Abstract
All four stereoisomers of 10,14-dimethyloctadec-1-ene, a sex pheromone component of the apple leafminer (Lyonetia prunifoliella: Lepidoptera), were synthesized starting from (R)- and (S)-propylene oxide by applying stereospecific inversion of chiral secondary tosylates as a key step. Field evaluation showed that male moths of the Japanese population were selectively attracted by the (10S,14S)-isomer and that the activity was not inhibited by the enantiomer.
A species-specific mating communication plays an important role in reproductive isolation of lepidopteran insects, and sex pheromones have been identified from female moths of about 650 species to date.Citation1–3) While most of them are composed of unsaturated fatty alcohols, hydrocarbons, and their derivatives with a straight chain, some species produce pheromones with a methyl-branched skeleton.Citation1) In addition to unique monomethyl epoxy and carbonyl compounds,Citation4,5) dimethyl hydrocarbons have been commonly found from leafminer moths in the family of Lyonetiidae and hemlock looper moths in the family of Geometridae.Citation1) The apple leafminer (Lyonetia prunifoliella Hübner, Lepidoptera: Lyonetiidae) is a pest of apple trees. From female moths of a North American population, Gries et al. identified the following three methyl-branched hydrocarbons as pheromone components; 10,14-dimethyloctadec-1-ene [1, Fig. (A)], 5,9-dimethyloctadecane, and 5,9-dimethylheptadecane.Citation6) Male moths were effectively attracted by a mixture of the three components synthesized without a stereoselective reaction in the field. In contrast, a lure baited with only the main component 1 attracted the males in Korea, suggesting different mating communication systems developed in North American and Korea populations.Citation7) Furthermore, the field test in Korea showed that only the (10S,14S)-isomer was responsible for the activity of 1.Citation7) The apple leafminer also inhabits Japan,Citation8) and the Japanese population is distinguished from the North American population as a subspecies named L. p. malinella (Matsumura). However, it is not clear whether the Japanese and Korean populations are the same, because the Korean population has not been taxonomically defined. Since chemical attraction of the Japanese population was still unknown, we considered the fieldwork in Japan.
Fig. 1. Chemical structure of (10S,14S)-isomer of 10,14-dimethyloctadec-1-ene (1) (A) and synthetic routes to four stereoisomers using stereospecific inversion of secondary tosylates [(B) and (C)].
Note: Reagents, conditions, and yields: (i) CH2=CH(CH2)6MgBr, Li2CuCl4, THF, 86%; (ii) TsCl, Et3N, Me3N·HCl, CH2Cl2, 90%; (iii) NaCH(CO2Me)2, DME, 93%; (iv) (1) LiCl, H2O, DMSO, 180 °C, 83%; (2) LiAlH4, THF, 95%; (v) I2, PPh3, imidazole, THF, 95%, (vi) (1) n-C3H7MgBr, Li2CuCl4, THF; (2) TsCl, Et3N, Me3N·HCl, CH2Cl2, 81% after 2 steps; (vii) LiCH2SO2Ph, THF, 93%; (viii) BuLi, THF, HMPA, 96%; (ix) Mg, MeOH, 90%.
![Fig. 1. Chemical structure of (10S,14S)-isomer of 10,14-dimethyloctadec-1-ene (1) (A) and synthetic routes to four stereoisomers using stereospecific inversion of secondary tosylates [(B) and (C)].Note: Reagents, conditions, and yields: (i) CH2=CH(CH2)6MgBr, Li2CuCl4, THF, 86%; (ii) TsCl, Et3N, Me3N·HCl, CH2Cl2, 90%; (iii) NaCH(CO2Me)2, DME, 93%; (iv) (1) LiCl, H2O, DMSO, 180 °C, 83%; (2) LiAlH4, THF, 95%; (v) I2, PPh3, imidazole, THF, 95%, (vi) (1) n-C3H7MgBr, Li2CuCl4, THF; (2) TsCl, Et3N, Me3N·HCl, CH2Cl2, 81% after 2 steps; (vii) LiCH2SO2Ph, THF, 93%; (viii) BuLi, THF, HMPA, 96%; (ix) Mg, MeOH, 90%.](/cms/asset/98e59e8b-4fc2-4bd8-a412-d9d19ba8f97f/tbbb_a_905187_f0001_b.gif)
The first enantiospecific synthesis was accomplished by coupling two chiral methyl-branched building blocks derived from citronellal and methyl 3-hydroxy-2-methylpropanoate,Citation9) and the modified route from only the latter chiral source was reported.Citation10) Subsequently, other synthetic routes applying Cu-phosphoramidite-catalyzed asymmetric addition of Me2ZnCitation11) and Evans’s stereo selective alkylationCitation12) have been reported. Recently, we achieved a simple method of constructing a chiral methyl-branched skeleton by an SN2 reaction with a tosylate of 2-alkanol, which was derived from commercialized (R)- and (S)-propylene oxide (2) with a high enantiomeric excess (ee).Citation13) Two kinds of chiral building blocks with a methyl branch at the 3- or 2-position could be obtained by selecting an appropriate carbanion as a nucleophile, and a 1,5-dimethyl structure was produced by their coupling. We successfully confirmed perfect configurational inversion by enantioselective HPLC analysis and accomplished enantiospecific synthesis of 5,9-dimethylheptadecane,Citation13) which was a main pheromone component of another leafminer species Leucoptera scitella. To demonstrate the usefulness of the SN2 reaction of secondary tosylates and develop the study about methyl-branched pheromones, we aimed to synthesize all four stereoisomers of 1 and evaluate the activity against the Japanese population of the apple leafminer.
We coupled a short-chain iodide with a methyl branch at the 3-position and a long-chain sulfonyl compound with a methyl branch at the 2-position to synthesize 5,9-dimethylheptadecane.Citation13) In this study, we applied the opposite strategy in order to avoid isomerization of a terminal double bond, which was expected to be caused by a strong basic condition for preparation of a sulfonyl compound. Long-chain iodides (S)-7 and (R)-7 were synthesized from (R)-2 and (S)-2, respectively [Fig. (B)], in a similar manner as that for the synthesis of two stereoisomers of 1-iodo-3-methylheptane.Citation13) The reaction between (R)-2 and a Grignard reagent prepared from 8-bromooct-1-ene with a catalytic amount of Li2CuCl4 produced a secondary alcohol (R)-3, which was converted to a tosylate (R)-4. To achieve chain elongation by two carbons and create a methyl group at the 3-position, (R)-4 was treated with the enolate of dimethyl malonate. This SN2 reaction formed geminal ester (S)-5 with a new C–C bond formation associated with complete inversion of the original stereochemistry. Decarboxylation of (S)-5 by Krapcho’s methodCitation14) and subsequent reduction with LiAlH4 produced primary alcohol (S)-6, which was converted into the desired building block (S)-7 by treatment with a mixture of I2, PPh3, and imidazole in THF. The overall yield of (S)-7 was 54% from (R)-2. In the same manner, the enantiomeric iodide (R)-7 was prepared from (S)-2 in a 53% overall yield. While (S)-6 and (R)-6 were inseparable by enantioselective HPLC, their high optical purity (>99% ee) could be confirmed after hydrogenation of the alkenols over a Pd–C catalyst. The analysis was conducted with a Chiralpak AY-H column using 0.3% 2-propanol in hexane as an eluent (flow rate, 0.5 mL/min), and two stereoisomers of methyldodecan-1-ol were separately detected [(S)-isomer tR 27.8 min and (R)-isomer tR 26.1 min].
As another chiral building block, short-chain sulfones (S)-9 and (R)-9 were synthesized from (R)-2 and (S)-2, respectively [Fig. (C)]. The reaction between (R)-2 and n-C3H7MgBr with CuCN gave a secondary alcohol, which was converted into tosylate (R)-8 without any purification. To achieve one-carbon elongation and create a methyl branch at the 2-position, the SN2 reaction between (R)-8 and the anion, which was derived from methyl phenyl sulfone by treatment with butyllithium (BuLi), was carried out. The sulfone (S)-9 with a new C–C bond was also produced with a complete inversion of the original stereochemistry in a 75% yield from (R)-2. The enantiomeric sulfone (R)-9 was prepared in the same manner and in a 76% yield from (S)-2. Enantioselective HPLC analysis was conducted with a Chiralpak AS-H column using 10% 2-propanol in hexane as an eluent (flow rate, 0.5 mL/min), and their high optical purities (>99% ee) were confirmed [(S)-9 tR 27.7 min and (R)-9 tR 30.4 min]. Methyl phenyl sulfone was used in this experiment instead of methyl p-tolyl sulfone as in our previous synthesis of a long-chain block, 2-methyl-1-(4-methylphenylsulfonyl)decane.Citation13) While the reaction with methyl p-tolyl sulfone needed the low concentrated condition (0.07 m), excess of methyl phenyl sulfone (3.0 equivalent) was enable to increase product yield (96%) at high concentrated condition (0.5 m). The anion of methyl phenyl sulfone is easily reacted with a primary alkyl halide and usually used as a one-carbon linchpin.Citation10) It is noteworthy that the anion attached to a chiral secondary tosylate in this study and created a methyl branch with exact stereochemistry.
The two chiral blocks (S)-7 and (S)-9 were coupled by using BuLi as a base in THF-HMPA to give (10S,13SR,14S)-10 with a 10,14-dimethyl skeleton. Reductive removal of the sulfonyl group was accomplished using magnesium turning activated with a catalytic amount of MeMgBr. Purification of the crude product with a AgNO3–SiO2 column gave the desired terminal alkene (10S,14S)-1 in an 86% yield from (S)-9. The following chemical data of (10S,14S)-1 were almost identical to those published with regard to previous syntheses,Citation7,8,11,12) indicating that racemization did not occur after the coupling; = +1.9 (c = 2.0, CHCl3); IR (neat): νmax (cm−1) = 2970 (s), 2940 (s), 2854 (s), 1640 (m), 1464 (m), 1377 (m), 910 (m), 723 (w); 1H NMR (500 MHz, CDCl3): δ = 0.840 (3H, d, CH3CH, J = 6.4 Hz), 0.843 (3H, d, CH3CH, J = 6.8 Hz), 0.889 (3H, t, CH3CH2, J = 6.9 Hz), 1.05–1.40 (26H, m), 2.04 (2H, dt, CH2CH=CH2, J = 7.2, 6.6 Hz), 4.93 (1H, d, CH=CHH, J = 10.2 Hz), 4.99 (1H, d, CH=CHH, J = 16.9 Hz). 5.81 (1H, ddt, CH2CH=CH2, J = 16.9, 10.2, 6.6 Hz) ppm; 13C NMR (125 MHz, CDCl3): δ = 14.19 (C-18), 19.77 (×2, CHCH3), 23.08 (C-17), 24.49 (C-12), 27.08 (C-8), 28.98, 29.19, and 29.36 (C-4, 5, 6), 29.57 (C-7), 29.98 (C-16), 32.78 and 32.80 (C-10, 14), 33.85 (C-3), 36.77 (C-9), 37.07 (C-15), 37.46 (×2, C-11, 13), 114.09 (C-1), 139.29 (C-2) ppm; GC-MS: tR 16.33 min, m/z 280 (M+, 1%), 238 (1%), 168 (14%), 153 (17%), 140 (10%), 126 (19%), 111 (41%), 97 (64%), 83 (62%), 69 (65%), 57 (100%). In the same manner, three other stereoisomers were synthesized in similar yield; (10R,14S)-1 (
= +1.2, c = 1.8, CHCl3) from (R)-7 and (S)-9, (10S,14R)-1 (
= −1.3, c = 1.5, CHCl3) from (S)-7 and (R)-9, and (10R,14R)-1 (
= −2.0, c = 1.5, CHCl3) from (R)-7 and (R)-9. While the 1H NMR spectra of the four stereoisomers were identical, the diastereomers showed small but discernible differences in chemical shifts for some 13C signals; 13C NMR (125 MHz, CDCl3) of (10S,14R)-1: δ = 14.19 (C-18), 19.71 (×2, CHCH3), 23.08 (C-17), 24.49 (C-12), 27.10 (C-8), 28.98, 29.19, and 29.38 (C-4, 5, 6), 29.58 (C-7), 29.98 (C-16), 32.77 (×2, C-10, 14), 33.85 (C-3), 36.87 (C-9), 37.17 (C-15), 37.40 (×2, C-11, 13), 114.09 (C-1), 139.28 (C-2) ppm. We tentatively assigned each 13C signal, and the assignments indicated differences for the carbons of two branched-methyl groups and the carbons around the branches, reflecting the conformational differences of the diastereomers as indicated for 5,9-dimethylpentadecane.Citation13)
This synthesis confirmed that two building blocks derived from chiral propylene oxide were useful to construct not only 1,5-dimethyl alkanes but also alkenes. We have applied a similar synthetic strategy to synthesize all four stereoisomers of 6,10,13-trimethyltetradecan-2-one.Citation15) In addition to the 2-ketone with a 1,5-dimethyl structure identified as a male sex pheromone of a stink bug,Citation16) we have synthesized four stereoisomers of (S)-2-methylpent-3-yl 3,13-dimethylpentadecanoate.Citation17) The acid moiety with a 1,11-dimethyl structure was also constructed from chiral propylene oxide as a chiral source. While the SN2 reaction is a well-known and fundamental chemical matter, it has rarely been utilized for pheromone synthesis because partial epimerization was feared. These experiments, however, indicated that the SN2 reaction of secondary tosylates is one of the most simple and reliable tools to make chiral methyl-branched compounds.
The synthetic pheromone was evaluated in apple orchards in Morioka City (Iwate Prefecture, Japan) in 2012. Each test compound was applied to a rubber septum (white rubber, 9 mm OD; Sigma-Aldrich), and the lure was placed at the center of a sticky board trap (SE-trap®, 30 cm × 27 cm bottom plate with a roof; Sankei Chemical), which was hung at a 1.5 m height. Table shows the results. The males of L. p. malinella could be attracted by the dimethyl alkene 1 without mixing two other components identified from the North American population, indicating agreement with the result of a field test in Korea. Among the four stereoisomers, (10S,14S)-1 most strongly attracted the males of L. p. malinella (Test I). While each stereoisomer of 1 was evaluated in the presence of the other components in Korea, this result in Japan revealed effective attraction by the lure baited with only (10S,14S)-1. The males were also captured by traps baited with (10S,14R)- and (10R,14S)-isomers, but the numbers were significantly lower than the number of males captured by (10S,14S)-1. Next, we examined the effect of the (10R,14R)-isomer on the activity of (10S,14S)-1 (Test II). The (10S,14S)-isomer unmixed and mixed with the enantiomer showed the same strong attraction activity, indicating that the (10R,14R)-isomer acted as neither a synergist nor an inhibitor. The result indicates that the males have an ability to recognize strictly the stereostructure of the pheromone component. It is an interesting future subject to examine whether the females of L. p. malinella secrete only (10S,14S)-1.
Table 1. Field attraction of L. prunifoliella males by synthetic lures baited with stereoisomers of 10,14-dimethyloctadec-1-ene (1) in apple orchards in Morioka.Table Footnotea
Fig. 2. Pheromone-trap monitoring of male moths of the apple leafminer, L. prunifoliella, in apple orchards in Morioka.
Note: The monitoring in 2012 was started in early August using three traps baited with (10S,14S)-1 (1.0 mg/septum). The captured males were counted every five days. The monitoring in 2013 was started in mid-April using the same two traps until September and one trap in October and November. The males were counted weekly. Each lure was renewed every month.
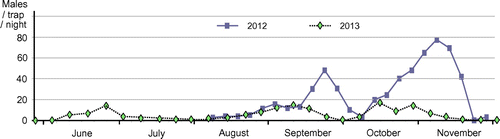
Fig. shows results of monitoring of the male moths by pheromone traps in 2012 and 2013. The males were captured from early June to late November in the apple orchards in Morioka, and clear peaks of attraction were observed in June and September, and from October to November. This species passes winter as an adult. It has been estimated that the female mates before hibernation and survives the winter, but the male dies after mating.Citation8) The monitoring with the synthetic pheromone confirmed this interesting life history; i.e. no males were captured in spring. Males of the first generation, which grow from eggs laid by females after hibernation, were captured in June. The first flight term was appropriately monitored by the pheromone trap. The mature larva comes out of the mine and pupates on the undersurface of the leaf in a hammock-shaped web. Since the web formation was observed around late May, early July, late July, late August, and mid-September in apple orchards, five generations per year have been expected in Aomori Prefecture.Citation8) The attraction peaks in September and from October to November might correspond to the fourth and fifth generations. While population density in summer seems to be very low and flight terms of the second and third generations were undetermined using the pheromone trap, Fig. indicates that the synthetic pheromone is a reliable tool for the monitoring of male moths in the field. Future work utilizing the sex pheromone should clarify habitation fields and other ecological aspects of L. p. malinella, including the extent to which the number of trapped males correlates with larval damage.
Funding
This work was supported in part by grant-aid for scientific research from the Ministry of Education, Culture, Sports, Science, and Technology (MEXT) of Japan [Grant-in-Aid for Scientific Research (C) No. 24580158].
References
- Ando T, Inomata S, Yamamoto M. Lepidopteran sex pheromones. Top. Curr. Chem. 2004;239:51–96.10.1007/b96138
- Ando T. Internet database 2013 [Internet]. Available from: http://www.tuat.ac.jp/~antetsu/LepiPheroList.htm
- El-Sayed AM. Internet database 2013 [Internet]. Available from: http://www.pherobase.com/
- Bierl BA, Beroza M, Collier CW. Potent sex attractant of the gypsy moth: its isolation, identification, and synthesis. Science. 1970;170:87–89.10.1126/science.170.3953.87
- Yamamoto M, Kamata T, Do ND, Adachi Y, Kinjo M, Ando T. A novel lepidopteran sex pheromone produced by females of a lithosiinae species, Lyclene dharma dharma, in the family of Arctiidae. Biosci. Biotechnol. Biochem. 2007;71:2860–2863.10.1271/bbb.70551
- Gries R, Gries G, King GGS, Maier CT. Sex pheromone components of the apple leafminer, Lyonetia prunifoliella. J. Chem. Ecol. 1997;23:1119–1130.10.1023/B:JOEC.0000006390.43868.3b
- Park JH, Han KS, Mori K, Boo KS. Right stereoisomers for sex pheromone components of the apple leafminer, Lyonetia prunifoliella, in Korea. J. Chem. Ecol. 2002;28:2515–2525.10.1023/A:1021488103491
- Sekita N, Yamada M. Life history of Lyonetia prunifoliella Hübner subsp. malinella (Matsumura) (Lepidoptera: Lyonetiidae). Appl. Entomol. Zool. 1979;14:285–292.
- Tamagawa H, Takikawa H, Mori K. Pheromone synthesis, CXCII. Synthesis of all the stereoisomers of 10,14-dimethyloctadec-1-ene, 5,9-dimethyloctadecane and 5,9-dimethylheptadecane, the sex pheromone components of the apple leafminer, Lyonetia prunifoliella. Eur. J. Org. Chem. 1999;1999:973–978.10.1002/(ISSN)1099-0690
- Nakamura Y, Mori K. Pheromone synthesis, CCIV. Synthesis of the enantiomers of anti-2,6-dimethylphentane-1,7-diol monotetrahydropyranyl ether and their conversion into the enantiomers of the sex pheromone components of the apple leafminer, Lyonetia prunifoliella. Eur. J. Org. Chem. 2000;2000:2745–2753.10.1002/(ISSN)1099-0690
- van Summeren RP, Reijmer SJW, Feringa BL, Minnaard AJ. Catalytic asymmetric synthesis of enantiopure isoprenoid building blocks: application in the synthesis of apple leafminer pheromones. Chem. Commun. 2005;2005:1387–1389.10.1039/b419268k
- Yadav JS, Gayathri KU, Thrimurtulu N, Prasad AR. Stereoselective synthesis of 10,14-dimethyloctadec-1-ene, 5,9-dimethyloctadecane, and 5,9-dimethylheptadecane, the sex pheromones of female apple leafminer. Tetrahedron. 2009;65:3536–3544.10.1016/j.tet.2009.01.093
- Taguri T, Yamakawa R, Fujii T, Muraki Y, Ando T. Stereospecific inversion of secondary tosylates to yield chiral methyl-branched building blocks, applied to the asymmetric synthesis of leafminer sex pheromones. Tetrahedron: Asymmetry. 2012;23:852–858.10.1016/j.tetasy.2012.05.023
- Krapcho AP, Weimaster JF, Eldridge JM, Jahngen EGE Jr, Lovey AJ, Stephens WP. Synthetic applications and mechanism studies of the decarbalkoxylations of geminal diesters and related systems effected in Me2SO by water and/or by water with added salts. J. Org. Chem. 1978;43:138–147.10.1021/jo00395a032
- Muraki Y, Taguri T, Yamamoto M, Zarbin PHG, Ando T. Synthesis of all four stereoisomers of 6,10,13-trimethyltetradecan-2-one, a sex pheromone component produced by males of the stink bug Pallantia macunaima. Eur. J. Org. Chem. 2013;2013:2209–2215.10.1002/ejoc.201201688
- Fávaro CF, Soldi RA, Ando T, Aldrich JR, Zarbin PH. (6R,10S)-Pallantione: the first ketone identified as sex pheromone in stink bugs. Org. Lett. 2013;15:1822–1825.10.1021/ol400413w
- Taguri T, Yamamoto M, Fujii T, Muraki Y, Ando T. Synthesis of four stereoisomers of (S)-2-methylpent-3-yl 3,13-dimethylpentadecanoate, a sex pheromone of the bagworm moth clania variegate, using stereospecific inversion of secondary sulfonates as a key step. Eur. J. Org. Chem. 2013;2013:6924–6933.10.1002/ejoc.v2013.30
