Abstract
Posttranslational isoprenylation of a tryptophan residue identified from Bacillus quorum sensing pheromone, ComX pheromone, is unique and essential for the bioactivity. A modifying enzyme, ComQ, forms ComX pheromone from the ComX precursor and isoprenyl pyrophosphate and exhibits moderate similarity to isoprenyl pyrophosphate synthases. We investigated non-conserved region in ComQ, corresponding to isopentenyl pyrophosphate binding region of the synthases, using in vitro cell-free isoprenylation. These results suggested that the only conserved aspartic acid residue in the region of ComQ is critical for enzyme activity and responsible for ComX binding. Our findings should contribute to basic understanding of the mechanism of tryptophan isoprenylation.
Graphical Abstract
ComQRO-E-2 is a unique enzyme that mediates post-translational geranylation of Trp. The 187th Asp in ComQRO-E-2 was found to be critical for activity.
Posttranslational modification generates structural diversity and frequently affects protein function. Various patterns of modification have been identified at various amino acid residues. One of them is isoprenylation, which generally refers to the attachment of a farnesyl or a geranylgeranyl group to a C-terminal cysteine residue through a sulfide bond.Citation1) Cysteine isoprenylation is widely distributed in eukaryotes and plays a critical role in protein function. Although the modification has not yet been reported in prokaryotes, we have identified tryptophan geranylation of a quorum sensing pheromone, ComXRO-E-2 pheromone,Citation2) secreted by Bacillus subtilis strain RO-E-2.Citation3) ComXRO-E-2 pheromone has a unique modified tryptophan residue with a geranyl group at the 3rd position on the indole ring, resulting in the formation of a tricyclic structure that contains a newly formed proline-like five-membered ring (Fig. (A)). Posttranslational isoprenylation of a tryptophan residue, including other amino acid residues except cysteine, is unprecedented in living organisms. In particular, the presence of geranylated compounds is rare in primary and secondary metabolites outside the plant kingdom, and only a few geranyl-derived secondary metabolites have been identified in microorganisms.Citation4,5) Successive studies of six Bacillus species strains revealed that posttranslational tryptophan isoprenylation of ComX pheromones was classified into two types: geranylation and farnesylation.Citation6,7) Thus, the occurrence of posttranslational isoprenylation has been discovered in prokaryotes as well as eukaryotes.Citation8)
Fig. 1. Chemical Structure of the Modified Tryptophan Residue and Amino Acid Sequence of ComXRO-E-2.
Note: (A) The attached geranyl group is indicated in bold. (B) Amino acid residues of ComXRO-E-2 pheromone are underlined. The target tryptophan for geranylation is boldfaced. Sequence source was as follows: ComXRO-E-2; AAL67740 (B. strain RO-E-2).
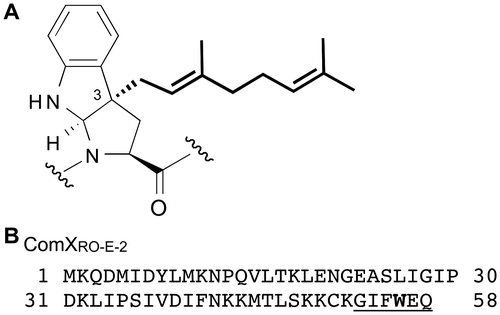
In a process known as quorum sensing, bacteria modulate gene expressions in response to cell density, and they secrete signaling molecules to sense their cell density.Citation9) Quorum sensing in bacteria causes various phenomena. In bacilli, ComX pheromone is a strain-specific extracellular signaling oligopeptide, which stimulates natural genetic competence controlled by quorum sensing.Citation2,10) Molecular genetic studies have suggested that a modifying enzyme, ComQ,Citation11) that forms ComX pheromones from the ComX precursor (Fig. (B)), is necessary for genetic competence.Citation12,13) In addition, our chemical studies indicated that the correctly isoprenylated tryptophan residue, including its stereostructure, played a critical role in the biological activity of the ComX pheromone in contrast to other unmodified amino acid residues.Citation14Citation–Citation17) Therefore, posttranslational tryptophan isoprenylation is essential for the function of the peptide pheromone.
We have recently established a cell-free system permitting in vitro geranylation of the tryptophan residue of chemically synthesized ComXRO-E-2, using recombinant ComQRO-E-2 overexpressed by Escherichia coli in the presence of geranyl pyrophosphate (GPP) and magnesium ion (Mg2+).Citation18) We investigated in vitro cell-free enzyme reactions of various chemically synthesized ComXRO-E-2 derivatives, finding that ComQRO-E-2 recognized the eleven residues from the 47th position to 57th position of the ComXRO-E-2 ([47–57]ComXRO-E-2) as a minimum substrate unit for geranylation. Furthermore, we recognized that the tryptophan residue must be located between second and fourth residues from the C-terminal in order to be modified by ComQRO-E-2.Citation19)
ComQ exhibits no similarity to cysteine farnesyltransferases or geranylgeranyltransferases, but moderate similarity to farnesyl pyrophosphate (FPP) synthases and geranylgeranyl pyrophosphate (GGPP) synthases, which catalyze the formation of extended isoprenoidal pyrophosphates through the condensation of GPP or FPP and isopentenyl pyrophosphate (IPP).Citation20) These enzymes have two conserved aspartate-rich motifs containing “DDXXD” residues (where X refers to any amino acid), named as first aspartate-rich motif (FARM) and second aspartate-rich motif (SARM).Citation20) FARM and SARM are essential for catalytic function and the binding of the substrates GPP (or FPP) and IPP, respectively, in the presence of Mg2+. FARM in ComQ is highly conserved, and mutation of the first or fifth aspartic acid of FARM in ComQ eliminates pheromonal signaling. FARM of ComQ was necessary for production of ComX pheromone and bound GPP (or FPP) by FPP and GGPP synthases in the presence of Mg2+.Citation12) In contrast, the amino acid residues corresponding to SARM in ComQ are quite different. To characterize the differences between tryptophan isoprenyltransferase and isoprenyl pyrophosphate synthase, we investigated non-conserved region corresponding to SARM in ComQ using site-directed mutagenesis of ComQ and in vitro cell-free isoprenylation.
Materials and methods
Site-directed mutagenesis
Site-directed mutagenesis was performed using a KOD-Plus-Mutagenesis Kit (Toyobo, Tokyo, Japan) with the ComQRO-E-2 expression plasmid DNA, as previously described,Citation18) and the mutagenic primers presented in Table , where the underlined nucleotides represent mutated codons. The coding sequences of all constructed plasmids were verified by DNA sequencing. DNA manipulation, cloning, and transformation were performed by the standard protocols.
Table 1. Primer sequences used in the site-directed mutagenesis study.
Preparation of ComQRO-E-2 analogs
Methods for overexpression and preparation of ComQRO-E-2 analogs were those used for the wild type, as previously described.Citation18) Briefly, each Escherichia coli mutant containing an appropriate plasmid was grown in M9 medium. After optical density at 620 nm of the culture reached approximately 0.6, isopropyl-β-d-thiogalactopyranoside (IPTG) (1 mm) was added. After incubation for 4 h, the cultured cells were collected by centrifugation and suspended in 25 mm Tris–HCl buffer (pH 7.4) containing 0.1 mm MgCl2 and EGTA. The suspension was homogenized by sonication. After the slurry was ultracentrifuged, the precipitate was collected by decantation and resuspended in the above buffer with the addition of 2 mg/mL dodecyl-β-d-maltoside.
SDS-PAGE
SDS-PAGE was performed using NuPAGE 4–12% Bis–Tris Gel (1.0 mm × 12 well, Invitrogen, Carlsbad, USA), and 75 μL broth equivalent of ComQRO-E-2 analogs was loaded. The gels were stained with CBB staining for 1 h, rinsed in deionized water overnight, and then analyzed.
In vitro geranylation and detection of geranylated peptides
In a final volume of 50 μL, ComXRO-E-2, GPP (10 pmol), and ComQRO-E-2 analogs suspension (7.5 mL broth equivalent) were incubated for 2 h at 37 °C in TAPS–NaOH buffer (50 mm, pH 8.5) containing MgCl2 (5 mm). After addition of 200 μL of CH3CN, the mixture was centrifuged. To 30 μL of the solution containing internal standard (synthetic Ala-ComXRO-E-2, 2.5 pmol), 10 μL of the supernatant was added, and the mixture was analyzed by LC-MS (HCTplus, Bruker Daltonics, Bremen, Germany), as previously described.Citation18,19) The amount of geranylated peptide was calculated from integration of the peak area. All experiments were carried out with triplicate samples, and the activity of the in vitro enzyme reaction represented the average of the triplicate samples. For investigation of the substrate specificity of [K182F]ComQRO-E-2, [47–58]ComXRO-E-2 (2.5 pmol), or [47–57]ComXRO-E-2 (2.5 pmol) was used as substrate instead of ComXRO-E-2. Relative activities were calculated on the basis of the activity of ComQRO-E-2 with ComXRO-E-2 as the substrate.
Results and discussion
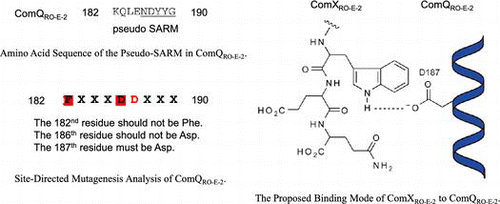
Substitution of five amino acid residues of a pseudo-SARM in ComQ with alanine
The amino acid sequences around the SARM in FPP synthase, GGPP synthase, and ComQs of six B. strains were aligned, as presented in Table . At least two or three aspartic acid residues are highly conserved in FPP and GGPP synthases from all living organisms for the essential active site, but each alignment of ComQ contained only one aspartic acid residue.
Table 2. Alignment of ComQ variants with SARM in FPP synthase and GGPP synthase with the ComQ variants from Six B. Strains.
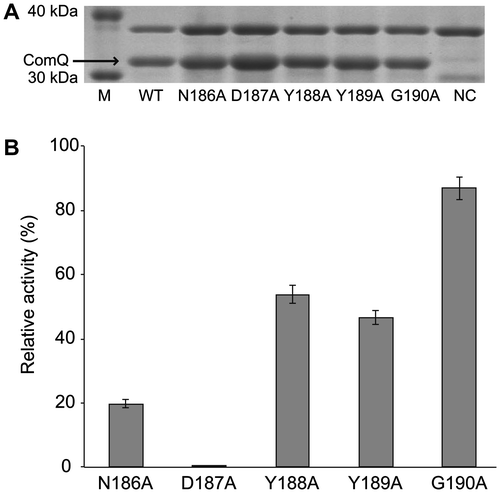
We first prepared individually alanine-substituted ComQRO-E-2 analogs for the five amino acid residues of a pseudo-SARM in ComQ and performed in vitro geranylation using these analogs and chemically synthesized ComXRO-E-2 in the presence of GPP and Mg2+ ion. As illustrated in Fig. , all mutants were appropriately overexpressed, and all alanine-substituted analogs exhibited less activity than wild-type ComQRO-E-2. [D187A]ComQRO-E-2, in which the aspartic acid residue at the 187th position was changed to alanine in ComQRO-E-2, lost enzymatic activity almost completely (0.03%). This result clearly demonstrated that the aspartic acid at the 187th position in ComQRO-E-2 played a critical role in enzymatic function as well as FPP and GGPP synthases. [N186A]ComQRO-E-2 exhibited only 20% of the level of relative activity of the wild type. This result suggested that the asparagine at the 186th position was important for activity, although not as important as the first aspartic acid in SARM of FPP and GGPP synthases. In contrast, three mutants, [Y188A]ComQRO-E-2, [Y189A]ComQRO-E-2, and [G190A]ComQRO-E-2, exhibited significant activity (54, 47, and 87%, respectively). The tyrosine at the 188th position is conserved in ComQ, but is likely to be less important than other conserved amino acid residues of the pseudo-SARM in ComQ. Presumably, these three amino acid residues (Y, Y, and G) are replaceable without greatly influencing original function of ComQ.
Fig. 2. Effects of Substitution of Five Amino Acid Residues of the Pseudo-SARM in ComQ with Alanine.
Note: (A) SDS-PAGE analysis of alanine-substituted mutants. NC is negative control carried out without IPTG addition for wild-type ComQRO-E-2. (B) Enzymatic activities of alanine-substituted mutants. The activities are represented by percent yield of geranylated peptides relative to the wild type. The values are the means of triplicate samples and error bars represent standard deviations.
Substitution of three amino acid residues in ComQ corresponding to aspartic acid of SARM with aspartic acid or similar amino acids
With the aim of investigating the role of conserved and non-conserved aspartic acids of the pseudo-SARM in detail, the asparagine at the 186th and the glycine at the 190th positions in ComQRO-E-2 were substituted with aspartic acid, and the aspartic acid at the 187th position in ComQRO-E-2 was substituted with a similar amino acid, asparagine, or glutamic acid. As illustrated in Fig. (A) and B, all mutants were appropriately overexpressed. As illustrated in Fig. (C), [N186D]ComQRO-E-2 exhibited only 9% of the level of activity relative to the wild type, and this was equivalent to 44% of the level of [N186A]ComQRO-E-2 activity. We attempted to prepare [N186G]ComQRO-E-2, but the recombinant protein was not expressed at all (data not shown). We conclude that the first amino acid residue of the pseudo-SARM in ComQ is very important for ComQ function and in particular must not be aspartic acid. Some FPP and GGPP synthases have sequences of DDXXG in SARM, but it is an unsuitable sequence for tryptophan isoprenylation.
Fig. 3. Effects of Substitution of Three Amino Acid Residues in ComQ Corresponding to Aspartic Acid of SARM.
Note: (A, B) SDS-PAGE analysis of mutants. NC is negative control without IPTG addition for wild-type ComQRO-E-2. (C) Enzymatic activities of mutants. The activities are represented by percent yield of geranylated peptides relative to the wild type. The values are the means of triplicate samples and error bars represent standard deviations.
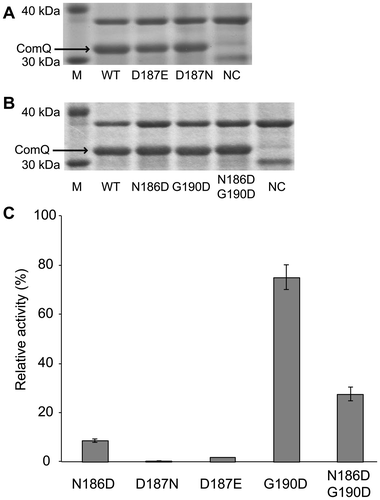
[D187 N]ComQRO-E-2 and [D187E]ComQRO-E-2 exhibited extremely weak enzymatic activity (0.7% and 2.0%, respectively) as well as [D187A]ComQRO-E-2. These results confirmed that the aspartic acid at the 187th position in ComQRO-E-2 could not be replaced with any amino acid and appeared to be involved in the binding of ComX. In contrast, because [G190D]ComQRO-E-2 exhibited significant activity (75%), the amino acid residue corresponding to the aspartic acid at the 5th position of SARM does not play an important role for ComQ function.Citation21) The double mutant, [N186D+G190D]ComQRO-E-2, exhibited weak enzymatic activity (28%), but the activity was higher than [N186D]ComQRO-E-2. Although the detailed reason is unknown, we estimated that this difference is related to the stability of the enzyme. These findings suggested that the difference between tryptophan geranyltransferase and FPP synthase was not only caused by mutation of the aspartic acid residues in SARM. We investigated FPP synthase activity using IPP instead of ComXRO-E-2 and each ComQRO-E-2 analog in the presence of GPP and Mg2+, but no activity was exhibited by any ComQRO-E-2 analogs including wild-type ComQRO-E-2 (data not shown).
Substitution of the forward amino acid residues of the pseudo-SARM in ComQ
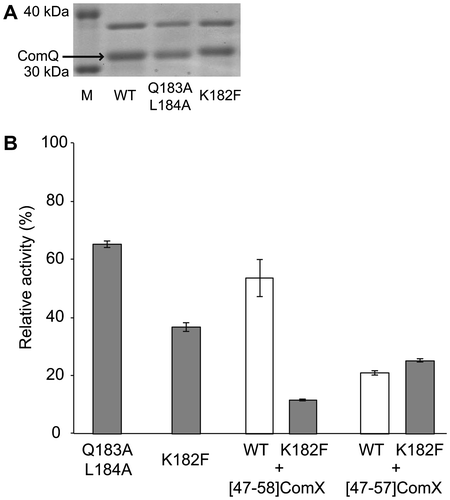
To confirm that the aspartic acid at the 187th position in ComQRO-E-2 is responsible for ComXRO-E-2 binding, we investigated the effect of the forward amino acid residues of the pseudo-SARM in ComQRO-E-2 on enzyme activity, as presented in Fig. . The glutamine at the 183rd and the isoleucine at the 184th positions are conserved in ComQ as well as in FPP and GGPP synthases (Table ). An alanine-substituted double mutant of the amino acids, [Q183A+I184A]ComQRO-E-2, was prepared and investigated. The mutant exhibited moderate activity (65%), indicating that the two amino acid residues did not strongly affect activity. In contrast, the amino acid residue at the 4th position before the pseudo-SARM is not conserved in ComQ, but highly conserved as phenylalanine in FPP and GGPP synthases (Table ). [K182F]ComQRO-E-2, a substitution of the 182nd lysine with phenylalanine, exhibited weak enzymatic activity (37%). This result suggested that bulkiness of the side chain hindered ComX binding. In FPP and GGPP synthases, the residues at the 4th and the 5th positions before FARM are the key residues determining product chain length, and the product specificity of the synthases is altered depending on the steric hindrance of the residues at the 4th and the 5th positions before FARM.Citation21–Citation23) If the pseudo-SARM in ComQ is responsible for ComX binding, we expected that substrate specificity of [K182F]ComQRO-E-2 would be distinct from that of wild-type ComQRO-E-2 because [K182F]ComQRO-E-2 would tend to favor shorter ComXRO-E-2 derivatives as a substrate than wild-type ComXRO-E-2. We accordingly investigated the enzyme activity of [K182F]ComQRO-E-2 and ComQRO-E-2 using [47–58]ComXRO-E-2, which is a dodecapeptide of the C-terminal ComXRO-E-2 and the shortest N-truncated ComXRO-E-2 that functions as a substrate, and [47–57]ComXRO-E-2, which is an undecapeptide next to the C-terminal ComXRO-E-2 and the minimum unit of substrate.Citation19) In comparison with ComXRO-E-2 and ComQRO-E-2, the activity ratio of [47–58]ComXRO-E-2 and ComQRO-E-2 was 54%, whereas that of [47–58]ComXRO-E-2 and [K182F]ComQRO-E-2 was 12%. The enzyme activity of [K182F]ComQRO-E-2 for [47–58]ComXRO-E-2 was drastically reduced compared to ComQRO-E-2. In contrast, the ratio of [47–57]ComXRO-E-2 and [K182F]ComQRO-E-2 was 25%, twofold greater than that of [47–58]ComXRO-E-2 and [K182F]ComQRO-E-2, which was increased more than that of wild-type ComQRO-E-2 (21%). Because the 182nd phenylalanine residue of [K182F]ComQRO-E-2 adversely affects the C-terminus glutamine residue of [47–58]ComX as well as that of ComX, this result indicates that [47–57]ComXRO-E-2 was a more suitable substrate for [K182F]ComQRO-E-2 than [47–58]ComXRO-E-2 (Fig. ). Together with previous studies on FPP and GGPP synthases,Citation20−Citation24) these findings suggest that 182nd lysine of ComQRO-E-2 is possibly located at the bottom of the ComXRO-E-2 binding site and next to the C-terminal of ComXRO-E-2 during isoprenylation. In fact, other ComQs have a smaller amino acid residue at the position than ComQRO-E-2, which is alanine or serine instead of lysine. The previous finding that the tryptophan residue modified by ComQRO-E-2 must be located between the second and fourth residues from the C-terminal is further supported. These results indicate that the aspartic acid residue of the pseudo-SARM in ComQ is responsible for ComX binding and that the C-terminal of ComX penetrates into the interior of the binding pocket consisting of the pseudo-SARM and the forward amino acid residues, owing to isoprenylation.
Fig. 4. Effects of Substitution of the Forward Amino Acid Residues of the Pseudo-SARM in ComQ.
Note: (A) SDS-PAGE analysis of mutants. (B) Enzymatic activities of mutants. The activities are represented by percent yield of geranylated peptides relative to the wild-type ComQRO-E-2 for ComXRO-E-2. The values are the means of triplicate samples and the error bars represent their standard deviations.
In conclusion, it was revealed that the only conserved aspartic acid residue corresponding to SARM in ComQ is critical for tryptophan isoprenylation activity. In addition, it was suggested that the unique single-aspartate motif of ComQ is responsible for ComX binding. Our findings should contribute to basic understanding of the mechanism of tryptophan isoprenylation. Considering that there must be other distinct regions of ComQ compared to FPP and GGPP synthases that account for their differences, further studies are needed to reveal the precise molecular mechanisms of tryptophan isoprenylation. Elucidation of the function of ComQ will lead to the molecular production of isoprenylated tryptophan derivatives by enzyme engineering and to the discovery of novel tryptophan isoprenyltransferases and tryptophan-isoprenylated proteins.
Supplemental material
The supplemental material for this paper is available at http://dx.doi.org/10.1080/09168451.2014.891932.
Funding
This work was supported by JSPS KAKENHI [grant number 24688011]; MEXT KAKENHI [grant number 25108724].
Supplementary Fig. 1
Download MS Power Point (239.5 KB)References
- Tamanoi F, Hrycyna CA, Bergo MO, editors. Protein prenylation. The Enzymes. Vol. 29. Burlington: Academic Press; 2011.
- Magnuson R, Solomon J, Grossman AD. Biochemical and genetic characterization of a competence pheromone from B. subtilis. Cell. 1994;77:207–216.10.1016/0092-8674(94)90313-1
- Okada M, Sato I, Cho SJ, Iwata H, Nishio T, Dubnau D, Sakagami Y. Structure of the Bacillus subtilis quorum-sensing peptide pheromone ComX. Nat. Chem. Biol. 2005;1:23–24.10.1038/nchembio709
- Kuzuyama T, Noel JP, Richard SB. Structural basis for the promiscuous biosynthetic prenylation of aromatic natural products. Nature. 2005;435:982–987.
- Chooi Y-H, Wang P, Fang J, Li Y, Wu K, Wang P, Tang Y. Discovery and characterization of a group of fungal polycyclic polyketide prenyltransferases. J. Am. Chem. Soc. 2012;134:9428–9437.10.1021/ja3028636
- Okada M, Yamaguchi H, Sato I, Tsuji F, Dubnau D, Sakagami Y. Chemical structure of posttranslational modification with a farnesyl group on tryptophan. Biosci. Biotechnol. Biochem. 2008;72:914–918.10.1271/bbb.80006
- Okada M, Yamaguchi H, Sato I, Tsuji F, Qi J, Dubnau D, Sakagami Y. Acid labile ComX pheromone from Bacillus mojavensis RO-H-1. Biosci. Biotechnol. Biochem. 2007;71:1807–1810.10.1271/bbb.70245
- Okada M. Post-translational isoprenylation of tryptophan. Biosci. Biotechnol. Biochem. 2011;75:1413–1417.10.1271/bbb.110087
- Bassler BL, Losick R. Bacterially speaking. Cell. 2006;125:237–246.10.1016/j.cell.2006.04.001
- Hamoen LW, Venema G, Kuipers OP. Controlling competence in Bacillus subtilis: shared use of regulators. Microbiology. 2003;149:9–17.10.1099/mic.0.26003-0
- Weinrauch Y, Msadek T, Kunst F, Dubnau D. Sequence and properties of comQ, a new competence regulatory gene of Bacillus subtilis. J. Bacteriol. 1991;173:5685–5693.
- Schneider K, Palmer TM, Grossman AD. Characterization of comQ and comX, two genes required for production of ComX pheromone in Bacillus subtilis. J. Bacteriol. 2002;184:410–419.10.1128/JB.184.2.410-419.2002
- Ansaldi M, Marolt D, Stebe T, Mandic-Mulec I, Dubnau D. Specific activation of the Bacillus quorum-sensing systems by isoprenylated pheromone variants. Mol. Microbiol. 2002;44:1561–1573.10.1046/j.1365-2958.2002.02977.x
- Tsuji F, Kobayashi K, Okada M, Yamaguchi H, Ojika M, Sakagami Y. The geranylmodified tryptophan residue is crucial for ComXRO-E-2 pheromone biological activity. Bioorg. Med. Chem. Lett. 2011;21:4041–4044.10.1016/j.bmcl.2011.04.123
- Okada M, Yamaguchi H, Sato I, Cho SJ, Dubnau D, Sakagami Y. Structure-activity relationship studies on quorum sensing ComX(RO-E-2) pheromone. Bioorg. Med. Chem. Lett. 2007;17:1705–1707.10.1016/j.bmcl.2006.12.070
- Okada M, Sato I, Jeong Cho SJ, Dubnau D, Sakagami Y. Chemical synthesis of ComX pheromone and related peptides containing isoprenoidal tryptophan residues. Tetrahedron. 2006;62:8907–8918.10.1016/j.tet.2006.06.074
- Okada M, Sato I, Cho SJ, Suzuki Y, Ojika M, Dubnau D, Sakagami Y. Towards structural determination of the ComX pheromone: synthetic studies on peptides containing geranyltryptophan. Biosci. Biotechnol. Biochem. 2004;68:2374–2387.10.1271/bbb.68.2374
- Tsuji F, Ishihara A, Kurata K, Nakagawa A, Okada M, Kitamura S, Kanamaru K, Masuda Y, Murakami K, Irie K, Sakagami Y. Geranyl modification on the tryptophan residue of ComXRO-E-2 pheromone by a cell-free system. FEBS Lett. 2012;586:174–179.10.1016/j.febslet.2011.12.012
- Tsuji F, Ishihara A, Nakagawa A, Okada M, Kitamura S, Kanamaru K, Masuda Y, Murakami K, Irie K, Sakagami Y. Lack of the consensus sequence necessary for tryptophan prenylation in the ComX pheromone precursor. Biosci. Biotechnol. Biochem. 2012;76:1492–1496.10.1271/bbb.120206
- Poulter CD. A paradigm for understanding structure and function relationships in E-polyprenyl diphosphate synthases. Phytochem. Rev. 2006;5:17–26.10.1007/s11101-005-4887-1
- Wang K, Ohnuma S. Chain-length determination mechanism of isoprenyl diphosphate synthases and implications for molecular evolution. Trends Biochem. Sci. 1999;24:445–451.10.1016/S0968-0004(99)01464-4
- Ohnuma S, Hirooka K, Ohto C, Nishio T. Conversion from archaeal geranylgeranyl diphosphate synthase to farnesyl diphosphate synthase. Two amino acids before the first aspartate-rich motif solely determine eukaryotic farnesyl diphosphate synthase activity. J. Biol. Chem. 1997;272:5192–5198.
- Ohnuma S, Narita K, Nakazawa T, Ishida C, Takeuchi Y, Ohto C, Nishio T. A role of the amino acid residue located on the fifth position before the first aspartate-rich motif of farnesyl diphosphate synthase on determination of the final product. J. Biol. Chem. 1996;271:30748–30754.
- Tarshis LC, Yan M, Poulter CD, Sacchettini JC. Crystal structure of recombinant farnesyl diphosphate synthase at 2.6-A resolution. Biochemistry. 1994;33:10871–10877.10.1021/bi00202a004

![Fig. 5. The proposed binding mode of [47–58]ComXRO-E-2 to [K182F]ComQRO-E-2.](/cms/asset/fdbc6b40-ab23-4f80-8461-92e9b6fd47bc/tbbb_a_891932_f0005_b.gif)