nd ed. Shanghai: Shanghai Scientific and Technologic Press; 2006. p. 1887–1820.
- Duan H, Kawazoe K, Bando M, Kido M, Takaishi Y. Di- and triterpenoids from Tripterygium hypoglaucum. Phytochemistry. 1997;46:535–543.10.1016/S0031-9422(97)00288-4
- Fujita R, Duan H, Takaishi Y. Terpenoids from Tripterygium hypoglaucum. Phytochemistry. 2000;53:715–722.10.1016/S0031-9422(99)00557-9
- Li CJ, Xie FG, Yang JZ, Luo YM, Chen XG, Zhang DM. Two sesquiterpene pyridine alkaloids and a triterpenoid saponin from the root barks of Tripterygium hypoglaucum. J. Asian Nat. Prod. Res. 2012;14:973–980.10.1080/10286020.2012.729049
- Duan H, Takaishi Y. Sesquiterpene evoninate alkaloids from Tripterygium hypoglaucum. Phytochemistry. 1999;52:1735–1738.10.1016/S0031-9422(99)00179-X
- Li W, Li B, Chen Y. Sesquiterpene alkaloids from Tripterygium hypoglaucum. Phytochemistry. 1999;50:1091–1093.10.1016/S0031-9422(98)00618-9
- Duan H, Takaishi Y. Structures of sesquiterpene polyol alkaloids from Tripterygium hypoglaucum. Phytochemistry. 1998;49:2185–2189.10.1016/S0031-9422(98)00439-7
- Duan H, Kawazoe K, Takaishi Y. Sesquiterpene alkaloids from Tripterygium hypoglaucum. Phytochemistry. 1997;45:617–621.10.1016/S0031-9422(96)00875-8
- Xu J, Li Y, Guo Y, Guo P, Yamakuni T, Ohizumi Y. Isolation, Structural Elucidation, and Neuroprotective Effects of Iridoids from Valeriana jatamansi. Biosci. Biotechnol. Biochem. 2012;76:1401–1403.10.1271/bbb.120097
- Xu J, Guo P, Liu C, Sun Z, Gui L, Guo Y, Yamakuni T, Ohizumi Y. Neuroprotective Kaurane Diterpenes from Fritillaria ebeiensis. Biosci. Biotechnol. Biochem. 2011;75:1386–1388.10.1271/bbb.110146
- Guo P, Li Y, Xu J, Liu C, Ma Y, Guo Y. Bioactive neo-clerodane diterpenoids from the whole plants of Ajuga ciliata Bunge. J. Nat. Prod. 2011;74:1575–1583.10.1021/np2001557
- Xu J, Guo Y, Xie C, Li Y, Gao J, Zhang T, Hou W, Fang L, Gui L, Hou W, Fang L, Gui L. Bioactive myrsinol diterpenoids from the roots of Euphorbia prolifera. J. Nat. Prod. 2011;74:2224–2230.10.1021/np200591h
- Xu J, Jin D, Liu C, Xie C, Guo Y, Fang L. Isolation, characterization, and NO inhibitory activities of sesquiterpenes from Blumea balsamifera. J. Agric. Food Chem. 2012;60:8051–8058.10.1021/jf302530u
- Xu J, Xie C, Jin D, Guo Y, Zhao P, Wang S, He Y. Three new dihydroagarofuran sesquiterpenoids from Celastrus orbiculatus. Phytochem. Lett. 2012;5:713–716.10.1016/j.phytol.2012.07.009
- Xu J, Jin D, Zhao P, Song X, Sun Z, Guo Y, Zhang L. Sesquiterpenes inhibiting NO production from Celastrus orbiculatus. Fitoterapia. 2012;83:1302–1305.10.1016/j.fitote.2011.12.026
- Duan HQ, Takaishi Y, Jia YF, Li D. Sesquiterpene polyol esters from Tripterygium wilfordii. Phytochemistry. 2001;56:341–346.10.1016/S0031-9422(00)00369-1
- Luo Y, Zhou M, Ye Q, Pu Q, Zhang G. Dihydroagarofuran derivatives from the dried roots of Tripterygium wilfordii. J. Nat. Prod. 2012;75:98–102.10.1021/np200493t
- Takaishi Y, Wariishi N, Tateishi H, Kawazoe K, Miyagi K, Li K, Duan H. Phenolic diterpenes from Tripterygium wilfordii var. regelii. Phytochemistry. 1997;45:979–984.10.1016/S0031-9422(96)00861-8
- Duan H, Takaishi Y, Momota H, Ohmoto Y, Taki T, Jia Y, Li D. Immunosuppressive diterpenoids from Tripterygium wilfordii. J. Nat. Prod. 1999;62:1522–1525.10.1021/np9902183
- Schmidt HH, Kelm M. Determination of nitrite and nitrate by the Griess reaction. In: Feelisch M, Stamler JS, editors. Methods in nitric oxide research. West Sussex, Wiley; 1996. p. 491–497.
