Abstract
Potential mechanisms underlying the high palatability of fat can be assessed by reviewing animal studies on fat detection and brain patterns during reward behavior. Fatty acids are likely recognized by receptors on taste buds, with the signals transmitted to the brain through taste nerves. Ingested oil is broken down and absorbed in the gastrointestinal tract, which also sends signals to the brain through unknown mechanisms. Information from both sensory receptors and peripheral tissue is integrated by the brain, resulting in a strong appetite for fatty foods via a reward system. Understanding mechanisms of fat recognition will prove valuable in the development of strategies to manage the high palatability of foods.
Graphical Abstract
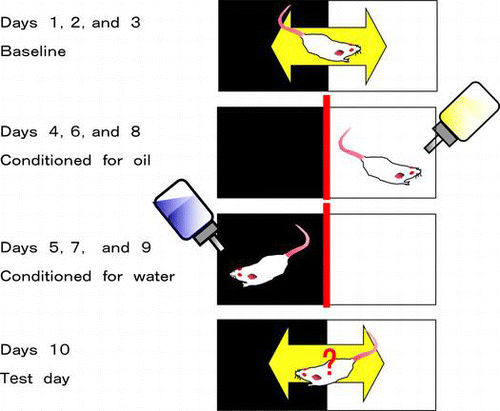
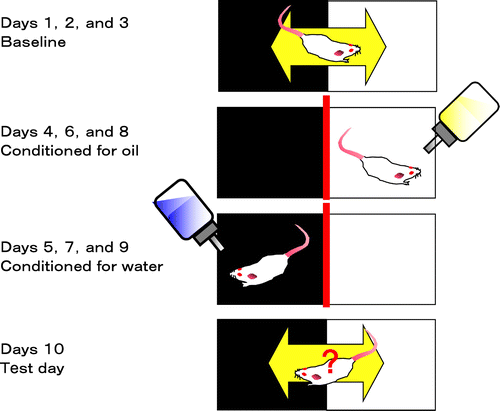
Spontaneous corn oil intake induces conditioned place preference. An addiction to the corn oil was indicated as the difference between time spent in the light box before and after the conditioning.
Key words:
Oily and fatty foods are preferably consumed due to their taste and flavor. Excessive energy intake from fat is currently a major health concern in developed countries, but fat consumption has decreased little in response. When mice were offered fried potatoes and boiled potatoes at the same time, they significantly preferred fried potatoes.Citation1) Mice also preferred corn oil solution to vehicle solution in a two-bottle choice test.Citation2) These results could be due to oils releasing favorable stimuli in the oral cavity. Moreover, Imaizumi et al. suggested that voluntary intake of corn oil by mice elicited rewarding (reinforcing) effectsCitation3) in a conditioned place preference (CPP) test, which is used to study the rewarding effects of addictive drugs. Several studies have proposed mechanisms underlying the preference for dietary fat. This review primarily examines animal studies focused on fat detection and reward behavior. Previous reviews may be helpful for understanding related articles in more detail.Citation4Citation−Citation6)
Release of gastrointestinal hormones
Anatomists include the mouth as part of the digestive tract. In the primitive hydra, a tiny freshwater coelenterate, there is no apparent distinction between the mouth and the digestive tract. Fujita categorized taste cells on the tongue and intestinal endocrine cells as paraneurons.Citation7) Both cells have an apical brush border where they receive chemical stimuli; then they transmit this information by releasing neurotransmitters or hormones at their base.
It is not surprising that fat detection studies started in the small intestine. A common system for fatty acid recognition was found in enteroendocrine cells, since fatty acids stimulate the release of most gastrointestinal hormones, such as somatostatin,Citation8) CCK,Citation9) secretin,Citation10) and peptide YY.Citation11)
Fatty acid carboxyl groups and carbon chain length in fat detection
Shintani et al.Citation12) determined the structural requirements of a fat molecule for inducing pancreatic enzyme secretion, which is the result of CCK release, by administration of fat-related materials into the duodenum of rats. They found that carboxyl groups of fatty acids were essential, while methyl ester did not stimulate pancreatic enzyme secretion. A long carbon chain length, as in oleic acid, linolenic acid, and linoleic acid, was also needed for secretion. Intermediate (C6–C10) and short fatty acid chain lengths did not induce enzyme secretion (Table ).
Table 1. Pancreatic enzyme secretion following intraduodenal administration of fats.
The above findings closely match those obtained from short-term selectivity behavior experiments, where rats were offered a choice of two bottles. Rats chose the three long-chain fatty acids (oleic, linoleic, and linolenic) and corn oil over vehicle solution, but did not choose trioliein, trilinolein, or acrylic acids with or without methylated carboxyl groups.Citation13) Together, these results suggest that fatty acid stimulation in the oral cavity may provide the chemical information underlying such selective behavior, and that relevant information is induced by the least fatty acid chain length and the presence or absence of carboxyl groups.
Fatty acid structure recognition in the mouth
Ever since Pavlov first reported that chemical and physical stimuli in the oral cavity and esophagus reflexively triggered an immediate transitory elevation of digestive juice secretion in the digestive tract, many reports have discussed the cephalic phase of pancreatic enzyme secretion in response to taste stimuli.Citation14–18) Ohara et al. observed a cephalic response only to palatable taste stimuli.Citation16Citation−Citation18)
Hiraoka et al.Citation19) found that orally administering fats to rats, with the esophagus surgically diverted outside the body and away from the stomach, induced the cephalic phase of pancreatic enzyme secretion. This finding revealed the existence of a mechanism for chemical reception of fats in the oral cavity (Fig. ). In the experiment, oleic, linoleic, and linolenic acids produced a transitory elevation in pancreatic enzyme secretion into the duodenum. Caprylic acid, a fatty acid with a short carbon chain, and three fatty acids with methylated carboxyl groups did not produce this response. These results closely match those obtained from the previously mentioned short-term selectivity experiments that offered rats a choice of two bottlesCitation13).
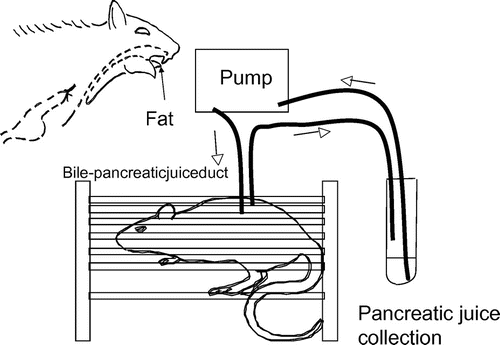
Fig. 1. Evidence for fatty acid recognition in the oral cavity.
Note: Oral administration of fats to rats with the esophagus surgically diverted outside the body, away from the stomach, induced the cephalic phase of pancreatic enzyme secretion. Oleic, linoleic, and linolenic acids produced a transitory elevation in pancreatic enzyme secretion. Long-chain fatty acid methylesters did not produce this response (Hiraoka et al., Physiol & Behav. 2003Citation19)).
Many studies have suggested that fatty acids are detected via specific receptors on the tongue; however, there is currently no appropriate expression to describe fatty taste. It is still unclear whether the fatty sensation in the oral cavity is related to taste, although fatty acid detection is an important cue in fat palatability.
Transmission of information regarding fat intake from the mouth to the brain
Fukuwatari et al.Citation20) reported that rats with bilateral transection of the glossopharyngeal nerve lost their appetite for fats, which suggests that fatty acids trigger chemical reception in the oral cavity and use nerves to transmit this information to the brain. Several studies have provided evidence that ingestion of a palatable meal may alter β-endorphin neuronal activity, although β-endorphin concentration was measured only in the brain and cerebrospinal fluid.Citation21–23)
Matsumura et al. recently reported that spontaneous dietary fat and sugar ingestion activated β-endorphin neurons in the hypothalamus, indicating that orosensory stimulation from fat may activate these neurons and promote β-endorphin release.Citation24) They also found that ingestion of fat emulsion increased c-fos expression in β-endorphin neurons, but intragastric infusion of fat emulsion only slightly increased c-fos expression. Moreover, dissection of the glossopharyngeal nerve, which innervates posterior tongue taste buds, partially but significantly decreased fat emulsion-induced c-fos expression.
Interestingly, intraduodenal injection of fat in rats suppressed the adrenal efferent sympathetic nerve activity and stimulated the gastric efferent parasympathetic nerve.Citation25) These results indicate a postingestive effect from dietary oil intake, as well as cephalic effects.
Fatty acid receptors on the tongue
Accumulating evidence suggests that fatty acids are detected via specific tongue receptors. CD36 is an 88-kDa glycoprotein originally discovered as a fatty acid-binding protein in adipocytes,Citation26) with CD36/FAT, reported as a possible fatty acid recognition receptor on the tongueCitation27). Fukuwatari et al.Citation27) found CD36 to be expressed in circumvallate papillae, localized to the apical part of taste bud cells. In support of these results, CD36-null mice had an attenuated preference for linoleic acid solution, and CD36-null mice with esophageal ligation showed abolished pancreatic secretion in response to fatty acids.Citation28) Furthermore, c-fos activation, in the nucleus of solitary tract neurons stimulated by fatty acids on the tongue of wild-type mice, was abolished in CD36-null mice. These findings suggest that CD36 expressed on the tongue functions as a fatty acid receptor.
In addition to CD36, Matsumura et al. found that G-protein-coupled receptor 120 (GPR120) is expressed in the epithelium of not only circumvallate papillae, but also fungiform and foliate papillae.Citation29) GPR120 was first found in the colon as a long-chain fatty acid recognition receptor.Citation30) It has a seven-transmembrane structure, which is different from the two-transmembrane structure of CD36, but similar to bitter, sweet, and umami receptors, which are also seven-transmembrane GPRs.
Strong GPR120 ligands include the polyunsaturated long-chain fatty acidsCitation30) preferred by mice in the selectivity experiments, suggesting that GPR120 also serves as a fat recognition receptor. GPR40 was recently found in circumvallate, foliate, and a small number of fungiform papillae; it may also be involved in fatty acid recognition by the tongue.Citation31)
TRPM5, a member of the transient receptor potential (TRP) family, has been reported as a downstream signal transducer of fatty acid receptors, and is co-expressed with GPR40 and GPR120.Citation31) TRPM5 is a calcium-activated cation channel expressed in taste receptor cells and functions as a signal transducer of many tastants.Citation32,33) TRPM5-null mice showed no licking response to a sweet tastant, a diminished preference ratio for sweet and umami tastants, and a reduced response to bitter tastants.Citation32) Sclafani et al. reported that TRPM5 KO mice showed no preference for soybean oil emulsion in a two-bottle choice test, while gustducin (an important signaling molecule in taste cells) KO mice showed a normal response.Citation34) These findings suggest that TRPM5, but not gustducin, is a member of the signaling network for fat recognition by the tongue.
Behavioral studies to prove the physiological importance of fatty acid receptors have been performed, but candidates were identified only under limited experimental conditions. Fat recognition studies are still at the beginning stages, and the signaling pathway for fatty acid recognition by the tongue has yet to be determined. Further research will be needed to understand the oral perception of fatty acids.
Recognition of fatty acids, but not triacylglycerols, as fat by the tongue
Dietary oil consists of more than 90% triacylglycerols, with some monoacylglycerols or diacylglycerols and fatty acids. This fact raises the question of, which components of oil are recognized when we perceive the taste of fat. CD36 and GPR120 did not interact with triacyl-, monoacyl-, or diacylglycerols (Tsuzuki et al., unpublished), and other triacylglycerol receptors have not been identified.
Kawai et al. discovered that lingual lipase released from Ebner’s gland digested triacylglycerol on the tongue for a few seconds, which released free fatty acids.Citation35) When rats preferring triolein were offered with the lipase inhibitor orlistat, they no longer preferred triolein. A small percentage of free fatty acids in dietary oil or released from triacylglycerol were thus recognized on rodent tongues. It may be difficult to translate these results to humans, who have lower levels of lingual lipase than rodents. Free fatty acids released from triacylglycerol in food during cooking and processing might be the important fat cue for humans, or an orosensory mechanism may be used to detect fatty acids and their derivatives, and perceive them as attractive ingredients.
Behaviors induced by dietary fat palatability: two-bottle choice and licking behavior
In the two-bottle choice test between vehicle solution and dietary oil, mice significantly preferred the oil. This preference was also true for vegetable oils such as canola oil and mixed vegetable oil,Citation2) and a higher concentration of corn oil compared to a lower concentration.Citation36) The most preferred solution was 100% dietary oil, suggesting that mice were able to discriminate between oil concentrations in the oral cavity.
When considering only oral stimulation caused by dietary oil, the licking test is useful because it evaluates reaction to a sample solution within 60 s. The initial licking rate for corn oil was significantly high, and licking count increased in a concentration-dependent manner.Citation36) In the two-bottle choice test, mice preferred not only dietary oil, but also 1% linoleic acid, which is the main fatty acid in corn oil. The optimal concentration of linoleic acid for mice is 0.25–1%, but a concentration greater than 2% was not preferred.Citation36) With the finding that lingual lipase digested triacylglycerolCitation35) and released small amounts of fatty acids on the tongue, it is reasonable to conclude that mice prefer low concentrations of fatty acids. The fact that mice preferred not only dietary oil but also a low concentration of fatty acids suggests that fatty acids in fatty foods play a key role in fat detection in the oral cavity.
Reward behavior
Animal studies have demonstrated the role of a reward system that underlies the seeking and overconsumption of high-calorie foods. When rats were given a mixture of glucose, saccharin, and corn oil solution, they lost calorie regulation abilities, although they could regulate calorie intake when given only water and food or glucose solution.Citation37) Lucas et al. reported that rats consumed more high-fat than high-carbohydrate isocaloric diets, suggesting that the postingestion action of a high-fat diet stimulated food intake.Citation38)
Ingestion of dietary fat appears to disrupt the regulation of energy intake in rats. This phenomenon could be explained by the rewarding and reinforcing effects of dietary oil. CPP tests for corn oil reveal its rewarding effect (Fig. ).Citation3) Time spent in the light box (conditioned for corn oil) significantly increased, suggesting that corn oil stimuli elicited the rewarding effect. A reinforcing effect by stimulation of corn oil was also observed in the operant task.Citation39,40) In the progressive ratio (PR) schedule for the operant task, the break point for corn oil significantly increased as indicated by the reinforcing effect of the PR test. This reinforcing effect increased in a concentration-dependent manner, with 50 and 100% corn oil being significantly higher compared to 0% corn oil.Citation40) The rewarding or reinforcing effect of dietary fat may be a reason why animals lose appropriate calorie self-regulation and overeat fatty foods.
Fig. 2. CPP test to assess rewarding effects.
Note: The test chamber consisted of a box with lighting (light box) and a box without lighting (dark box). Each box was separated by a guillotine door. Mice were acclimated to the boxes (Day 1–3), and the time on Day 3 spent in the light box was used as the basal spent time (baseline). Mice were alternatively conditioned from Day 4 to Day 9 to a test sample in the light box or to water/vehicle in the dark box. On the test day (Day 10), mice were freely moved to both boxes which lacked samples, and the time spent in the light box was measured. The CPP index was calculated as the difference between time spent in the light box on Day 3 (baseline) and on Day 10 (test day).
Rewarding high fat behavior: dopaminergic system
It is well known that the dopaminergic system is involved in the reinforcing effects of foodsCitation41,42) and many addictive drugs. Imaizumi et al.Citation3) therefore studied the relationship between food reward and the dopaminergic system in the brain. They determined that dopamine receptor types D1 and D2 are related to the rewarding effect. Pretreatment with D1 antagonists SCH23390 (0.03 mg/kg) and haloperidol (0.1 mg/kg) antagonized the rewarding effect in the CPP test. The D2 antagonist (±)-sulpiride, however, caused no change, suggesting that the rewarding effect elicited by dietary oil is mediated by the D1 receptor.Citation3) Sawano et al.Citation43) reported that intraoral injection of corn oil increased dopamine turnover in the cortex and midbrain. In contrast, Yoneda et al. showed that the break point for corn oil in the operant PR test was attenuated by pretreatment with (–)-sulpiride, another D2 antagonist, while SCH23390 had no effect.Citation40) Thus, the reinforcing effect of corn oil in the operant task appears to be mediated by D2 receptors. The discrepancy between the CPP and operant tests is likely attributed to the detecting system. Recent studies found that D1 receptors in particular contributed to the instrumental and reward-related incentive learning process.Citation44,45) D1 antagonists could also affect the learning process in the CPP test, since subjects were pretreated with those drugs before they were conditioned for corn oil.
Using microdialysis methods, Liang et al.Citation46) found that oral corn oil stimulation released dopamine in the nucleus accumbens. The rats studied were implanted with gastric fistulae, suggesting that the increased dopamine came from the corn oil stimulation. This finding also implies that taste stimulation by corn oil affects brain mechanisms.
The opioidergic system in reward behavior
The opioidergic system has also been suggested to be involved in reward behavior in response to high fat intake. Naloxone, an opioid receptor antagonist, was found to reduce the preference for high-fat foods in human subjects. In other studies, opioid agonists influenced the intake of high-fat diets.Citation47,48) In the CPP test, Imaizumi et al.Citation49) reported that voluntary intake of corn oil in the light box showed place preference in mice. The acquisition of place preference by corn oil intake was blocked by intraperitoneal injections of opioid μ and δ antagonists 15 min before conditioning. The opioid κ agonist also blocked corn oil-induced CPP, suggesting that the rewarding effects were at least partially mediated via the opioidergic system through μ and δ receptors. These data imply that both the dopaminergic and opioidergic systems are involved in the reinforcing effects of fat.
In a study of opioid release and fat intake, Mizushige et al. reported that mRNA levels of pro-opiomelanocortin (POMC), a β-endorphin precursor, increased in the hypothalamus of rats that were given corn oil for five consecutive days. Interestingly, this increase was found just before ingestion of the corn oil. After ingestion, POMC mRNA levels decreased. If rats were kept away from corn oil on the test day, high POMC mRNA levels were maintained for more than 30 min.Citation50) These results suggest that the β-endorphin system is related to the anticipation of corn oil. In accordance with these results, daily corn oil ingestion with daily naloxone treatment for five days did not lead to an increased preference for corn oil in the licking test, supporting the notion that the strong palatability of corn oil is at least partially induced by the opioidergic system. As discussed above, Matsumura et al.Citation24) reported that spontaneous dietary fat ingestion activated β-endorphin neurons in the hypothalamus, indicating that orosensory stimulation from fat may activate those neurons to promote β-endorphin release. These results also support the importance of the brain-signaling mechanism that is activated after ingestion of corn oil in inducing an appetite for more corn oil.
Postingestive stimuli as an inducer of rewarding effects
The postingestive effects of fat are unclear. To better understand its role, Suzuki et al.Citation51) used the fat substitute sorbitol fatty acid ester in an animal study. The substitute, which consists of fatty acids esterified to a sorbitol molecule, is both non-digestible and low in calories (1.5 kcal/g). In a long-term two-bottle choice test between 100% corn oil and 100% sorbitol fatty acid ester, mice drank equal amounts of each solution during the first hour. After 1–24 h, however, they showed a preference for corn oil, suggesting that mice considered sorbitol fatty acid ester to be similar to corn oil in the first hour, but not after. The reason for the preference of corn oil over sorbitol fatty acid ester one hour after presentation was not related to oral stimulation, but might be due to the postingestive effect of corn oil.
To further investigate this postingestive effect, mice were intragastrically administered corn oil before CPP for sorbitol fatty acid ester to provide additional calories. Interestingly, sorbitol fatty acid ester with the additional calories elicited a rewarding effect. Neither an intragastric injection of corn oil alone nor oral stimulation of sorbitol fatty acid ester alone elicited that response, suggesting that both oral stimulation and calories are needed to elicit rewarding effects in CPP tests.Citation51 To understand the mechanism for the reinforcing effect of dietary oil, mercaptoacetate (MA), a β-oxidation blocker, was used to block the β-oxidation pathway for fatty acid conversion into energy. Intraperitoneal injection of MA before conditioning in the CPP test attenuated the reinforcing effect of corn oil, but did not affect the reinforcing effect of sucrose,Citation52) suggesting that β-oxidation in mitochondria after ingesting corn oil plays an important role as energy information, although no information was obtained from related sources. Matsumura et al.Citation53) found that injection of MA decreased the number of corn oil licks within a 60-s period, but did not affect licks for the sucrose solution. Thus, β-oxidation may not only provide postingestive information, but may also be involved in mechanisms underlying the oral acceptance of fat as an energy source.
A rewarding effect is induced when animals take up food extremely high in fat, and it produces seeking behavior. This effect is not the same as preference, however, because animals also show a preference for lower concentrations of fat without the rewarding effect. Sakamoto et al. recently advocated at least two levels of fat preference, a rewarding level and a homeostatic mechanism level (unpublished). The mechanisms underlying fat preference in the latter level remain unclear.
Palatability-induced olfactory stimuli of fatty acids: irrelevance to high-fat rewarding effects
In addition to low-level fat preference, an olfactory preference for fat has been suggested. Odor is an important signal, telling us whether we can eat and swallow certain foods. Fatty foods have a particularly distinct odor. While the flavors in fatty foods/dietary oils mainly come from free fatty acids or flavor components from other ingredients, the responsible odor in dietary oils is not well-defined.
Deep-fried foods have a distinctive and attractive odor. Warner et al.Citation54) reported that some of the volatile compounds used to produce deep-fried oil might contribute to its distinctive odor. This indicates that the odor in fat foods might come in part from cooking by-products.
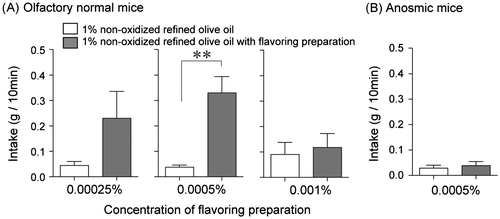
While Kinney et al. reported that olfactory nerve-sectioned mice did not show any preference for high-fat foods,Citation55) Ramirez reported that rats with anosmia induced by olfactory bulbectomy showed a reduced, but not abolished, preference for 1% corn oil.Citation56) Supporting these data, Takeda et al. reported that zinc sulfate-induced, olfactory-blocked mice showed a preference for corn oil concentrations greater than 5%, but did not discriminate between vehicle solution and 1% corn oil.Citation57) These results suggest that mice chose corn oil via an olfactory cue when corn oil was provided at lower concentrations. Nakano et al.Citation58) recently reported that some volatile compounds in olive oil produced by heat-processing enhanced olive oil palatability in mice (Fig. ). They found that olive oil oxidized at room temperature for three weeks and heated olive oil both were significantly preferred over non-oxidized olive oil. This preference was enhanced with an additive of oxidized refined olive oil flavoring, suggesting that the aroma of oxidized fat could be present in most fats and may act as a signal for detection of fats or fatty acid sources.
Fig. 3. Volatile compounds from oxidized olive oil (flavoring preparation) increased the intake of fresh olive oil in olfactory normal mice (A; n = 8) and anosmic mice (B; n = 10). A flavoring preparation was added to 1% non-oxidized olive oil, and intake was measured for 10 min. Intake values are expressed as mean ± SEM. *p < 0.05, **p < 0.01. (Nakano et al., Biosci. Biotechnol. Biochem. 2013Citation58)).
These data collectively suggest that rodents can detect oil without olfaction, although the odor effect of oil as a signal cannot be ignored, especially with low oil concentrations.
Conclusion
The high palatability of dietary fats occurs early in the oral cavity. Fatty acids originating from food, processing or released from triglycerides by lingual lipase on the tongue are likely recognized through receptors on taste buds. Signals are then transmitted to the brain through taste nerves. Ingested oil is digested and absorbed in the gastrointestinal tract, which also sends signals to the brain through unknown mechanisms. The information from both sensory receptors and peripheral tissue is integrated in the brain, resulting in a strong appetite for fatty foods. This preference is induced due to several fat concentration-dependent mechanisms, i.e. rewarding preference via homeostatic mechanism, and preference via olfactory stimulation.
Understanding the mechanisms of fat recognition can help develop a strategy for coping with the high palatability of attractive foods, which will be conducive to the prevention of overeating.
Acknowledgments
This study was supported by the Program for the promotion of Basic and Applied Researches for Innovation in Bio-oriented Industry and Food Science Institute Foundation, Science and technology research promotion program for agriculture, forestry, fisheries, and food industry, and in part by JSPS KAKENHI Grant (B) Number 25292071.
Notes
Abbreviations: CPP, conditioned place preference; TRP, transient receptor potential.
References
- Imaizumi M, Takeda M, Suzuki A, Sawano S, Fushiki T. Appetite. 2001;36:237–238.10.1006/appe.2001.0399
- Takeda M, Imaizumi M, Fushiki T. Life Sci. 2000;67:197–204.10.1016/S0024-3205(00)00614-7
- Imaizumi M, Takeda M, Fushiki T. Brain Res. 2000;870:150–156.10.1016/S0006-8993(00)02416-1
- Manabe, Y, Matsumura, S, and Fushiki, T, Fat detection taste, texture, and post ingestive effects. In: Montmayeur, JP, and Coutre, J, editor. New York, NY: CRC Press; 2009 p. 243–264.
- Fushiki T, Kawai T, Suzuki A. Biology of the intestinal growing animals. In: Zabielski R, Gregory PC, Westrom B, editor. Amsterdam: Elsevier; 2002. p. 409–426.
- Mizushige T, Inoue K, Fushiki T. J. Nutr. Sci. Vitaminol. 2006;53:1–4.
- Fujita T. Physiol. Behav. 1991;49:883–885.10.1016/0031-9384(91)90198-W
- Ensinck JW, Vogel RE, Laschansky EC, Francis BH. Gastroenterology. 1990;98:633–638.
- Lewis LD, Williams JA. Am. J. Physiol. 1990;258:G512–G518.
- Li P, Lee KY, Chang TM, Chey WY. Am. J. Physiol. 1990;259:G960–G965.
- Aponte GW, Fink AS, Meyer JH, Tatemoto K, Taylor IL. Am. J. Physiol. 1985;249:G745–G750.
- Shintani T, Takahashi N, Fushiki T, Sugimoto E. Biosci. Biotechnol. Biochem. 1995;59:1428–1432.10.1271/bbb.59.1428
- Tsuruta M, Kawada T, Fukuwatari T, Fushiki T. Physiol. Behav. 1999;66:285–288.10.1016/S0031-9384(98)00299-6
- Sarles H, Dani R, Prezalin G, Souville C, Frigarella C. Gut. 1968;9:214–221.10.1136/gut.9.2.214
- Preshaw RM, Cooke AR, Grossman MI. Gastroenterelogy. 1966;50:171–178.
- Ohara I, Kimura M, Itokawa Y. J. Nutr. Sci. Vitaminol. 1990;36:497–503.10.3177/jnsv.36.497
- Ohara I, Otsuka SI, Yugari Y. J. Nutr. 1979;109:2098–2105.
- Ohara I, Otsuka S, Yugari Y. Am. J. Physiol. 1988;254:G424–G428.
- Hiraoka T, Fukuwatari T, Imaizumi M, Fushiki T. Physiol. Behav. 2003;79:713–717.10.1016/S0031-9384(03)00201-4
- Fukuwatari T, Shibata K, Iguchi K, Saeki K, Iwata A, Tani K, Sugimoto E, Fushiki T. Physiol. Behav. 2003;78:579–583.10.1016/S0031-9384(03)00037-4
- Triscari J, Nelson D, Vincent GP, Li CH. Int. J. Pept. Protein Res. 1989;34:358–362.
- Mizushige T, et al. Life Sci. 2009;84:760–765.10.1016/j.lfs.2009.03.003
- Yamamoto T, Sako N, Maeda S. Physiol. Behav. 2000;69:345–350.10.1016/S0031-9384(99)00252-8
- Matsumura S, Eguchi A, Okafuji Y, Tatsu S, Mizushige T, Tsuzuki S, Inoue K, Fushiki T. FEBS Lett. 2012;586:1231–1235.10.1016/j.febslet.2012.03.028
- Matsumura S, et al. Neurosci. Res. 2010;67:236–244.10.1016/j.neures.2010.03.010
- Abumrad NA, el-Maghrabi MR,Amri EZ, Lopez E, Grimaldi PA. J. Biol. Chem. 1993;268:17665–17668.
- Fukuwatari T, Kawada T, Tsuruta M, Hiraoka T, Iwanag T, Sugimoto E, Fushiki T. FEBS Lett. 1997;414:461–464.10.1016/S0014-5793(97)01055-7
- Laugerette F, Passilly-Degrace P, Patris B, Niot I, Febbraio M, Montmayeur JP, Besnard P. J. Clin. Invest. 2005;115:3177–3184.10.1172/JCI25299
- Matsumura S, Mizushige T, Yoneda T, Iwanaga T, Tsuzuki S, Inoue K, Fushiki T. Biomed. Res. 2007;28:49–55.10.2220/biomedres.28.49
- Hirasawa A, Tsumaya K, Awaji T, Katsuma S, Adachi T, Yamada M, Sugimoto Y, Miyazaki S, Tsujimoto G. Nat. Med. 2005;11:90–94.10.1038/nm1168
- Cartoni C, Yasumatsu K, Le Coutre J, Ninomiya Y, Damak S. Proceedings of the 5th Internal Symposium on Molecular and Neural Mechanisms of Taste and Olfactory Perception (Fukuoka City, Japan). 2007; p. 29.
- Damak S, et al. Chem. Senses. 2006;31:253–264.
- Zhang Y, Hoon MA, Chandrashekar J, Mueller KL, Cook B, Wu D, Zuker CS, Ryba NJ. Cell. 2003;112:293–301.10.1016/S0092-8674(03)00071-0
- Sclafani A, Zukerman S, Glendinning JI, Margolskee RF. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007;293:R1504–R1513.10.1152/ajpregu.00364.2007
- Kawai T, Fushiki T. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003;285:R447–54.
- Yoneda T, Saitou K, Mizushige T, Matsumura S, Manabe Y, Tsuzuki S, Inoue K, Fushiki T. Physiol. Behav. 2007;91:304–309.10.1016/j.physbeh.2007.03.006
- Takeda M, Imaizumi S, Sawano S, Manabe Y, Fushiki T. Nutrition. 2001;17:117–120.10.1016/S0899-9007(00)00513-X
- Lucas F, Ackroff K, Sclafani A. Am. J. Physiol. 1998;275:R1511–R1522.
- Ward SJ, Walker EA, Dykstra LA. Neuropsychopharmacology. 2007;32:2592–2600.10.1038/sj.npp.1301384
- Yoneda T, Taka Y, Okamura M, Mizushige T, Matsumura S, Manabe Y, Tsuzuki S, Inoue K, Fushiki T. Life Sci. 2007;81:1585–1592.10.1016/j.lfs.2007.09.020
- Guyon A, Assouly-Besse F, Biala G, Puech AJ, Thiebot MH. Psychopharmacology. 1993;110:460–466.10.1007/BF02244653
- Spyraki A, Fibiger HC, Phillips AG. Psychopharmacology. 1982;77:379–382.10.1007/BF00432775
- Sawano S, Takeda M, Imaizumi M, Manabe Y, Kuroda K, Fushiki T. Methods Find Exp. Clin. Pharmacol. 2000;22:223–227.10.1358/mf.2000.22.4.584454
- Dalley JW, Laane K, Theobald DE, Armstrong HC, Corlet PR, Chudasama Y, Robbin TW. Proc. Natl. Acad. Sci. USA. 2005;102:6189–6194.10.1073/pnas.0502080102
- Kelley AE, Smith-Roe SL, Holahan MR. Proc. Natl. Acad. Sci. USA. 1997;94:12174–12179.10.1073/pnas.94.22.12174
- Liang NC, Hajnal A, Norgren R. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006;291:R1236–R1239.10.1152/ajpregu.00226.2006
- Ookuma K, Barton C, York DA, Bray GA. Peptides. 1998;19:141–147.10.1016/S0196-9781(97)00255-6
- Zhang M, Gosnell BA, Kelley AE. J. Pharmacol. Exp. Ther. 1998;285:908–914.
- Imaizumi M, Takeda M, Sawano S, Fushiki T. Behav. Brain Res. 2001;121:129–136.10.1016/S0166-4328(00)00388-0
- Mizushig T, Kawai T, Matsumura S, Yoneda T, Kawada T, Tsuzuki S, Inoue K, Fushiki T. Biomed. Res. 2006;27:227–232.10.2220/biomedres.27.227
- Suzuki A, Yamane T, Imaizumi M, Fushiki T. Nutrition. 2003;19:36–40.10.1016/S0899-9007(02)00838-9
- Suzuki A, Yamane T, Fushiki T. Nutrition. 2006;22:401–407.10.1016/j.nut.2005.10.002
- Matsumura S, et al. Am. J. Physiol. Regul. Inter. Comp. Physiol. 2008;295:R82–R91.10.1152/ajpregu.00060.2008
- Warner K, Neff WE, Byrdwell WC, Gardner HW. J. Agric. Food Chem. 2001;49:899–905.10.1021/jf000822f
- Kinney NE, Antill RW. Physiol. Behav. 1996;59:475–478.10.1016/0031-9384(95)02086-1
- Ramirez I. Am J. Physiol. 1993;265:R1404–R1409.
- Takeda M, Sawan S, Imaizumi M, Fushiki T. Life Sci. 2001;69:847–854.10.1016/S0024-3205(01)01180-8
- Nakano K, Kubo H, Matsumura S, Saito T, Fushiki T. Biosci. Biotechnol. Biochem. 2013;77:1166–1170.10.1271/bbb.120861
