Abstract
New approaches in the treatment of skeletal defects may benefit from the use of soluble biological factors. We previously standardized a derivative of bovine colostrum (SBCD), deprived of casein and fat and rich in cytokines. In the present study, we tested its possible use as an adjuvant in bone healing. SBCD contained factors involved in stromal cell stimulation and differentiation and induced cytokine production from stimulated mesenchymal stem cells (MSCs). In vitro, SBCD promoted proliferation, migration and, in association with osteogenic factors, osteogenic differentiation of osteoblastic and MSCs. In in vivo experiments of subcutaneous Matrigel injection in mice, SBCD plus hydroxyapatite, but not hydroxyapatite nor SBCD alone, induced recruitment of macrophages and stromal cells. After 60 days, plugs containing SBCD and hydroxyapatite were densely calcified and diffusely positive for osteocalcin, supporting the occurrence of an early osteogenic process. These results indicate that SBCD is a rich source of factors with osteoinductive properties.
Graphical Abstract
Induction of osteogenic differentiation by a standardized a derivative of bovine colostrum was tested in vitro and in vivo.

Replacement and regeneration of bone tissue are major topics of interest in several disciplines, traumatology, orthopaedics, neurosurgery, and dentistry being, among others, potentially involved. Standard treatment of bone defects entails grafting bone, which is the second most commonly transplanted tissue after blood, with over 2.2 million bone grafting procedures performed annually worldwide.Citation1) Autologous bone grafting is considered the gold standard for reconstruction of bony skeletal defects and for stimulation of bone regeneration. This procedure presents no risk of disease transmission or graft rejection and has the advantage to potentially transfer live osteocytes and osteoblasts to begin new bone formation.Citation2) However, it may generate a wound at the harvest site, with possible consequences in terms of extending the surgical time and increasing patient morbidityCitation3) and it is limited by the quantity of material available.Citation4) Allografts may instead cause immune reactions, have the potential risk of transmitting infectious diseases, and their sterilization procedures may diminish the biomechanical quality of the material.Citation1,5) In order to avoid these disadvantages, a broad spectrum of synthetic biomaterials serving as synthetic bone substitutes has been developed during the last years. Especially, calcium phosphate ceramics, such as hydroxyapatite, are increasingly introduced in clinical practice in virtue of their good biocompatibility and bioactivity.Citation6)
No matter which is the source of hard, bone-inducing material, an essential role in the process of bone formation is going to be played by stimulating factors, either endogenous or exogenous, favoring proliferation and differentiation of stromal cells. This has spurred extensive research into the potential of growth factors, which have been shown to have the ability to stimulate the differentiation of mesenchymal stem cells (MSCs) into osteogenic cells (osteoinduction). Biofactors have recently been tested for the clinical treatment of musculoskeletal diseases and open fractures with loss of tissue, opening up the considerable potential offered by tissue engineering to orthopaedic and maxillofacial surgeons.Citation7,8)
A large number of growth factors are known to be present in human and bovine milkCitation9) and particularly in the first milk produced after birth, i.e. colostrum. Non-peptide hormones, peptide hormones, and growth factors have been isolated and quantified in colostrum.Citation10) However, the variable source of the product and the lack of standardized composition have so far prevented a reproducible use of colostrum as a natural source of growth factors. We recently generated and characterized a lyophilized and standardized bovine colostrum derivative (SBCD), deprived of casein and fat.Citation11) SBCD contained several bioactive factors, being basic fibroblast growth factor (FGF-b), transforming growth factor (TGF), tumor necrosis factor (TNF), and vascular endothelial growth factor (VEGF), the most represented among growth factors.
In the present study, we evaluated the potential use of SBCD as a possible adjuvant in bone healing. The in vitro capacity to stimulate proliferation and migration of MSCs and osteoblasts was assessed, along with experiments on an in vivo model of ectopic osteogenesis.
Materials and methods
Colostrum
SBCD, a standardized lyophilized product of bovine colostrum, deprived of lipids and casein, was obtained from Biofer S.p.A. (Medolla, Italy). Preparation and characterization of SBCD, as well as concentration of specific cytokines and of a vast number of growth factors was reported elsewhere.Citation11)
Cell culturing
The effects of the SBCD on cell proliferation and differentiation were evaluated in vitro by using human osteoblastic cell lines (Saos-2, MG-63), murine bone marrow-derived MSCs (D1 ORL UVA), and primary cells obtained from lipoaspirates (human adipose tissue-derived stem cells, henceforth ASC). ASCs were isolated from fat tissue obtained from three different donors as described previouslyCitation12) and maintained in ‘control medium’ consisting of Dulbecco’s minimum essential medium enriched with sodium pyruvate and supplemented with 10% fetal bovine serum (FBS, Gibco BRL), 100 U/mL penicillin, 100 μg/mL streptomycin, and 250 ng/mL amphotericin B. The non-adherent cell population was removed after 48 h and the adherent cell layer was washed twice with fresh medium; cells were then continuously cultured since their harvest until sixth passage. D1 bone marrow-derived MSCs were purchased from ATCC (ATCC number: CRL-12424), as were human osteoblastic cell lines Saos-2 (ATCC number: HTB-85) and MG-63 (ATCC number: CRL-1427). D1 cells were kept in Dulbecco’s minimum essential medium supplemented with 10% FBS (Gibco BRL), 100 U/mL penicillin, 100 μg/mL streptomycin. Saos-2 and MG-63 cells were, respectively, cultured in McCoy’5A (Gibco, Life Technologies) with 15% FBS, (Benchmark, Gemini Bio-Products) and in Dulbecco’s Modified Eagle’s Medium (Gibco, Life Technologies) with 10% FBS. Both media were supplemented with 1% penicillin–streptomicin (MD Biomedicals, Thermo Fisher Scientific). Cells were always passaged at subconfluency to prevent contact inhibition and were kept under a humidified atmosphere of 5% CO2 in air, 37 °C.
Cell vitality assay (3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyl tetrazolium bromide test
Cells were seeded in 96-well plates (103 cells/well) and incubated in suitable media in presence of 2% FBS, 2% FBS + 0.1 mg/mL SBCD, 2% FBS + 1 mg/mL SBCD, 2% FBS + 2.5 mg/mL SBCD, 2% FBS + 5 mg/mL SBCD and 10% FBS, for 1, 3, 5, and 7 days. To evaluate the mitochondrial activity of the seeded cells, i.e. the cell viability at different SBCD concentrations, a test with 3-(4,5-dimethylthiazole-2-yl)-2.5-diphenyl tetrazolium bromide (MTT) (Chemicon International, Billerica, MA, USA) was performed on days 1, 3, 5, and 7 as previously reported.Citation13) Samples were measured at 570 nm by a microplate reader (BioRad Laboratories, Hercules, CA, USA).
In vitro wound healing scratch plate assay
The in vitro wound healing assay was performed according to established protocols.Citation14) Briefly, cells were seeded in six-well plates and allowed to grow in their media to approximately 90% confluence. In vitro wounds were created by drawing lines down the well with a 20 μL plastic pipette. After washing with PBS, cells were maintained in 5 mg/mL SBCD, 10% FBS, and 0.5% FBS (control condition). The digital images were captured after 6 and 12 h of incubation and the area occupied by the cells within the scratch was measured (NIH Image J version 1.42).
Cytokine and growth factors
The presence of specific growth factors in SBCD as well as in ASCs and Saos-2 cells after stimulation with SBCD was evaluated. Cells were seeded at the concentration of 104 per well in 96-well plates in control medium. The following day, after washings, cells were incubated for 3 h with RPMI or RPMI containing 5 mg/mL SBCD. After the stimulus, cells were washed and incubated with RPMI alone for further 4 h. Cell media or resuspended SBCD were collected and analyzed using the flexible Bio-Plex system (Bio-Rad Laboratories, Hercules, CA, USA), which is based on a capture sandwich immunoassay, for the simultaneous dosage of different biomolecules. At least three independent repetitions in duplicate were made per experimental condition type. Analyte concentrations were expressed in pg/mL. A standard curve ranging on average from 0.15 to 3700 pg/mL (High Photomultiplier Tube Setting—PMT setting) was prepared and then fitted by Bio-Plex Manager software. The presence of bone morphogenic protein-2 (BMP-2) was evaluated using an ELISA kit (R&D System, Abingdon, UK), following the manufacturer’s instructions.
In vitro osteogenic differentiation tests
Cells were seeded in 96-well plates (103 cells/well) and incubated in DMEM in the presence of 2% FBS, 2% FBS + 5 mg/mL SBCD, 2% FBS + osteogenic factors (OM) (50 μM Ascorbic Acid, 10 mm Beta Glycerophosphate, and 100 nM Dexamethasone), and 5 mg/mL SBCD + OM (50 μM Ascorbic Acid, 10 mM Beta Glycerophosphate, and 100 nM Dexamethasone) for 1, 3, 5, 7, and 14 or alternatively 21 days. In vitro osteogenic differentiation was determined by a series of assays aiming at revealing established bone markers.
Alkaline Phosphatase activity assay. Alkaline Phosphatase activity was determined using a colorimetric end point assay,Citation15) which measures the conversion of the colorless substrate p-nitrophenol phosphate by the enzyme alkaline phosphatase to the yellow product p-nitrophenol. To measure alkaline phosphatase activity, cells were lysed with 0.05% Triton X-100 and incubated with the reagent solution containing phosphatase substrate (Sigma-Aldrich, Milan, Italy) at 37 °C for 15 min. The rate of color change corresponds to the amount of enzyme present in solution. Optical density was measured at a wavelength of 405 nm (reference 620 nm). Samples were compared against the calibration curve of p-nitrophenol standards. The final alkaline phosphatase concentration was adjusted per total protein content, to avoid biases due to the cell number. Therefore, part of the cell lysates obtained for ALP quantification was incubated with BCA™ (Thermo Fisher Scientific, Waltham, MA, USA) Protein Assay, following to the manufacturer’s instructions. Optical density was measured at a wavelength of 570 nm and results were adjusted to a calibration curve made by known number of cells. Alkaline phosphatase values were determined and normalized on whole protein content at day 3 in Saos-2, MG-63, D1 and at day 7 in ASCs.
Calcium content assay. Cell calcium content was determined at day 14 for Saos-2 and D1 or 21 for MG-63 and ASC by Calcium colorimetric assay kit (BioVision Research Products, Mountain View, CA, USA), according to the manufacturer’s protocol. The OD was measured at 575 nm within 20 min since preparation. A calibration curve was always made.
Osteocalcin detection in cell-conditioned media. Human osteocalcin was measured in human cell-conditioned media by Osteocalcin Elisa kit (KAQ1381 Invitrogen Corporation, Camarillo, CA, USA) at day 14 for SaOs-2 and at day 21 for MG-63 and ASCs, following the manufacturer’s protocol.
Collagen and calcium staining. At the established time points, cells grown in six-plate wells were washed once with PBS and fixed with 4% paraformaldehyde for 10 min at room temperature. The solution was removed and cells were washed with PBS. To stain collagen, Sirius red dye (Direct Red 80, Sigma Aldrich) dissolved (1 mg/mL) in a saturated aqueous solution of picric acid (Sigma Aldrich) was added to the fixed cell cultures. After being kept under mild shaking for 2 h, samples were quickly rinsed in acid water (0.5% acetic acid in pure water) and then abundantly washed with distilled water. Calcium salts were stained after von Kossa following published protocols.Citation16) For both picro-Sirius Red and von Kossa stains, the cultures were observed under light microscopy and representative pictures were captured by an Olympus camera.
In vivo osteogenesis model in Matrigel plugs plus hydroxyapatite
Animal experiments were performed according to the guidelines for the care and use of research animals and were approved by the local Ethics Committee. Five-week-old male/female Balb-C mice (n = 35) were enrolled in this study. Matrigel (8.13 mg/mL, BD, Buccinasco Milan, Italy), in liquid form at 4 °C, was mixed with heparin (64 U/mL, Sigma Aldrich) and SBCD (0.1 mg/mL), in the presence or absence of an injectable nanocrystalline paste (0.3% Ostim®, Osartis, Obernburg, Germany, final concentration 0.6%) and injected (0.4 mL) into the abdominal subcutaneous tissue of mice, along the peritoneal midline, as previously described.Citation17) Mice were killed at different times (10, 20, or 60 d) and subcutaneous plug samples were formalin-fixed and processed for light microscopy and immunohistochemistry. Briefly, sections from paraffin-embedded blocks were collected onto poly-L-lysine-coated slides and processed with histological and histochemical staining reactions (hematoxylin and eosin, Masson’s trichromic and van Gieson stain and von Kossa reaction) using the Artisanlink Apparatus (DAKO). For immunohistochemistry, the following Abs were used: anti-osteocalcin polyclonal Ab (Takara Bio Inc., Shiga, Japan; 1:250 dilution) and anti-collagen IV polyclonal Ab (dilution: 1:500), and anti-F4/80 rat mAb (dilution 1:100) (all from Cambridge Science Park, Cambridge, UK). Endogenous peroxidase activity was blocked with 6% H2O2 for 8 min at room temperature. Primary antibodies were applied to slides overnight or for 1 h at 4 °C. Horseradish peroxidase-labeled anti-rabbit Envision polymers (Dako) or anti-rat IgG (Abcam, Cambridge, MA, USA) were incubated for 1 h. The reaction product was developed using 3,3-diaminobenzidine. Omission of the primary antibody or substitution with an unrelated rabbit serum or mouse IgG served as negative control.
Statistical analysis
Data from MTT assays and in vitro osteogenic differentiation tests (with the exception of the picro-Sirius Red and von Kossa stains) were all analyzed by GraphPad Prism 5 (GraphPad Software Inc, La Jolla, CA, USA). Each experiment consisting of eight samples per condition was repeated at least three times, for each cell line. As for the primary cells, the experiments were run three times independently with cells from each one of three different donors (thus reaching a total of nine tests for each assay where ASC were used). Statistical analysis was performed by using the one-way analysis of variance (ANOVA) with post hoc Dunnett’s test or the Student t test, as appropriate. A p value of <0.05 was considered significant.
Results
Presence of MSC-stimulating factors in the SBCD
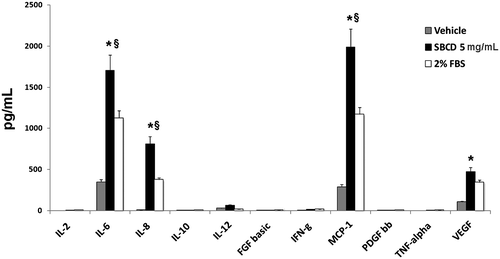
We previously reported that SBCD contains a great number of cytokines and growth factors, possibly involved in MSC proliferation, migration, and differentiation,Citation11) including FGF-b, TGF, TNF, and VEGF. We here tested whether SBCD also contained the osteogenic protein BMP-2.Citation18) We found that BMP-2 was present at 53 ± 13 pg/mg SBCD. In addition, after a 3 h incubation, SBCD induced the release of several cytokines in ASCs, as depicted in Fig. . Interleukin (IL)-6, IL-8, monocyte chemotactic protein-1 (MCP-1), and VEGF were increased by SBCD in ASCs as well as in SaOs-2 (not shown), in respect to unstimulated cells or to cells stimulated with 2% FBS (Fig. ).
Fig. 1. Growth factor release after cell stimulation with SBCD.
Notes: Cytokines and growth factors present in the media of ASCs after 3 h incubation with SBCD (5 mg/mL) or with vehicle alone (RPMI) or with RPMI plus 2% FBS were detected by Bio-Plex system. Data are mean ± SD of three different experiments performed in triplicate. Three cell lines from different donors were used. Student’s t test was performed: * = p < 0.05 vs. RPMI; § = p < 0.05 vs. 2% FBS.
Effect of SBCD on proliferation and migration of osteoblasts and MSC
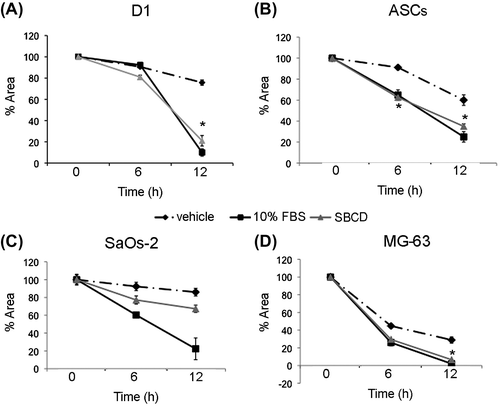
We subsequently evaluate the functional effect of SBCD on two osteoblastic cell lines (Saos-2 and MG-63) and on MSC from bone marrow (D1) and adipose tissue (ASCs) in terms of proliferation and migration. In osteoblasts and MSC cultured in basal conditions, proliferation was increased dose-dependently by the supplementation of SBCD, as shown by MTT analysis (Fig. ). At 2.5 and 5 mg/mL SBCD, cell proliferation resulted superior to that achieved in conventional maintenance conditions at 10% FBS for all the cell types, but MG-63. For this cell line, 5 mg/mL SBCD only induced a proliferation rate higher than 10% FBS (Fig. ). In addition, 5 mg/mL SBCD promoted cell proliferation, as evaluated by wound assay (Fig. ). The effect was comparable to that of 10% FBS (positive control) and significantly higher than the vehicle, with the only exception of SaOs-2 cells (Fig. ).
Fig. 2. Proliferative effect of SBCD.
Notes: MTT Assay shows the proliferative effect of SBCD elicited on different cell types (D1, ASCs, SaOs-2, and MG-63) at different concentrations (0.1, 1, 2.5, and 5 mg/mL) and time points (1, 3, 5, and 7 days), when added to media with low serum concentration. Standard 10% FBS medium was used as positive control. Data are mean ± SD of three different experiments performed in eight different tests. Three lines from different donors were used for ASCs. ANOVA with Dunnett’s comparison test was performed: * = p < 0.05 vs. 2% FBS.
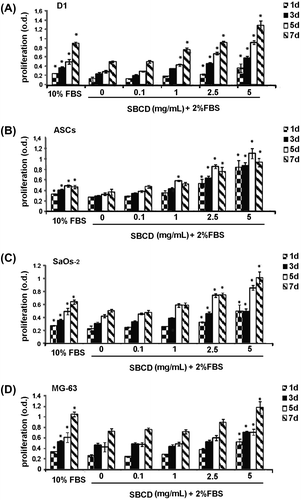
Fig. 3. Effect of SBCD on cell migration.
Notes: Wound healing scratch test was performed at 6 and 12 h on D1, ASCs, SaOs-2, and MG-63 cells. Data are mean ± SD of three different experiments performed in triplicate. Three lines from different donors were used for ASCs. ANOVA with Dunnett’s comparison test was performed: * = p < 0.05 vs. vehicle.
In vitro osteogenesis in presence of SBCD
We next evaluated the osteogenic potential of SBCD per se and in association with OM. No differentiation and minimal cell survival were observed when cells were cultured in the presence of OM alone (Figs. (A) and (A) and (B)). SBCD (5 mg/mL) displayed a significant potential to induce osteogenesis when added to OM, but not alone. This osteogenic effect was comparable to that of 2% FBS plus OM. The osteogenic activity was detected by the early markers alkaline phosphatase activity (Fig. (A)) and picro-Sirius Red (Fig. (B)), the latter detecting collagen I expression. In addition, this effect was confirmed by the detection of the late osteogenic marker calcium deposition evaluated by calcium content (Fig. (A)), osteocalcin release within the culture medium (Fig. (B)) and von Kossa stain (Fig. (C)).
Fig. 4. Induction of early osteogenic markers by SBCD.
Notes: (A) Effect of SBCD and OM in low serum media (2% FBS) on alkaline phosphatase activity measured in D1, ASCs, SaOs-2, and MG-63 cells. Data are expressed as fold increase over control (2% FBS alone) and are mean ± SD of three different experiments performed in eight different samples. Three different cell lines from different donors were used for ASCs. Cells cultured for the same time in the presence of OM alone, omitting both 2% FBS and SBCD, did not survive. (B) Representative micrographs showing the effect of SBCD and OM in low serum media on the collagen deposition detected in D1, ASCs, SaOs-2, and MG-63 cells, as detected by Picro-Sirius Red stain. Original magnification: 100×.
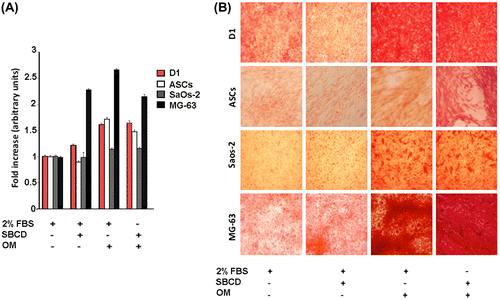
Fig. 5. Induction of late osteogenic markers by SBCD.
Notes: (A) Effect of SBCD and/or OM in low serum media (2% FBS) on calcium deposition as detected in D1, ASCs, SaOs-2, and MG-63 cells based on a quantitative colorimetric assay. Data are expressed as fold increase over control (2% FBS alone) normalized to one. (B) Effect of SBCD and OM in low serum media (2% FBS) on human osteocalcin secretion in ASCs, SaOs-2, and MG-63 cells. Data are expressed as fold increase over control (2% FBS alone) normalized to one. The mean ± SD of three different experiments performed in eight different samples. Three cell lines from different donors were used for ASCs. (C) Representative micrographs showing the effect of SBCD and OM in low serum media (2% FBS) on the calcium deposition as detected by Von Kossa stain, in D1, ASCs, SaOs-2, and MG-63 cells. Cells cultured for the same time in the presence of OM alone, omitting both 2% FBS and SBCD, did not survive. Original magnification: ×100.
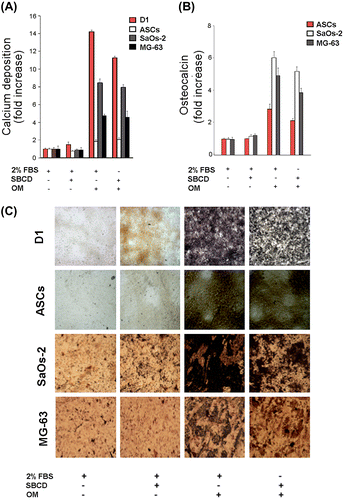
In vivo osteogenesis model in Matrigel plugs with hydroxyapatite
When SBCD (0.1 mg/mL) was added to Matrigel and injected subcutaneously in mice, a modest effect on cell recruitment was observed in respect to the control plugs treated with Matrigel alone (n = 6 for each group, Fig. (A) and (B)). In particular, after 10 days, few inflammatory cells and some endothelial cells, organized in vessels containing erythrocytes, infiltrated the Matrigel plug (Fig. (A) and (B)). In subsequent experiments, hydroxyapatite crystals (0.6%) were added to the Matrigel preparation and the effect of SBCD (0.1 mg/mL) addition was investigated at time intervals in plugs collected at 10, 20, and 60 days (n = 4 for each time). In plugs containing Matrigel and hydroxyapatite alone, only few macrophage-like cells were detected around crystals that maintained their original structure (n = 3 plugs for each time interval, Fig. (D)). On the contrary, in plugs supplemented with SBCD a marked inflammatory reaction could be detected (Fig. (C)). After 20 days, most cells were F4/80+ macrophages and reactive giant cells surrounding the crystals (Fig. (E)). Spindle-shaped, fibroblast-like cells were diffusely present. Staining for osteocalcin was weakly positive in these cells, while a heavy deposit was detected in the stromal areas surrounding foci of calcium deposits (Fig. (F)). At 60 days, the plugs appeared as a hard, subcutaneous plug. Sectioning, in cases requiring decalcification, revealed a poorly cellular stroma, with heavy and extensive von Kossa positive granular calcium deposits (Fig. (A) and (C)). They were surrounded by dense stroma, positive at Masson’s trichromic and van Gieson staining procedures (Fig. (B)). Staining for osteocalcin was heavily and diffusely positive (Fig. (D)). Proper formation of lamellar bone could not be appreciated.
Fig. 6. In vivo osteogenic effect of SBCD in Matrigel plus hydroxyapatite at day 20.
Notes: Matrigel was mixed with 0.1 mg/mL SBCD and 0.6% hydroxyapatite and subcutaneously injected in mice. (A–B) Representative hematoxylin/eosin staining showing a modest effect of SBCD alone (B) as compared to vehicle (A). SBCD induced a slight cellular infiltrate (B), with some vessels (B, inset). (C–D) Representative hematoxylin/eosin staining showing a dense cellular infiltrate after 20 days in plugs containing SBCD plus hydroxyapatite (C) and not hydroxyapatite alone (D). (E–F) Representative micrographs showing the immunohistochemical staining of F4/80+ macrophages surrounding the crystals (E) and osteocalcin deposition (F) in Matrigel plugs containing 0.1 mg/mL SBCD and 0.6% hydroxyapatite. Original magnification: A–D: ×100; E and F: ×200.
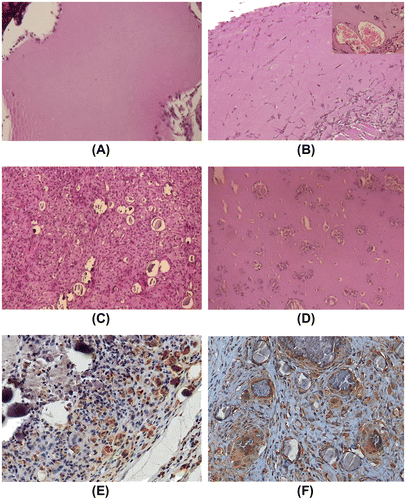
Fig. 7. In vivo osteogenic effect of SBCD in Matrigel plus hydroxyapatite at day 60.
Notes: Representative micrographs of Matrigel plugs containing 0.1 mg/mL SBCD and 0.6% hydroxyapatite 60 days after subcutaneous injection in mice. Large calcifications were detected (A, H/E staining), surrounded by stroma (B, Trichromic reaction). Calcium deposits, as shown by von Kossa stain (C) and presence of osteocalcin (D), indicate induction of an osteogenic process. Original magnification: ×40.
Discussion
Several factors and biological preparations have extensively been investigated in the search for bone induction and regeneration, a topic of high biological and clinical interest.Citation19) The present study demonstrates the osteogenic potential of a natural, extensively available, and fully standardized biological product. In fact, we give evidence of the ability of a standardized bovine colostrum derivative to induce in vitro proliferation and migration of mesenchymal osteoblast progenitors and to promote osteogenesis in vitro and in vivo in association with either soluble factors or synthetic bone substitute materials, respectively.
In respect to mature milk, bovine colostrum contains higher amounts of fats, proteins, peptides, fat-soluble vitamins, and various enzymes, hormones, growth factors, cytokines, minerals, and nucleotides. Except for lactose, the levels of these compounds rapidly decrease during the first 3 days of lactation to those typical for mature milk.Citation20,21) In this study, colostrum was harvested within the fifth hour, as in preliminary experiments this was found the most biologically active fraction.Citation11) Indeed, after the ultrafiltration and purification process and in fully stable conditions, SBCD contains a number of growth factors and cytokines, which are known to regulate proliferation, differentiation, adhesion, migration, and other functions in many cell types.Citation11) In addition, we here found that SBCD contained the osteogenic protein BMP-2, known to enhance the activity of osteoblasts and to induce bone formation.Citation18)
Recently, bovine colostrum was reported to display a wound healing feature that was specifically ascribed to the growth factor-induced activation of tyrosine kinase receptors supporting keratinocyte proliferation and migration.Citation22) We here reported the ability of an innovative colostrum derivative to promote proliferation and migration of osteoblasts and mesenchymal cells in vitro. Similar mitogenic and motogenic effects were observed in fibroblasts treated with mare’s colostrum.Citation23) When added to conventional in vitro OM, in the absence of FBS, SBCD sustained the osteoblastic differentiation of mesenchymal cells. Interestingly, SBCD could promote in vivo osteogenesis, in the presence of hydroxyapatite, in an ectopic osteogenic model. Neither SBCD nor the hydroxyapatite was able to induce the formation of a properly calcified tissue, when injected separately into mice. Instead, when associated and co-injected, an extensive stromal calcification was observed, associated with presence of osteocalcin, an osteoblast-derived protein expressed only in fully differentiated osteoblasts.Citation24,25) The generation of a connective tissue within the plugs thus enriched was consistently observed at early times (20 d). The progression toward a calcified tissue was subsequently indicated by calcium deposition and staining for osteocalcin, which is known as a marker of terminal osteoblast differentiation corresponding with matrix deposition and mineralization.Citation25) In the basic experiments performed in the present study, heavy calcium and osteocalcin deposits in collagenous areas were interpretable as woven bone formation. This is consistent with previous results showing osteocalcin detection in woven bone in a rabbit model of osteogenesis.Citation26) We were instead unable to reach evidence of end-stage osteogenesis with lamellar bone formation, possibly due to the absence of additional mechanical stimuli or relatively short time of observation.
The cooperative effect of SBCD with hydroxyapatite on osteogenesis appears as a conductive event in a specifically determined osteogenic microenvironment. In this context, SDBC appears to be mainly involved in mesenchymal precursors’ recruitment, proliferation, and to promote differentiation only in with the presence of other osteogenic stimuli. Indeed, hydroxyapatite alone is not able to recruit cells as colostrum, but it is endowed with osteoinductive properties.Citation27,28) This is also in accordance with the observation that nanosized hydroxyapatite particles,Citation29–31) mimicking those naturally occurring in bone,Citation32) increased the alkaline phosphatase synthesis and calcium-containing mineral deposition by osteoblasts.Citation33) A number of growth factors and cytokines present in SBCD are known to be involved in mesenchymal precursors’ recruitment, proliferation, and differentiation in vivo.Citation34) In the present study, we did not determine which of the factors identified might be responsible for the osteoinductive effect observed in vivo, but it is conceivable that their simultaneous presence and interaction may lead to a sequence of events favoring the osteogenic process in specifically determined cells. In analogy, dietary supplementation of a growth factor rich fraction of bovine colostrum (1–30 kDa fraction) was shown to promote bone development in juvenile rats.Citation35)
We are fully aware that SBCD represents a relatively rough approach because of the heterogeneity of its components. Although immunologic reaction to the SBCD was not evident in our experimental model, as shown by the limited inflammatory infiltrate around Matrigel and SBCD plugs in absence of hydroxyapatite, this aspect should be carefully taken into consideration for further clinical studies. Indeed, the use of SDBC totally deprived of immunoglobulins would be regarded as mandatory to prevent immune reaction in humans. SBCD might therefore represent a convenient source of specific agents effective on the osteogenic process, whose identification and separation might lead to purified and highly active osteoinducing biological products.
In conclusion, additional studies and experimental models are needed, as a pre-requisite for future clinical application of the present approach. The data presented here indicate that the association of efficient biomaterials and an adequate supply of cells (MSCs and osteoprogenitors) in presence of a potent source of biological factors, as provided by SBCD, might lead to the goal.
Acknowledgments
The authors would like to thank Prof. Paolo Bianco (University of Rome) for his critical suggestions, and Dr Alessandro Mautino (Bioclarma) for his kind assistance during the Bio-Plex analysis. We thank AIM (Advances in Medicine, Bologna, IT) for providing the standardized colostrum derivative (SBCD).
Notes
Abbreviations: MSCs, mesenchymal stem cells; SBCD, standardized bovine colostrum derivative; FGF-b, fibroblast growth factor; TGF, transforming growth factor; BMP-2, bone morphogenic protein-2; TNF, tumor necrosis factor; VEGF, vascular endothelial growth factor; ASCs, Adipose tissue-derived stem cells; FBS, fetal bovine serum; MTT, 3-(4,5-dimethylthiazole-2-yl)-2.5-diphenyl tetrazolium bromide; ANOVA, analysis of variance; IL, Interleukin.
References
- Giannoudis PV, Dinopoulos H, Tsiridis E. Bone substitutes: an update. Injury. 2005;36:S20–S27.10.1016/j.injury.2005.07.029
- Hubble MJ. Bone grafts. Surg. Technol. Int. 2002;10:261–265.
- Kurz LT, Garfin SR, Booth RE. Harvesting autogenous iliac bone grafts. Spine. 1989;14:1324–1331.10.1097/00007632-198912000-00009
- Femyhough JC, Schimandle JJ, Weigel MC. Chronic donor site pain complicating bone graft harvesting from the posterior iliac crest for spinal fusion. Spine. 1992;17:1474–1480.10.1097/00007632-199212000-00006
- Solomon L. Bone grafts. J. Bone Joint. Surg. Br. 1991;73:706–707.
- Bloemers FW, Blokhuis TJ, Patka P, Bakker FC, Wippermann BW, Haarman HJ. Autologous bone versus calcium-phosphate ceramics in treatment of experimental bone defects. J. Biomed. Mater. Res. 2003;66B:526–531.10.1002/(ISSN)1097-4636
- Schliephake H. Bone growth factors in maxillofacial skeletal reconstruction. Int. J. Oral Maxillofac. Surg. 2002;31:469–484.10.1054/ijom.2002.0244
- Mussano F, Ciccone G, Ceccarelli M, Baldi I, Bassi F. Bone morphogenetic proteins and bone defects: a systematic review. Spine. 2007;32:824–830.
- Koldovský O, Thornburg W. Hormones in milk. J. Pediatr. Gastroenterol. Nutr. 1987;6:172–196.
- Koldovský O, Illnerová H, Macho L, Strbák V, Stĕpánková R. Milk-borne hormones, possible tools of communication between mother and suckling. Physiol. Res. 1995;44:349–351.
- Sacerdote P, Mussano F, Franchi S, Panerai AE, Bussolati G, Carossa S, Bartorelli A, Bussolati B. Biological components in a standardized derivative of bovine colostrum. J. Dairy Sci. 2013;96:1745–1754.10.3168/jds.2012-5928
- Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211–228.10.1089/107632701300062859
- Mussano F, Lee KJ, Zuk P, Tran L, Cacalano NA, Jewett A, Carossa S, Nishimura I. Differential effect of ionizing radiation exposure on multipotent and differentiation-restricted bone marrow mesenchymal stem cells. J. Cell. Biochem. 2010;111:322–332.10.1002/jcb.v111:2
- Nanney LB, Woodrell CD, Greives MR, Cardwell NL, Pollins AC, Bancroft TA, Chesser A, Michalak M, Rahman M, Siebert JW, Gold LI. Calreticulin enhances porcine wound repair by diverse biological effects. Am. J. Pathol. 2008;173:610–630.10.2353/ajpath.2008.071027
- Holtorf HL, Sheffield TL, Ambrose CG, Jansen JA, Mikos AG. Flow perfusion culture of marrow stromal cells seeded on porous biphasic calcium phosphate ceramics. Ann. Biomed. Eng. 2005;33:1238–1248.10.1007/s10439-005-5536-y
- Yang R, Davies CM, Archer CW, Richards RG. Immunohistochemistry of matrix markers in Technovit 9100 New-embedded undecalcified bone sections. Eur. Cell Mater. 2003;6:57–71.
- Bussolati B, Ahmed A, Pemberton H, Landis RC, Di Carlo F, Haskard DO, Mason JC. Bifunctional role for VEGF-induced heme oxygenase-1 in vivo, induction of angiogenesis and inhibition of leukocytic infiltration. Blood. 2004;103:761–766.
- Chen D, Zhao M, Mundy GR. Bone morphogenetic proteins. Growth Factors. 2004;22:233–241.10.1080/08977190412331279890
- Barradas AM, Yuan H, van Blitterswijk CA, Habibovic P. Osteoinductive biomaterials, current knowledge of properties, experimental models and biological mechanisms. Eur. Cell Mater. 2011;21:407–429.
- Blum JW, Hammon HM. Colostrum effects on the gastrointestinal tract, and on nutritional, endocrine and metabolic parameters in neonatal calves. Lifest. Prod. Sci. 2000;66:1151–1159.
- Campana WM, Baumrucker CR. Hormones and growth factors in bovine milk. In: Jensen RG, editor. Handbook of milk composition. San Diego (CA): Academic Press; 1995. p. 476–494.10.1016/B978-012384430-9/50022-6
- Kovacs D, Cardinali G, Aspite N, Picardo M. Bovine colostrum promotes growth and migration of the human keratinocyte HaCaT cell line. Growth Factors. 2009;27:448–455.10.3109/08977190903211077
- Zava S, Barello C, Pessione A, Garoffo LP, Fattori P, Montorfano G, Conti A, Giunta C, Pessione E, Berra B, Giuffrida MG. Mare’s colostrum globules stimulate fibroblast growth in vitro: a biochemical study. J. Med. Food. 2009;12:836–845.10.1089/jmf.2008.0139
- Nakamura A, Dohi Y, Akahane M, Ohgushi H, Nakajima H, Funaoka H, Takakura Y. Osteocalcin secretion as an early marker of in vitro osteogenic differentiation of rat mesenchymal stem cells. Tissue Eng. Part C Methods. 2009;15:169–180.10.1089/ten.tec.2007.0334
- Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G. Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell. 1997;89:747–754.10.1016/S0092-8674(00)80257-3
- Hu Z, Peel SA, Ho SK, Sándor GK, Su Y, Clokie CM. The expression of bone matrix proteins induced by different bioimplants in a rabbit sinus lift model. J. Biomed. Mater. Res Part A. 2010;95A:1048–1054.10.1002/jbm.a.v95a:4
- Eyckmans J, Roberts SJ, Schrooten J, Luyten FP. A clinically relevant model of osteoinduction, a process requiring calcium phosphate and BMP/Wnt signalling. J. Cell Mol. Med. 2010;14:1845–1856.
- Cheng L, Ye F, Yang R, Lu X, Shi Y, Li L, Fan H, Bu H. Osteoinduction of hydroxyapatite/β-tricalcium phosphate bioceramics in mice with a fractured fibula. Acta Biomater. 2010;6:1569–1574.10.1016/j.actbio.2009.10.050
- Zeitler T, Cormack A. Interaction of water with bioactive glass surfaces. J. Cryst. Growth. 2006;294:96–102.10.1016/j.jcrysgro.2006.05.029
- Pereira M, Jones J, Hench L. Bioactive glass and hybrid scaffolds prepared by sol–gel method for bone tissue engineering. Adv. Appl. Ceram. 2005;104:35–42.10.1179/174367605225011034
- LeGeros R. Calcium phosphate-based osteoinductive materials. Chem. Rev. 2008;108:4742–4753.10.1021/cr800427g
- Robinson RA. An electron microscope study of the crystalline inorganic components of bone and its relationship to the organic matrix. J. Bone Joint. Surg. 1952;34A:389–434.
- Webster TJ, Ergun C, Doremus RH, Siegel RW, Bizios R. Enhanced functions of osteoblasts on nanophase ceramics. Biomaterials. 2000;21:1803–1810.10.1016/S0142-9612(00)00075-2
- Tang JM, Wang JN, Zhang L, Zheng F, Yang JY, Kong X, Guo LY, Chen L, Huang YZ, Wan Y, Chen SY. VEGF/SDF-1 promotes cardiac stem cell mobilization and myocardial repair in the infarcted heart. Cardiovasc. Res. 2011;91:402–411.10.1093/cvr/cvr053
- Lee J, Kwon SH, Kim HM, Fahey SN, Knighton DR, Sansom A. Effect of a growth protein-colostrum fraction on bone development in juvenile rats. Biosci. Biotechnol. Biochem. 2008;72:1–6.10.1271/bbb.60695
