Abstract
Two novel phenolic glucosides (1–2), as well as seven known compounds (3–9), were isolated from the stem of Acanthopanax koreanum; their chemical structures were determined by chemical and spectroscopic methods and subsequently compared with previously reported data. Their inhibition of nuclear factor kappa B (NF-κB) was measured in human embryonic kidney (293T) cells by using an NF-κB luciferase assay.
Key words:
Acanthopanax koreanum (Araliaceae) is a native plant distributed in South Korea. The root and stem bark of A. koreanum has been used as a tonic and prophylactic, as well as to treat rheumatism, paralysis, hepatitis, and diabetes in traditional Korean medicine.Citation1,2) The primary constituents of A. koreanum contain diterpenoids, lignans, triterpenoids, polyacetylenes, phenylpropanoids, and flavonoids.Citation3) The bioactive effects of diterpenoids and triterpenoids are well known. Previous pharmacological studies have shown that the diterpenoids from A. koreanum can treat diabetes by inhibiting the protein tyrosine phosphatase 1B (PTP1B) activityCitation4) and have been used to treat inflammatory and immune diseases by modulating excessive activation of the nuclear factor of activated T-cells.Citation5) In addition, a previous study has shown that acanthoic acid could be used to attenuate murine and ulcerative colitis.Citation6) Triterpenoids have an immunomodulatory effect on spleen lymphocyte IL-2 and IFN-c, as well as an inhibitory effect on LPS-stimulated pro-inflammatory cytokine production in bone marrow-derived dendritic cells.Citation7,8) A. koreanum components therefore have various documented anti-inflammatory effects. In particular, a recent study has shown that impressic acid, isolated from the leaves of A. koreanum, significantly inhibited the nuclear factor kappa B (NF-κB) activity.Citation9) However, the effects of phenolic components from A. koreanum on NF-κB transcriptional inhibitory activity have not been reported.
NF-κB is an inducible transcription factor of the Rel family, which is sequestered in the cytoplasm by the IκB family of proteins.Citation10) The NF-κB family consists of a group of inducible transcription factors that regulate immune and inflammatory responses and prevent cells from undergoing apoptosis in response to cellular stress. A number of signal transduction cascades can activate the NF-κB pathway, resulting in translocation of the NF-κB proteins from the cytoplasm to the nucleus where they induce the expression of specific cellular genes.Citation11) The activation of NF-κB causes transcription at the κB site, which is involved in inflammatory disorders and cancer. Inhibiting NF-κB signaling has therefore become a therapeutic target for treating inflammatory diseases and cancer.Citation10,Citation12,13) The effects of compounds 1–9 on TNFα-induced NF-κB transcriptional activity in human embryonic kidney (293T) cells were evaluated in the present study by an NF-κB luciferase assay.
Dried stems of A. koreanum (3.3 kg) were extracted three times with MeOH under refluxing. The MeOH extract (210.0 g) was suspended in H2O (0.8 L) and partitioned with EtOAc and n-BuOH (each 0.8 L × 3). The BuOH extract (42.0 g) was subjected to silica gel column chromatography (60 × 350 mm) with a gradient of CHCl3–MeOH–H2O (15:1:0–1:1:0.1) to yield five fractions (Fr. 1–Fr. 5). Fr. 2 was further chromatographed in a silica gel column (30 × 580 mm), using a gradient of CHCl3–MeOH (20:1–10:1), to yield six subfractions (Fr. 2.1–Fr. 2.6). Further purification of Fr. 2.2 was conducted by chromatography in a reverse-phase (RP) column (15 × 600 mm) with an eluent gradient of MeOH–H2O (1:5) to yield compounds 6 (25.0 mg) and 8 (2.0 mg). Fr. 2.5 was further chromatographed in a RP column (20 × 600 mm) with MeOH–H2O (1:12–1:1) to yield compounds 3 (180.0 mg), 4 (240.0 mg), 7 (24.0 mg), and 9 (72.0 mg). Fr. 4 was chromatographed in a silica gel column (30 × 580 mm) with an eluent gradient of CHCl3–MeOH (10:1–1:1) to yield for fractions (Fr. 4.1–Fr. 4.4). Fr. 4.2 was column chromatographed in a RP column (20 × 600 mm), eluting with MeOH–H2O (1:20–1:1), to provide compounds 1 (3.0 mg), 2 (13.0 mg), and 5 (30.0 mg).
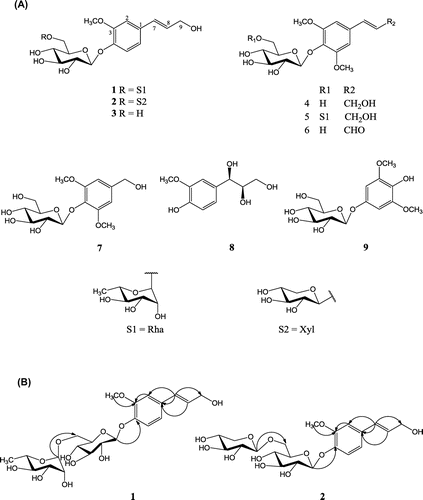
Compound 1 was obtained as a pale yellow solid, with the molecular formula C22H32O12 as deduced from the [M + Na]+ peak at m/z 511.1798 (calcd. for C22H32O12Na, 511.1786) by high-resolution electrospray ionization mass spectrometry (HRESIMS). The 1H-nuclear magnetic resonance (NMR) spectrum of 1 presented three aromatic signals at δH 7.66 (1H, d, J = 8.2 Hz, H-5), 7.20 (1H, dd, J = 8.2 Hz, 2.0 Hz, H-6), and δH 7.16 (1H, d, J = 2.0 Hz, H-2), belonging to an ABX system. Two olefinic proton signals at δH 6.82 (1H, d, J = 15.8 Hz, H-7) and δH 6.57 (1H, dt, J = 15.8 Hz, 4.1 Hz, H-8), with a large coupling constant (J = 15.8 Hz), were indicative of a trans-olefinic coupling pattern. A methylene signal at δH 4.54 (2H, d, J = 4.1 Hz, H-9) and a methoxy signal at δH 3.71 (3H, s) were observed. The 1H-NMR spectrum also contained two anomeric proton signals at δH 5.56 (1H, d, J = 7.6 Hz, H-1′) and 5.48 (1H, br, s, H-1″), indicating that compound 1 possessed two sugar moieties. These sugar units were identified as d-glucose and l-rhamnose after acid hydrolysis. The13C-NMR spectrum suggested that the sugar residues of 1 were composed of glucose and rhamnose by comparing the data with a reference.Citation14) The distortionless enhancement by polarization transfer and 13C-NMR spectra of 1 contained fifteen methine signals, two methene signals, and two methyl signals. Signals at δC 129.2 (C-7), 129.7 (C-8), and 62.7 (C-9) (Table ) were indicative of a propenyl group in compound 1. The key correlation between δH 6.82 (H-7) and δC 132.3 (C-1) in the heteronuclear multiple bond correlation (HMBC) spectrum revealed a propenyl group located at C-1. The anomeric proton of the glucosyl unit at δH 5.56 (1H, d, J = 7.6 Hz, H-1′) showed a long range correlation with δC 147.6 (C-4), indicating that the glucosyl unit was attached to C-4 of the aglycone. The anomeric proton of the rhamnosyl unit at δH 5.48 (1H, br, s, H-1″) was indicative of a cross-peak with δC 67.9 (C-6′) of a glucosyl, suggesting that the rhamnosyl unit was linked to C-6′ of the glucosyl group. The methoxy proton at δH 3.71, which was correlated with δC 150.2 (C-3) in the HMBC spectrum, suggests a methoxy group attached at C-3 (Fig. ). The structure of 1 was conclusively identified as 3-methoxy-1-(3-hydroxy-propen-1-yl)phenyl-4-O-α-l-rhamnopyranosyl-(1→6)-β-d-glucopyranoside.
Table 1. 1H-NMR (600 MHz) and 13C-NMR (150 MHz) spectroscopic data for compounds 1 and 2 in pyridine-d5.
Compound 2 was obtained as a pale yellow solid. The HRESIMS data of 2 showed a molecular ion peak at m/z 497.1653 [M + Na]+ (calcd. for C21H30O12Na, 497.1629), which was consistent with the molecular formula of C21H30O12. The 1H-NMR spectrum of 2 showed three aromatic signals at δH 7.76 (1H, d, J = 8.2 Hz, H-5), 7.20 (1H, dd, J = 8.2 Hz, 2.0 Hz, H-6), and δH 7.18 (1H, d, J = 2.0 Hz, H-2), two olefinic proton signals at δH 6.83 (1H, d, J = 15.8 Hz, H-7) and δH 6.53 (1H, dt, J = 15.8 Hz, 4.1 Hz, H-8), one methylene signal at δH 4.54 (2H, d, J = 4.1 Hz, H-9), and one methoxyl signal at δH 3.71 (3H, s) (Table ); it also contained a 3-methoxy-1-(3-hydroxy-propen-1-yl)phenyl. Two anomeric protons at δH 5.59 (1H, d, J = 7.6 Hz, H-1′) and 5.01 (1H, d, J = 7.6 Hz, H-1″) suggest two sugar units. Acid hydrolysis of 2 with 10% HCl yielded d-glucose, d-xylose, and a 3-methoxy-1-(3-hydroxy-propen-1-yl) phenol. The correlation between H-1′ and C-4 (δC 147.9) in the HMBC spectrum suggested that the glucose moieties were linked to C-4 of the aglycone moiety. The xylose moiety was connected to C-6′ (δC 70.0) based on the HMBC correlation from H-1″ (δH 5.01) to C-6′ (δC 70.1) of glucose (Fig. ). Compound 2 was therefore determined to be 3-methoxy-1-(3-hydroxy-propen-1-yl)phenyl-4-O-β-d-xylopyranosyl-(1→6)-β-d-glucopyranoside.
The other known compounds were identified as coniferin (3),Citation15) syringin (4),Citation15) 3,5-dimethoxy-1-(3-hydroxy-propen-1-yl)phenyl-4-O-α-l-rhamnopyranosyl-(1→6)-β-d-glucopyranoside (5),Citation14) sinapaldehyde glucoside (6),Citation16) di-O-methylcrenatin (7),Citation17) (−)-(7R, 8R)-guaiacylglycerol (8),Citation18) and koaburaside (9),Citation17) by comparing their physical and spectroscopic data with those reported in the literature. Compounds 5–9 were isolated for the first time from A. koreanum.
The effects of compounds (1–9) on the NF-κB transcriptional activity, which was induced by TNFα in 293T cells, were evaluated by an NF-κB luciferase assay. A CCK-8 cell-counting kit (Dojindo, Kumamoto, Japan) was used to analyze the cell toxicity of the compounds according to the manufacturer’s instructions. Human embryonic kidney cells were cultured overnight in a 96-well plate (about 1 × 104 cells/well). Cell toxicity was assessed after adding each compound in a dose-dependent manner. After 24 h of treatment, 10 μL of the CCK-8 solution was added to triplicate wells and incubated for 1 h. The absorbance at 450 nm was measured to determine the viable cell numbers. We found that compounds 1–9 did not show significant cytotoxicity to 293T cells at the tested concentrations (0.1, 1, and 10 μM; data not shown).
The luciferase vector was first transfected into 293T cells. After a limited amount of time, the cells were lysed, and luciferin (the substrate of luciferase) was added to the cellular extract together with Mg2+ and an excess of ATP. Luciferase enzymes expressed by the reporter vector could catalyze the oxidative carboxylation of luciferin under these conditions. Cells were seeded at 2 × 105 cells per well in 24-well plates. After 24 h, the cells were transfected with the inducible NF-κB firefly luciferase reporter constitutively expressing the Renilla reporter. After 24 h of transfection, the medium was changed to the assay medium (Opti-MEM + 0.5% FBS + 0.1 mM NEAA + 1 mM sodium pyruvate + 100 units/mL of penicillin + 10 μg/mL of streptomycin), and the cells were pretreated for 1 h with either vehicle alone (1% DMSO in water) or the compounds, before treating with 10 ng/mL TNF-α for 23 h. Unstimulated 293T cells were used as a negative control (−), and pyrrolidine dithiocarbamate (PDTC) was used as a positive control. The dual luciferase assay was performed 48 h after transfection, and promoter activity values are expressed as arbitrary units using a Renilla reporter for internal normalization. All compounds were pretreated with transfected 293T cells at various concentrations (0.1, 1, and 10 μM), before stimulating with TNF-α. The results show that compounds 4, 6, and 7 significantly inhibited the TNFα-induced NF-κB transcriptional activity in a dose-dependent manner, with IC50 values ranging from 26.05 to 41.49 μM. Compounds 2, 5, and 9 had negligible effects with IC50 values greater than 50 μM (Table ). However, compounds 1, 3, and 8 were inactive (IC50 > 100 μM) at the tested concentrations (data not shown).
Table 2. Inhibitory effects of compounds 2, 4-7, and 9 on the TNF-α induced NF-κB transcriptional activity.
Based on the structure-activity relationship of the isolated compounds (1–9), the effects of compounds 4, 6, and 7 were significantly increased when a glucose group was linked to C-4 of the skeleton. However, the effects decreased when a disaccharide group was linked to compound 5. These results suggested that a glucose unit at C-4 of 3,5-dimethoxy-1-(3-hydroxy-propen-1-yl) phenol may increase TNFα-induced NF-κB transcriptional activity. The glucose unit was therefore a key functional element in the phenolic derivatives.
Two new phenolic glucosides (1–2) and seven known compounds (3–9) were isolated in this study from a methanol extract of the stems of A. koreanum. Compounds 4, 6, and 7 significantly inhibited TNFα-induced NF-κB transcriptional activity. Overall, the phenolic glucosides were novel bioactive components of A. koreanum that inhibited the TNFα-induced NF-κB transcriptional activity. These compounds could be used as therapeutics for inflammation control. On the basis of the structure-activity relationship, further investigation and optimization of these derivatives might enable the preparation of potentially useful compounds for treating inflammation.
Supplemental material
The supplemental material for this paper is available at http://dx.doi.org/10.1080/09168451.2014.890036.
Supplementary figs. 1-12
Download PDF (610.1 KB)Funding
This work was financially supported by the Priority Research Centers Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology [2009-0093815] of the Republic of Korea.
References
- Yook CS. Coloured medicinal plants of Korean. Seoul: Academy Publishing; 1993. p. 371–372.
- Bae K. The medicinal plants of Korea. Seoul: Kyo-Hak Publishing; 2000. p. 361.
- Shin KH, Lee SH. The chemistry of secondary products from Acanthopanax species and their pharmacological activities. Nat. Prod. Sci. 2002;8:111–126.
- Na MK, Oh WK, Kim YH, Cai XF, Kim SH, Kim BY, Ahn JS. Inhibition of protein tyrosine phosphatase 1B by diterpenoids isolated from Acanthopanax koreanum. Bioorg. Med. Chem. Lett. 2006;16:3061–3064.10.1016/j.bmcl.2006.02.053
- Cai XF, Lee IS, Dat NT, Shen GH, Kim YH. Diterpenoids with inhibitory activity against NFAT transcription factor from Acanthopanax koreanum. Phytother. Res. 2004;18:677–680.10.1002/(ISSN)1099-1573
- Kang OH, Kim DK, Cai XF, Kim YH, Lee YM. Attenuation of experimental murine colitis by acanthoic acid from Acanthopanax koreanum. Arch. Pharm. Res. 2010;33:87–93.10.1007/s12272-010-2230-x
- Nhiem NX, Kiem PV, Minh CV, Tai BH, Tung NH, Ha DT, Soung KS, Kim JH, Ahn JY, Lee YM, Kim YH. Structure-activity relationship of lupane-triterpene glycosides from Acanthopanax koreanum on spleen lymphocyte IL-2 and INF-γ. Bioorg. Med. Chem. Lett. 2010;20:4927–4931.10.1016/j.bmcl.2010.06.044
- Kim JA, Yang SY, Koo JE, Koh YS, Kim YH. Lupane-type triterpenoids from the steamed leaves of Acanthopanax koreanum and their inhibitory effects on the LPS-stimulated pro-inflammatory cytokine production in bone marrow-derived dendritic cells. Bioorg. Med. Chem. Lett. 2010;20:6703–6707.10.1016/j.bmcl.2010.09.001
- Kim JA, Yang SY, Song SB, Kim YH. Effects of impressic acid from Acanthopanax koreanum on NF-кB and PPARγ activities.Arch. Pharm. Res. 2011;34:1347–1351.10.1007/s12272-011-0815-7
- Pande V, Ramos MJ. NF-kappa B in human disease: current inhibitors and prospects for de novo structure based design of inhibitors. Curr. Med. Chem. 2005;12:357–374.10.2174/0929867053363180
- Yamamoto Y, Gaynor RB. Role of the NF-kappa B pathway in the pathogenesis of human disease states. Curr. Mol. Med. 2001;1:287–296.10.2174/1566524013363816
- Baldwin AS. Control of oncogenesis and cancer therapy resistance by the transcription factor NF-kappa B. J. J. Clin. Invest. 2001;107:241–246.10.1172/JCI11991
- Pande V, Ramos M. Inhibitory effect of astragalin on expression of lipopolysaccharide-induced inflammatory mediators through NF-κB in macrophages. Curr. Med. Chem. 2005;12:357–374.10.2174/0929867053363180
- Kim MS, Kim SH. Studies on the constituents of the stem bark of Kalopanax pictus. Arch. Pharm. Res. 2011;34:2101–2107.10.1007/s12272-011-1213-x
- Sano K, Sanada S, Ida Y, Shoji J. Chemical constituents from Acanthopanax senticosus. Chem. Pharm. Bull. 1991;39:865–870.10.1248/cpb.39.865
- Li ZF, Yang JH, Zhang WG, Feng YL, Jian H, Luo XJ, Yang SL, Pei YH. Constituents of the barks of Fraxinus chinensis Roxb. Zhongcaoyao. 2011;42:852–855.
- Wei XL, Yang CH, Liang JY. Glycosides from the stems of Photinia pyrifolia. Zhongguo Tianran Yaowu. 2005;3:228–230.
- Zhang DM, Li Y, Yu SS. Enantioselective synthesis of phenylpropanetriols. Tianran Chanwu Yanjiu Yu Kaifa. 2004;16:496–499.
- DellaGreca M, Fiorentin A, Monaco P, Previtera L. Synth. Commun. 1998;28:3693–3700.10.1080/00397919808004916