Abstract
To examine the effect of dietary supplementation with 1-kestose on the IgA levels in milk, BALB/c mice were fed diets with or without 5% 1-kestose during pregnancy and lactation. The total and specific IgA levels in the milk were measured at 7 and 14 days after delivery. A two-way ANOVA with repeated measures resulted in a significant effect of 1-kestose-supplementation on total IgA concentrations (p < 0.05) and the level of anti-Bacteroides IgA (p < 0.05). A significant positive correlation was found between the mean count of Bacteroides spp. in maternal feces and the total IgA concentration in maternal milk (r = 0.55, p < 0.05), suggesting a potential link between the gut and mammary gland immune system. In conclusion, this study demonstrated the effects of dietary prebiotics on milk IgA production.
Graphical Abstract
Consumption of 1-kestose, a major component of fructooligosaccharides, by pregnant and lactating mice increased the total IgA concentration in the mouse milk.
Fructooligosaccharides (FOS) is a compound of 1-kestose, nystose, and 1F-ß-fructofuranosylnystose that is synthesized from sucrose by fungal-ß-fructofuranosidase.Citation1,2) The primary physiological effect of FOS consumption in animals, including humans,Citation2–5) is preferential stimulation of colonic Bifidobacteria, which is a criterion for defining a compound as a prebiotic. FOS consumption has also been shown to modulate lipid metabolismCitation6,7) and improve gastrointestinal conditions,Citation3,8,9) mineral absorption,Citation10) and ulcerative colitis in experimental models.Citation11) Several studies have shown that supplementation with 1-kestose or FOS leads to increased production of IgA in the digestive tract of rodents.Citation12–15)
In animals, intestinal microbiota can affect intestinal IgA levels. Previous reports have shown the existence of a relationship between the cecal lactobacilli count and intestinal IgA levels in rats.Citation15) Furthermore, Bacteroides spp. in the gut microbiota induce differentiation of B cells from Peyer’s patches into IgA+B cells,Citation16) and B lymphocytes from mucosa-associated lymphoid tissues have been shown to repopulate the mucosa,Citation17) a process referred to as “homing.”
Therefore, we postulate that the activation of intestinal microbiota following 1-kestose or FOS consumption may enhance mucosal IgA secretion in other exocrine tissues such as the mammary glands. To test this hypothesis, the present study aimed to determine whether supplementation with dietary 1-kestose, a major component of FOS, increases the level of IgA in mouse milk and to examine the relationship between intestinal microbiota counts and the level of IgA in mouse milk.
Materials and methods
Animals and diet
Pregnant BALB/c mice were purchased from Japan SLC (Shizuoka, Japan) and maintained individually in plastic cages. As shown in Table , the following 2 diets were used: the control diet consisting of only formula AIN-93GCitation18) and the 1-kestose-supplemented diet in which 5% of the cornstarch in formula AIN-93G had been replaced with 1-kestose (Meioligo CR®; Meiji Food Materia Co., Ltd.; Tokyo, Japan). Consequently, we used the following 2 groups of mice: control group (n = 11) and 1-kestose-supplemented group (n = 7). From the day of mating (approximately 19–21 days before delivery, with the day of delivery designated as Day 0) until analysis, the groups of mice were fed ad libitum with the appropriate diet. Following delivery, each litter size was adjusted to contain 4–6 pups, and the lactating dams continued ad libitum access to the respective diet. On days 7 and 14, the lactating mice were placed under anesthesia by subcutaneous injection with oxytocin (1 IU), and milk samples were collected. All experimental protocols were approved by the Animal Care Committee in the Division of Research and Development, Meiji Dairies Corporation.
Table 1. Components of the control and experimental diets fed ad libitum to mice during pregnancy and lactation to determine their effects on IgA levels in mouse milk.
Preparation of fecal and intestinal samples
On the last day of the feeding period (day 17), maternal feces were collected and blood samples were obtained immediately after the mice had been euthanized using an intraperitoneal injection containing a solution of ketamine hydrochloride (70 mg/kg body weight; Wako Pure Chemical Industries, Osaka, Japan) and xylazine hydrochloride (8 mg/kg body weight; ICN Biomedicals, Inc., Aurora, OH, USA). Following the euthanization, their intestines were carefully removed. The small intestine (pylorus to ileocecal junction) was segmented into the jejunum and ileum, with each segment of equal size. The colon component included the large intestine, excluding the cecum. After the luminal content of each gut segment had been flushed with ice-cold phosphate-buffered saline (PBS), each segment was weighed. PBS containing phenylmethylsulfonyl fluoride (1 mmol/L), EDTA (5 mmol/L), soybean trypsin inhibitor (100 mg/mL), leupeptin (100 mg/mL), and aprotinin (100 kIU/mL in 50 mmol/L Tris-HCl [pH 6.8]) was then mixed with the tissue in a volume 20-fold greater than the wash volume before the tissue was homogenized on ice. The suspension was then centrifuged for 15 min at 10,000 g, and the resulting supernatant was used as the tissue extract for the intestinal IgA assay.
Analysis of fecal microbiota
Bacterial DNA was extracted from freeze-dried fecal samples (20 mg) with the QIAamp stool mini kit (QIAGEN; Valencia, CA, USA) and then stored at −20 °C until analysis. Real-time polymerase chain reaction (PCR) was performed with locus-specific primers as previously described.Citation19,20) The primer sequences were as follows: Bifidobacterium spp. sense primer, 5′-CTCCTGGAAACGGGTGG-3′; Bifidobacterium spp. antisense primer, 5′-GGTGTTCTTCCCGATATCTACA-3′; Lactobacilli spp. sense primer, 5′-CTCAAAACTAAACAAAGTTTC-3′; Lactobacilli spp. antisense primer, 5′-CTTGTACACACCGCCCGTCA-3′; Bacteroides spp. sense primer, 5′-ATAGCCTTTCGAAAGRAAGAT-3′; and Bacteroides spp. antisense primer, 5′-CCAGTATCAACTGCAATTTTA-3′. Each PCR reaction mixture contained 20 pmol of each primer, 10 μL of 2 × QuantiFast SYBR Green PCR Master Mix (QIAGEN; Valencia, CA, USA), 1.2 μL MgCl2 (25 mM), and 1 μL DNA solution in a total volume of 20 μL. To measure the Bifidobacterium spp. and Lactobacilli spp. populations, the PCR amplification protocol included initial denaturation for 5 min at 95 °C followed by 45 cycles of melting, annealing, and extension at 94 °C for 1 min, 60 °C for 30 s, and 72 °C for 1 min, respectively. To measure the Bacteroides spp. population, the PCR amplification protocol was as follows: initial denaturation for 5 min at 95 °C followed by 45 cycles of melting, annealing, and extension at 94 °C for 1 min, 50 °C for 30 s, and 72 °C for 30 s, respectively. Both protocols included a final extension for 5 min at 72 °C.
Total IgA immunoassay
The total IgA concentration of the milk, serum, and tissue extracts was measured using enzyme-linked immunosorbent assay (ELISA). The initiation of ELISA included the coating of 96-well microtiter plates (Nunc A/S; Roskilde, Denmark) with 100 μL rat anti-mouse IgA antibodies (1 μg/mL in 0.15 M PBS; Pharmingen, San Diego, CA, USA) overnight at 4 °C. The unbound antibodies were removed from each well by 3 washes with 125 μL PBS containing 0.05% (w/v) Tween 20 (PBS-T). To block non-specific protein binding, the plates were incubated with 125 μL of 1% (w/v) bovine serum albumin (BSA; Intergen, Purchase, NY, USA) for 30 min at room temperature and washed again with 3 volumes of PBS-T. Diluted solutions of experimental samples or standard mouse IgA (mouse myeloma protein; ICN Biomedicals, Inc.; Costa Mesa, CA, USA) were prepared in PBS (0.01 mol/L; pH 7.2) containing NaCl (0.5 mol/L) and 0.1% Tween 20. Diluted and standard samples were added to the coated wells in duplicate. An uncoated well that had been blocked with 1% BSA was included as a control for non-specific binding for each sample and the standard. Following overnight incubation at 4 °C, the plates were washed, and 100 μL biotinylated rat anti-mouse IgA antibodies (50 ng/mL; Pharmingen) were added to each well. Following 2 h of incubation at room temperature, the plates were washed, and 100 μL alkaline phosphatase-conjugated avidin (1 mg/mL; Organon Teknika Corporation, Durham, NC, USA) was added to each well. To visualize the reaction, 100 μL p-nitrophenyl phosphate (PNPP; 1 mg/mL in diethanolamine buffer [pH 9.8]) was added to the wells before incubation at room temperature for 30 min. After termination of color development using the addition of 50 μL of 5 mol/L NaOH, the absorbance at 405 nm was measured for each sample/standard. Using the value obtained, a standard curve was developed to calculate the total IgA concentration of each sample.
Specific IgA levels
The specific IgA levels were measured using ELISA. The 96-well microtiter plates were coated with antigens of formalin-fixed Bacteroides spp. and Lactobacillus spp. that originally were isolated from mice feces in our laboratory and had been diluted in carbonate buffer (pH 9.6) to an optical density of 1.0 at 660 nm. The unbound antigens were removed from each well with 3 washes of 100 μL PBS-T. The wells were then blocked with 120 μL of gelatin (0.5% [w/v]) for 2 h at room temperature and washed again with 3 volumes of PBS-T. An uncoated well that had been blocked with gelatin (0.5% [w/v]) was included as a control for non-specific binding for each sample. Diluted milk samples were added to the wells in duplicate. IgA antibodies with bound antigens were detected using the same methods previously described for IgA immunoassay. Absorbance at 405 nm, which indicated the level of specific IgA acting against Bacteroides spp. or Lactobacillus spp., was measured for each sample.
Statistical analysis
Data are expressed as mean ± SD values. Statistical analyses were conducted using the Student’s t-test and a two-way ANOVA with repeated measures. The relationships among several parameters were assessed by Pearson’s correlation coefficient analysis. In all analyses, statistical significance was set at p < 0.05.
Results
The control and the 1-kestose-supplemented group dams gave birth to 5–10 pups and had a similar mean body weight at the late lactation stage before dissection (Table ). Although the mean weight of the small intestines, which included the combination of the ileum and jejunum, was similar between the 1-kestose-supplemented group and the control group, the mean weight of the colon and the counts of both Bifidobacterium spp. and Bacteroides spp. in the maternal feces were significantly higher in the 1-kestose-supplemented group when compared to the control group (Table , p < 0.05). The mean total IgA levels in the jejunum and colon of the 1-kestose-supplemented group mice were significantly greater than those of the control mice (Table , p < 0.05). The mean serum IgA concentration of the maternal mice was not significantly different between the groups (Table ).
Table 2. Maternal weight and fecal microbiota count of the control and 1-kestose-supplemented mice groups late in the lactation stage (day 17).Table Footnotea
Table 3. Total IgA levels in the intestinal tissue and serum samples of the control and 1-kestose-supplemented mice group at 17 days after delivery.Table Footnotea
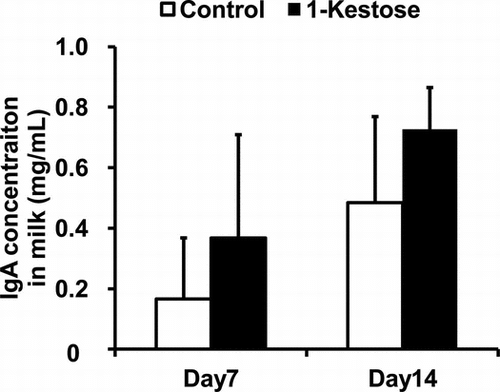
The IgA levels in the milk on days 7 and 14 after delivery are provided in Fig. and Table . The two-way ANOVA with repeated measures revealed a significant effect of 1-kestose-supplementation and measurement day during lactation on total IgA concentrations (Fig. , p < 0.05) and the level of anti-Bacteroides IgA (Table , p < 0.05) with no interaction between the factors.
Fig. 1. Effects of dietary supplementation with 1-kestose on total IgA concentration in mouse milk.
Notes: Enzyme-linked immunosorbent assay was used to measure the total IgA concentration in milk samples that had been collected from maternal mice that were fed a control diet (n = 11) or a diet supplemented with 1-kestose (n = 7) at 7 and 14 days after delivery. Data are expressed as mean ± SD values. A two-way ANOVA with repeated measures showed significant effects of 1-kestose supplementation and measurement day during lactation (p < 0.05), with no interaction between factors.
Table 4. Specific IgA levels, reported as the absorbance at 405 nm, in the milk of lactating mice in the control and 1-kestose-supplemented groups.Table Footnotea
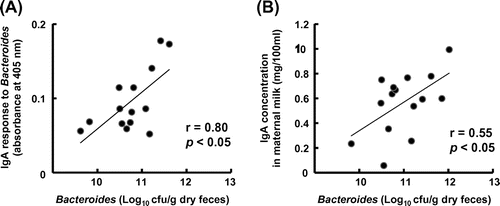
A significant positive correlation was found between the mean number of Bacteroides spp. in maternal feces and the mean level of anti-Bacteroides IgA in maternal milk (Fig. , r = 0.80, p < 0.05) and also between the mean number of Bacteroides spp. in the maternal feces and the total IgA concentration in maternal milk (Fig. , r = 0.55, p < 0.05).
Fig. 2. Correlation between Bacteroides spp. count in maternal feces and (A) the level of anti-Bacteroides IgA or (B) total IgA concentration in milk.
Notes: The Bacteroides spp. count in fecal samples collected at 17 days after delivery was measured. (A) Enzyme-linked immunosorbent assay was used to measure the level of anti-Bacteroides IgA in milk collected at 14 days after delivery. Bacteroides spp. that had been isolated from mice feces were used as the coating antigen. The absorbance at 405 nm, indicating the level of anti-Bacteroides IgA, was measured. (B) Enzyme-linked immunosorbent assay was used to measure the total IgA concentration in milk collected at 14 days after delivery.
The type of diet did not have any effect on either the count of Lactobacillus spp. in maternal feces or the level of anti-Lactobacillus IgA in milk between the control and 1-kestose supplemented groups.
Discussion
In this study, we found that consumption of 1-kestose, a major component of FOS, by pregnant and lactating mice increased the total and specific IgA levels in the mouse milk. To the best of our knowledge, this is the first report of the effect of dietary prebiotics on the IgA production in milk.
Interest in dietary supplementation with functional foods, which include foods that exert an immunomodulatory effect in the intestinal mucosa, has increased recently. The concept of functional foods is based on the understanding that the gut is the largest immune organ in the human body. Probiotics, the most representative of the functional foods, are live micro-organisms that confer a health benefit on the host by altering its microbiota. Previous studies have shown that probiotic supplementation during lactation increases the IgA levels in human milk and mouse milk.Citation21,22) However, there is a concern that probiotic consumption has the potential to cause systemic infections, which has been supported by several reports of safety concerns relating to probiotic use in immunocompromised hosts such as infants and pregnant women. Another concern is that probiotic bacteria cannot function as efficiently as desired in the absence of prebiotics, which are non-digestible dietary components that beneficially affect the host by selectively stimulating the growth and/or activity of one type or a limited number of types of bacteria.Citation1) Prebiotics are usually delivered in the form of oligosaccharides, and many of them occur naturally. Among them, FOS is frequently used as a prebiotic and added as a dietary supplement to foods, beverages, and infant formula. As it has been given a generally recognized as safe status, FOS or 1-kestose may be beneficial for pregnant and lactating women.
The present study showed that maternal supplementation with 1-kestose significantly increased the amount of total IgA in the intestinal tracts of lactating mice (Table ), supporting previous reportsCitation12–14) that dietary FOS or 1-kestose intensifies IgA production in the digestive tract. The authors had also previously confirmed that dietary FOS promotes IgA+B cell class switching in the Peyer’s patches of the small intestine,Citation13) and Yanagibashi et al. previously reported that Bacteroides spp. induce differentiation of B cells from Peyer’s patches into IgA+B cells.Citation16) Considering that the fecal Bacteroides count was higher in the 1-kestose-supplemented group than the control group, in the present study (Table ), the increase in IgA levels in the intestinal mucosa might be caused by an increased number of IgA-producing plasma cells in the digestive tract. Additionally, the increased fecal Bifidobacterium spp. count that was observed in the present study may have resulted in higher intestinal IgA levels; Bifidobacterium has previously been shown to induce IgA productionCitation23)
Two observations in the present study suggest enteromammary pathway involvement, which is characterized by the homing of immune cells. First, maternal supplementation with 1-kestose was found to increase the total IgA concentration in milk, but not in serum, at 17 days after delivery (Fig. and Table ). This observation is consistent with that of Halsey et al., who reported that IgA in mouse milk originates from blood during the initial stage of lactation but is produced locally in the mammary gland in the later stage of lactation.Citation17) It also supports observations that IgA+B cells from Peyer’s patches home to the mammary mucosa via the lymph and blood circulation before differentiating into IgA-producing plasma cells.Citation24,25) Second, we found a significant correlation between the fecal Bacteroides spp. count and the total IgA concentration in mouse milk; an analogous relationship between bacterial count and cecal IgA concentration has been previously reported in mice.Citation15) These results suggest that the increased level of milk IgA was involved in stimulating the activation of intestinal microbiota following 1-kestose consumption. Additional research should focus on determining the mechanisms underlying the homing of immune cells in the enteromammary pathway.
IgA, the most abundant Ig isotype in mucosal secretions, not only provides protection against microbial antigens at mucosal surfacesCitation26,27) but also binds to commensal bacteria to ensure permanent communication between the bacteria and components of the neighboring mucosal immune system.Citation28,29) The present study demonstrated that 1-kestose consumption increased the anti-Bacteroides IgA level in milk. As is the case with Bacteroides spp., increased levels of anti-Bifidobacterium IgA in milk might occur in 1-kestose-fed mice because of a higher fecal Bifidobacterium spp. count, which has been shown to induce the IgA production.Citation23) An increase in the levels of specific IgA in milk by 1-kestose consumption is expected to improve the gut immune response to commensal bacteria in neonates. However, further research should be conducted to clarify the effects on neonates.
We also confirmed that FOS consumption by pregnant and lactating mice increased the total IgA concentration in the mouse milk (data not shown). The composition of FOS is 36% 1-kestose, 51% nystose, and 9% 1F-β-fructofuranosylnystose,Citation2) each of which is highly beneficial to Bifidobacterium spp. in the digestive tract and promotes an increase in the Bifidobacterium population.Citation3) In addition, previous reports have indicated that supplementation with 5% of 1-kestose or FOS provides a sufficient amount of oligosaccharides.Citation12−Citation14) To identify the components and quantity of each FOS that are responsible for the effect on the levels of milk IgA, further studies should examine the effect of sole supplementation with each component of FOS at variable doses.
In conclusion, the results of this study provide the first evidence that dietary supplementation with 1-kestose in pregnant and lactating mice increases the specific and total IgA levels in milk accompanied by increasing levels of commensal bacteria in the intestines. Future research should examine the effects of increased milk IgA on the development of the neonatal gut immune response.
The authors’ contribution
The authors’ contributions were as follows: S. J., Y. N, M. N., and T. T. designed the study; S. J., Y. N, and M. N. conducted the experiments and analyzed the data; S. J., Y. N, M. N., and T. T. wrote the paper.
Notes
Abbreviations: BSA, bovine serum albumin; ELISA, enzyme-linked immunosorbent assay; FOS, fructooligosaccharides; GRAS, generally recognized as safe; PBS, phosphate-buffered saline; PBS-T, phosphate-buffered saline containing Tween 20; PCR, polymerase chain reaction; PNPP, p-nitrophenyl phosphate.
References
- Gibson GR, Roberfroid MB. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J. Nutr. 1995;125:1401–1412.
- Hidaka H, Eida T, Takizawa T, Tokunaga T, Tashiro Y. Effects of fructo-oligosaccharides on intestinal flora and human health. Bifidobact. Microflora. 1986;5:37–50.
- Mitsuoka T, Hidaka H, Eida T. Effect of fructo-oligosaccharides on intestinal microflora. Nahrung. 1987;31:427–436.10.1002/(ISSN)1521-3803
- Bouhnik Y, Flourié B, Riottot M, Bisetti N, Gailing MF, Guibert A, Bornet F, Rambaud JC. Effects of fructo-oligosaccharides ingestion on fecal bifidobacteria and selected metabolic indexes of colon carcinogenesis in healthy humans. Nutr. Cancer. 1996;26:21–29.10.1080/01635589609514459
- Bouhnik Y, Vahedi K, Achour L, Attar A, Salfati J, Pochart P, Marteau P, Flourié B, Bornet F, Rambaud JC. Short-chain fructo-oligosaccharide administration dose-dependently increases fecal bifidobacteria in healthy humans. J. Nutr. 1999;129:113–116.
- Tokunaga T, Oku T, Hosoya N. Influence of chronic intake of new sweetener fructooligosaccharide (Neosugar) on growth and gastrointestinal function of the rat. J. Nutr. Sci. Vitaminol. (Tokyo). 1986;32:111–121.10.3177/jnsv.32.111
- Fukasawa T, Murashima K, Nemoto T, Matsumoto I, Koga J, Kubota H, Kanegae M. Identification of marker genes for lipid-lowering effect of a short-chain fructooligosaccharide by DNA microarray analysis. J. Diet. Suppl. 2009;6:254–262.10.1080/19390210903070822
- Oku T, Tokunaga T, Hosoya N. Nondigestibility of a new sweetener, “Neosugar,” in the rat. J. Nutr. 1984;114:1574–1581.
- Juffrie M. Fructooligosaccharide and diarrhea. Biosci. Microflora. 2002;21:31–34.
- Ohta A, Ohtsuki M, Baba S, Adachi T, Sakata T, Sakaguchi E. Calcium and magnesium absorption from the colon and rectum are increased in rats fed fructooligosaccharides. J. Nutr. 1995;125:2417–2424.
- Cherbut C, Michel C, Lecannu G. The prebiotic characteristics of fructooligosaccharides are necessary for reduction of TNBS-induced colitis in rats. J. Nutr. 2003;133:21–27.
- Hosono A, Ozawa A, Kato R, Ohnishi Y, Nakanishi Y, Kimura T, Nakamura R. Dietary fructooligosaccharides induce immunoregulation of intestinal IgA secretion by murine Peyer’s patch cells. Biosci. Biotechnol. Biochem. 2003;67:758–764.10.1271/bbb.67.758
- Nakamura Y, Nosaka S, Suzuki M, Nagafuchi S, Takahashi T, Yajima T, Takenouchi-Ohkubo N, Iwase T, Moro I. Dietary fructooligosaccharides up-regulate immunoglobulin A response and polymeric immunoglobulin receptor expression in intestines of infant mice. Clin. Exp. Immunol. 2004;137:52–58.10.1111/j.1365-2249.2004.02487.x
- Yoshida N, Satou W, Hata S, Takeda Y, Onodera S, Ando K, Shiomi N. Effects of 1-kestose and nystose on the intestinal microorganisms ans immune system in mice. J. Appl. Glycosci. 2006;53:175–180.10.5458/jag.53.175
- Ito H, Takemura N, Sonoyama K, Kawagishi H, Topping DL, Conlon MA, Morita T. Degree of polymerization of inulin-type fructans differentially affects number of lactic acid bacteria, intestinal immune functions, and immunoglobulin A secretion in the rat cecum. J. Agric. Food. Chem. 2011;59:5771–5778.10.1021/jf200859z
- Yanagibashi T, Hosono A, Oyama A, Tsuda M, Hachimura S. Bacteroides induce higher IgA production than Lactobacillus by increasing activation-induced cytidine deaminase expression in B cells in murine Peyer's patches. Biosci. Biotechnol. Biochem. 2009;73:372–377.10.1271/bbb.80612
- Halsey JF, Mitchell C, Meyer R, Cebra JJ. Metabolism of immunoglobulin A in lactating mice: origins of immunoglobulin A in milk. Eur. J. Immunol. 1982;12:107–112.10.1002/(ISSN)1521-4141
- Kubota T, Shimojo N, Nonaka K, Yamashita M, Ohara O, Igoshi Y, Ozawa N, Nakano T, Morita Y, Inoue Y, Arima T, Chiba K, Nakamura Y, Ikegami S, Masuda K, Suzuki S and Kohno Y. Prebiotic consumption in pregnant and lactating women increases IL-27 expression in human milk. Br. J. Nutr. 2014;111:625–632.10.1017/S0007114513003036
- Matsuki T, Watanabe K, Fujimoto J, Takada T, Tanaka R. Use of 16S rRNA gene-targeted group-specific primers for real-time PCR analysis of predominant bacteria in human feces. Appl. Environ. Microbiol. 2004;70:7220–7228.10.1128/AEM.70.12.7220-7228.2004
- Dubernet S, Desmasures N, Guéguen M. A PCR-based method for identification of lactobacilli at the genus level. FEMS Microbiol. Lett. 2002;214:271–275.10.1111/fml.2002.214.issue-2
- Prescott SL, Wickensw K, Westcott L, Jung W, Currie H, Blackz PN, Stanley TV, Mitchellz EA, Fitzharrisk P, Siebersw R, Wuz L, Cranew J and the Probiotic Study Group. Supplementation with Lactobacillus rhamnosus or Bifidobacterium lactis probiotics in pregnancy increases cord blood interferon-γ and breast milk transforming growth factor-β and immunoglobin A detection. Clin. Exp. Allergy. 2008;38:1606–1614.10.1111/cea.2008.38.issue-10
- Fukushima Y, Kawata Y, Mizumachi K, Kurisaki J, Mitsuoka T. Effect of bifidobacteria feeding on fecal flora and production of immunoglobulins in lactating mouse. Int. J. Food. Microbiol. 1999;18:193–197.10.1016/S0168-1605(98)00183-4
- Yasui H, Nagaoka N, Mike A, Hayakawa K, Ohwaki N. Detection of Bifidobacterium strains that induce large quantities of IgA. Microb. Ecol. Health. Dis. 1992;5:155–162.10.3109/08910609209141310
- Tanneau GM, Hibrand-Saint Oyant L, Chevaleyre CC, Salmon HP. Differential recruitment of T- and IgA B-lymphocytes in the developing mammary gland in relation to homing receptors and vascular addressins. J. Histochem. Cytochem. 1999;47:1581–1592.10.1177/002215549904701210
- Salmon H. Immunophysiology of the mammary gland and transmission of immunity to the young. Reprod. Nutr. Dev. 2003;43:471–475.10.1051/rnd:2003033
- Macpherson AJ, McCoy KD, Johansen F-E, Brandtzaeg P. The immune geography of IgA induction and function. Mucosal Immunol. 2008;1:11–22.10.1038/mi.2007.6
- Cerutti A. Innate control of B cell responses. Nat. Rev. Immunol. 2008;8:421–434.10.1038/nri2322
- Fagarasan S, Muramatsu M, Suzuki K, Nagaoka H, Hiai H, Honjo T. Critical roles of activation-induced cytidine deaminase in the homeostasis of gut flora. Science. 2002;15:1424–1427.10.1126/science.1077336
- Rol N, Favre L, Benyacoub J, Corthésy B. The role of secretory immunoglobulin A in the natural sensing of commensal bacteria by mouse Peyer's patch dendritic cells. J. Biol. Chem. 2012;287:40074–40082.10.1074/jbc.M112.405001
