Abstract
We investigated the major leaf isoflavonoid contents of Pueraria mirifica from three different cultivars (PM-III, PM-IV, and PM-V) using reverse RP-HPLC analysis. The proportions and net levels of puerarin, daidzin, genistin, and daidzein in P. mirifica leaves were found to depend on the plant cultivar and to correlate with cultivation temperature and rainfall amount. The crude leaf-extracts were tested using the Yeast Estrogen Screen (YES) assay with both human estrogen receptors (hERα and hERβ). Their estrogenic activity was higher when determined by the YES system containing hERβ than that with hERα and was also higher when the Δsnq2 than the wildtype yeast was employed. The results open the possibility of selecting and cultivating certain P. mirifica cultivars at a farm scale to produce a sufficient supply of leaf material to act as a starting source for the commercial scale extraction of these major isoflavonoids.
Isoflavonoids, one of the common types of phytoestrogens, are found in relative abundance in many forms of edible legume seedsCitation1,2) legume-derived foodsCitation3,4) and dietary supplements.Citation5) They are of increasing interest to the food industry due to their potential health benefits, including for the potential chemoprevention of cancers.Citation6) The recent establishment of several phytoestrogen databases illustrates the growing interest of the food industry in phytoestrogens from natural sources and their bioactivities.Citation7)
Soy isoflavonoids have been well studied, with most of the results being obtained from daidzein and genistein,Citation8) which appear to harbor anti-cancer properties.Citation9) However, a new source of isoflavonoids would promote the alternative production and consumption of such chemicals from those new plant sources and relieve the pressure on the currently limited amount of available plant material and risks associated with sole large scale monoculture productionCitation10) or the in vitro culture of plants.Citation11)
Pueraria mirifica Airy Shaw et Suvatabhandu is a Thai indigenous legume herb with a long-term folk medicinal consumption among Thai women for menopausal treatment. The tuberous materials of the plant revealed strong estrogenic effects in the MCF-7 proliferation/antiproliferation,Citation12) uterotrophicCitation13,14) and Yeast Estrogen Screen (YES)Citation15) test assays. Among the phytoestrogen-rich plant materials, the tubers of kudzu (Pueraria lobata) are widely used in traditional Chinese, Japanese, and Korean medicines,Citation16) whereas in traditional Thai medicines the related P. mirifica is used and has been extensively studied. P. lobata has been analyzed for the purpose of the potential development of the plant products or chemicals for the benefit of the food industry,Citation17) whereas P. mirifica has been subjected to long-term studies to establish products for menopausal treatment.Citation18,19) At present, these two plants are used as the main botanical ingredients in cosmetic and dietary supplement products.
The estrogenic activity in P. mirifica was found to be stronger than that in P. lobata in the MCF-7 antiproliferation, uterotrophic, and ovariectomized rat assays.Citation20,21) However, the analysis of P. mirifica tubers collected from wild plant populations over a vast area in Thailand in comparison with that of P. lobata collected from China revealed that neither daidzin nor genistin, which are the isoflavonoid glycosides derived from daidzein and genistein, respectively, were the major isoflavonoids in these plants, but rather puerarin was.Citation22) Accordingly, it is of interest to find out which isoflavonoids or other phytoestrogens, if any, might be responsible for the beneficial effects observed in these assays, whilst the estrogen-like activity and long-term folk medical use make P. mirifica an interesting candidate as a potential phytoestrogen source for the development of commercialized products. However, the basic knowledge related to phytoestrogens, and especially the control of isoflavonoid synthetic pathways and transport/storage within the plant, is still largely unknown in this species.
In this study, we present the content levels for the five major isoflavonoids (puerarin, daidzin, genistin, daidzein, and genistein) from the leaves of three different farm-grown P. mirifica cultivars (PM-III, PM-IV, and PM-V) together with their estrogenic activity, as analyzed by the YES assay involving the human estrogen receptors hERα and hERβ. The use of leaves as the isoflavonoid source has the potential advantage over tubers in that the mature tubers need at least three years cultivation to reach maturity, while the plant leaves can be harvested monthly for 9 (cultivar PM-V) to 11 (cultivars PM-III and PM-IV) months a year. Thus, this research may open the possibility of introducing a new more economically viable plant source material for natural isoflavonoid supply.
Materials and methods
Chemicals, biochemicals, and yeast strains
The isoflavonoid standards puerarin, genistin, daidzein, genistein, and 17β-estradiol (E2) were purchased from Sigma (St. Louis, MO, USA), whereas daidzin was purchased from FlukaBiochemika (Buchs, Switzerland). The organic solvents for extraction (analytical grade) and chromatography (HPLC grade) were purchased from Merck (Germany). Water of over 16 M MΩ/cm, a component of the mobile phase of HPLC, was prepared by Maxima Ultra Pure Water Systems (ELGA). The Saccharomyces cerevisiae expression strain Y190 (Clontech®) (MATa, ura3–52, his3-Δ200, ade2–101, trp1–901, leu2–3, 112, gal4Δ gal80Δ, URA3::GALUAS-lacZ, cyhr2, LYS2::GALUAS-HIS3) containing the lacZ reporter gene fused to GALUAS in the chromosomes was used as the host yeast. For the YES-hERα assay, wildtype Y190 was transformed with the plasmid pGBT9-hERαLBD, encoding for amino acid residues 331–595 of the hERα fused to GAL4DBD, and pGAD424-hTIF2, encoding for amino acid residues 624–1287 of the transcriptional intermediary factor (TIF2) fused to GAL4AD. For the YES-hERβ assay, Y190 was transformed with pGBT9-hERβLBD, encoding for amino acid residues 213–477 of the hER β fused to GAL4DBD, and pGAD424-hSRC1, encoding for amino acid residues 231–1094 of steroid receptor co-activator 1 (SRC1) fused to GAL4AD.Citation15) In addition, the YES-hERαΔsnq2 and YES-hERβΔsnq2 systems were constructed from the YES-hERα and YES-hERβ systems as above except using the Y190-Δsnq2 as the host strain in place of the wildtype Y190 so as to increase the sensitivity of YES system.
Plant material
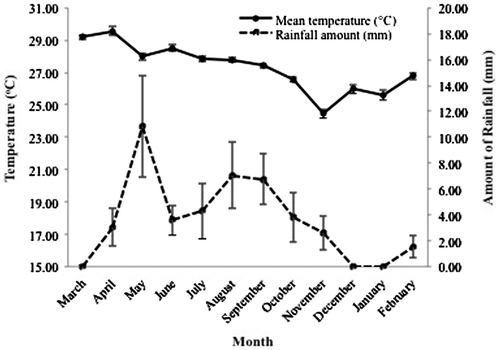
The fresh mature leaves of the P. mirifica cultivars (PM-III, PM-IV, and PM-V) were cultivated in a field trial in the Banpong District of Ratchaburi Province (E99°52°/N13°37°), Thailand, and were collected every month from March 2007 to February 2008. The leaf morphology was distinctive between each of the three cultivars, as previously reported.Citation23) The monthly record of daily mean temperatures and rainfall in the Ratchaburi Province was provided by the Meteorological Department, Ministry of Information and Communication Technology, Thailand and is shown in Fig. .
Fig. 1. The mean monthly temperature (°C) and rainfall amountCitation39) at Ratchaburi province, including the field trial area, during the study period (March 2007 to February 2008).
Data are shown as the mean ± S.E.M.
Extraction and isolation
The collected leaf samples were cleaned, dried in a hot air oven at 80 °C for 72 h, and subsequently ground into powder and filtered through a 120-mesh-size sieve. A 50 g aliquot of the leaf powder was extracted with methanol by vigorous shaking (3 × 500 mL, 2 h each, RT). After settling, the supernatant was filtered (Whatman filter paper No.4, Whatman, USA), and the pooled filtrates then evaporated in vacuo (Buchi, Germany) at 37 °C. The residual material was dissolved in 50 mL deionized water and then partitioned in 1:1 (v/v) chloroform (3 times) to remove the chlorophyll, harvesting the aqueous phase that was then mixed at 1:1 (v/v) ratio with n-butanol (3 times). After phase separation the butanol phase was harvested and evaporated in vacuo at 45 °C. The final residue, referred to, as the crude leaf-extract was stored at 4 °C.
Reverse phase HPLC analysis
The reverse phase HPLC system, a Waters™ Auto Sampler (Waters-717), controller (Waters-600) and photodiode array detector (Waters-2996), utilized a reversed phase C18 column (250 × 4.6 mm) filled with 5 μm ODS2 (Waters Spherisorb, Ireland), pre-filtered with a Waters Spherisorb S5 ODS2 (4.6 × 10 mm) guard cartridge. The filter set was Millipore membrane (0.45 μm, 13 mm for the sample and 47 mm for the mobile phase) of the HA type for aqueous solutions and HV type for organic solvents. The chromatography manager software was operated with a personal computer. The crude leaf-extract (1 g) was dissolved in methanol (1 mL) with the aid of sonication (30 min, RT), and then sequentially filtered through Whatman No.1 filter paper and a 0.45 μm (13 mm diameter) PTFE filter membrane. The isoflavonoid analysis was then performed by reverse phase HPLC as previously describedCitation16) with modification. Elution was performed with a linear gradient from 100:0 to 55:45 (v/v) 0.1% (v/v) acetic acid: acetonitrile as the mobile phase at a flow rate of 1 mL/min for 50 min, while the eluate was monitored at OD254. The five major isoflavonoid standards were mixed at appropriate proportions for use in chromatogram peak area calibration curves, and the comparison of their retention time with the test samples was performed for identification, and the peak area was derived for quantification using the Empower® program. The analyses of samples were run in triplicate.
YES assay
The methodology was described in previous study.Citation15) A stock solution of each crude leaf-extract was freshly prepared as serial dilutions (50–2,000 μg/mL) in DMSO. Standard compounds: E2, genistein, and genistin were also prepared in DMSO in dose ranges of 10−16–10−4 M, 10−8–10−4 M, and 10−8–10−3 M, respectively, by using DMSO as a negative control. The highest concentration of DMSO in each dilution was 1% (v/v). The YES assay was performed as described elsewhereCitation18) with modification as follows. Yeasts were grown in synthetic dextrose (SD) minimal medium supplemented with adenine (SDA; 0.67% (w/v) yeast nitrogen base without amino acids, 2% (w/v) glucose, and 0.002% (w/v) adenine sulfate) with vigorous shaking overnight (30 °C). Then 50 μL of the overnight culture was mixed with 200 μL of fresh SDA medium and 2.5 μL of either crude leaf-extract (dissolved in DMSO to the desired concentration), DMSO alone (negative control), or E2 (dissolved in DMSO to the desired concentration; positive control) in a 1.5-mL microtube and incubated at 30 °C for 4 h with shaking at 200 rpm. Thereafter, a 150 μL aliquot of the cultured cell solution was transferred into a 96-well microplate for measurement of the cell density at an OD660. Another aliquot (100 μL) of the cell suspension was centrifuged (10,000 × g, 5 min) to pellet the cells that were then resuspended in 200 μL of Z-buffer (0.2 mg/mL Zymolyase 100T in 0.1 M sodium phosphate pH 7.0, 10 mMKCl, 1 mM MgSO4, and 3.5 mMβ-mercaptoethanol) and incubated for 15 min at 30 °C. After centrifugation (10,000 × g, 5 min) the cell lysate (supernatant) was recovered and incubated with 40 μL of substrate solution (4 mg/mL oNPG) in 0.1 M sodium phosphate buffer pH 7.0), for 30 min at 30 °C.Citation24) The reaction was stopped by the addition of 100 μL of 1 M Na2CO3, and the cell debris centrifugally removed (8,600 × g for 5 min). Finally, 150 μL of the supernatant was transferred into a 96-well microplate for measurement of the absorbance at OD420 and OD550 to follow the formation of the ortho-nitrophenol (o-NP) product. One unit (U) of β-galactosidase was defined in terms of Miller U as follows:
where OD420 represents the absorbance of the oNP product, OD550 was the scatter from cell debris, which when multiplied by 1.75 approximated the scatter observed at OD420, OD660 was the cell density at the start of the assay, T was the reaction time (min), and V the culture volume used in the assay (mL).
The data for β-galactosidase activity (U) and the concentration of tested samples were fitted using the four parameters logistic dose-response model of the GraphPad Prism software version 4 (GraphPad Software Inc., USA), as a Sigmoidal dose-response (variable slope), and the EC50 value calculated. The Relative PotencyCitation25) of each crude leaf-extract was calculated to allow direct comparison between samples, and was calculated by dividing the obtained EC50 (μg/ml) for E2 by the EC50 of the test sample, and then multiplying the value by 100. Thus, the RP value of E2 was always 100 as the reference standard.
Statistical analysis
Statistical calculations were carried out with the Statistical Packages for Social Science (SPSS) software version 17.0 for Windows (SPSS Inc., USA). Data are shown as the mean ± 1 S.E.M. and differences between means, for the isoflavonoid contents of the crude leaf-extracts, β-galactosidase activities, EC50, and RP, were tested for statistical significance by the unpaired T-test, Pearson correlation analysis, and Duncan’s analysis of variance, accepting significance at the level of p < 0.05 and p < 0.01.
Results
Plant harvesting
The three cultivars of P. mirifica produced abundant numbers and masses of leaves for harvesting every month, except when no or too few leaves were present to harvest, which was in April for the PM-III cultivar, March for the PM-IV cultivar, and February, March, and April for the PM-V cultivar.
Crude leaf-extract yield
The different yields of the leaf crude extract obtained for each of the three P. mirifica cultivars over the assayed year are summarized in Table with highest mean temperature in April and rainfall amount in May. Within each month a significant variation in the crude leaf-extract yield between the cultivars was evident although cultivar PM-III typically had the highest monthly yields (in 7/12 months) and the highest average yearly yield, whilst PM-V had the lowest monthly (in 7/12 months) and yearly yield.
Table 1. The leaf crude extract yields obtained (g/100 g leaf powder) from each the three different P. mirifica cultivars in each month over the 12-month cultivation period (March 2007 to February 2008).
HPLC analysis
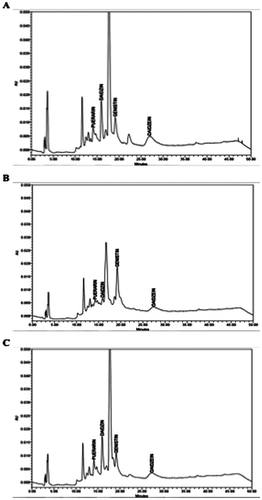
The five standard isoflavonoids were clearly separated and distinct when analyzed by RP-HPLC with the C18 column and monitoring the eluent at OD254 (Fig. ). The HPLC resolution of the crude leaf-extract of each P. mirifica cultivar was complicated, but resolution of the isoflavonoids was still clear with genistein being absent in all three cultivars in all months of growth. A representative example of HPLC traces derived from samples harvested during January 2008 is shown in Fig. .
Fig. 2. HPLC Fingerprints of the five synthetic isoflavonoid standards of puerarin (25 mg/mL, RT = 14.35 min), daidzin (30 mg/mL, RT = 15.93 min), genistin (30 mg/mL, RT = 18.96 min), daidzein (25 mg/mL, RT = 26.49 min), and genistein (50 mg/mL, RT = 34.64 min).
Profiles shown are representative of those seen from at least 27 independent trials.
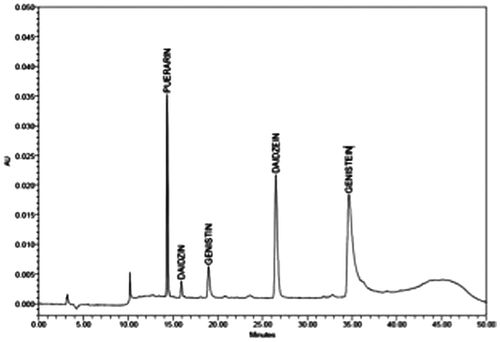
Fig. 3. HPLC fingerprints of the crude leaf-extract of P. mirifica cultivar (A) PM-III, (B) PM-IV, and (C) PM-V, derived from samples harvested during January 2008.
The profiles shown are representative of those seen from at least 27 independent trials, and from leaves harvested in the other months.
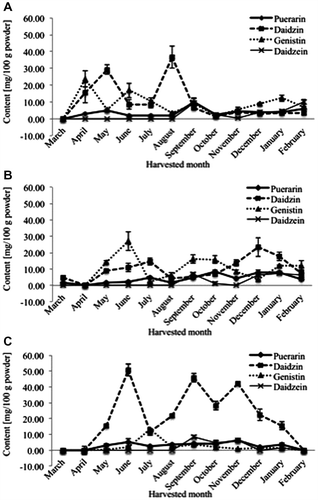
The calculated amount of each isoflavonoid found in each P. mirifica cultivar over the 12-month cultivation period (Fig. ) revealed the highest mean total isoflavonoid content across all three cultivars was found in June (41.7 ± 4.0 mg/100 g leaf powder) followed by September (37.9 ± 4.3 mg/100 g leaf powder) but this varied between the individual cultivars. Cultivar PM-V had the highest total isoflavonoid contents in September (61.6 ± 4.0 mg/100 g powder), but for PM-III the optimal month was June, while for PM-IV it was equally optimal in April and August. Daidzin in the PM-V cultivar was the most prevalent of the isoflavonoids, where the highest concentration was found in June (50.6 ± 3.7 mg/100 g powder), however, levels of daidzin in the other two cultivars (PM-III and PM-IV) were significantly lower in this and most other months. In contrast, genistin levels were very low in the PM-V cultivar except in July (13.0 ± 0.1 mg/100 g powder), but was the isoflavonoid with the highest concentration in the PM-III and PM-IV cultivars for seven and five months, respectively, and with its levels being second to those of daidzin in several other months. In contrast, puerarin and daidzein remained at relatively low concentrations over all 12 months in all three cultivars.
Fig. 4. Major isoflavonoid profile (mg/100 g powder) of the crude leaf-extracts from the three different cultivars of P. mirifica Harvested in different months.
(A) PM-III, (B) PM-IV, and (C) PM-V. Data are shown as the mean ± S.E.M.
The monthly rainfall level strongly correlated with, and so potentially had a significant impact upon the genistin content in the leaves each month (p < 0.05), whilst the temperature likewise strongly correlated with the puerarin, daidzin, and daidzein contents (p < 0.01) in the PM-III cultivar (Table S1 in supplementary information). In contrast, in the PM-V cultivar the rainfall strongly correlated with the puerarin content (p < 0.01) and the temperature with the daidzin content (p < 0.01). Note, however, that in all four of the above correlations an actual causation has yet to be established.
YES assay
Because the YES assay is a suitable and simple method for the in vitro screening of estrogenic activity that reduces the required time and provides an easy means for monitoring compared with the in vivo cancer cell test, we employed this assay in this study using the S. cerevisiae wildtype and Δsnq2Y190 yeast strains, each expressing part of either the hERα or hERβ receptor. If the compound binds to ligand binding domain (LBD) of hER fused to DNA binding domain (DB) of GAL4, it will turn on β-galactosidase, harboring GALUAS. The readout is then achieved by performing a β-galactosidase assay through the conversion of oNPG to oNP, which is monitored by absorbance of the oNP at 420 nm and compared to that for E2 as a positive control and reference standard.Citation15)
For the standardization of the YES-hER assay with the wildtype and Δsnq2 yeast strains for the evaluation of estrogenic activity, the EC50 values of E2 in the YES-hERα and YES-hERβ assays (Table ) were found to agree fairly well (1.2-fold lower activity) with that previously reported for YES-hERα (2.25 × 10−10 M), but was some 51.3-fold less active than that reported before for YES-hERβ (2.3 × 10−10 M). In this study here, the YES-hERα exhibited a 44.2-fold higher estrogenic activity than the YES-hERβ system, whilst the YES-hERαΔsnq2 assay exhibited a 283.6-fold higher estrogenic activity than that in the YES-hERβΔsnq2 system. When the SNQ2 deletion was employed, the estrogenic activity was significantly increased in both the hERα and hERβ based assays (Table ). Thus, for E2 detection the sensitivity was 16.2- and 2.5-fold higher for the YES-hERαΔsnq2 and YES-hERβΔsnq2 systems compared to the YES-hERα and YES-hERβ assays, respectively, while for genistein and genistin the sensitivity increased by 1.2- and 4.5-fold when evaluated by YES-hERαΔsnq2 compared to YES-hERα, respectively, and 11.3- and 8.0-fold higher, respectively, for the YES-hERβΔsnq2 system compared to the YES-hERβ one. In contrast to E2, genistein and genistin both showed a higher estrogenic activity against the hERβ-based systems than in the hERα ones, for both the wildtype and Δsnq2 yeast strains (Table ). However, the sensitivity of genistein and genistin detection in the YES-hERβΔsnq2 assay was significantly lower than that of E2 by about 13.1- and 1887-fold, respectively. The estrogenic activity of puerarin, daidzein, and daidzin could not be detected using any of the four YES systems within the range of concentrations used in this assay (10−8–10−3 M), and so they were excluded from the sensitivity assessment.
Table 2. The estrogenic activity, as the EC50 Value and derived relative potencyCitation25) using E2 as the reference standard, of the standard genistein and genistin and reference E2, as determined by the four different YES systems.
The evaluation of the estrogenic activity of the P. mirifica crude leaf-extracts revealed that the highest estrogenic activity was always detected with the ERβ systems as opposed to the ERα ones, and with the Δsnq2 yeast strain compared to the wildtype, and so the YES-hERβ Δsnq2 assay was the most sensitive for all the cultivars (Fig. ). With respect to the three P. mirifica cultures individually, in the PM-III cultivar the highest estrogenic activity detected by the wildtype YES-hERα and YES-hERβ was in September (RP of ~10−2 and 10−4, respectively), and this declined steadily each month thereafter to a minimum in August. (This and the subsequent analysis of course assume that this 12-month period from March 2007 to February 2008 represents the pattern seen each year, which remains to be established). A broadly similar trend was observed when assayed by the YES-hERαΔsnq2 system, but in contrast, when assayed by the YES-hERβΔsnq2 system, no such clear monthly pattern over the 12-month period was evident with instead the highest activity being seen in November followed by that in August (Fig. ).
Fig. 5. Relative potency values of the crude leaf-extracts from the three different cultivars of P. mirifica harvested in different months.
(A) PM-III, (B) PM-IV, and (C) PM-V. RP values, relative to the EC50 of the E2 standard, were derived from the estrogen-like activity EC50 values as ascertained using the wild type (YES) and snq2∆ (YES-snq2∆) Y190 yeast strains with hERα or hERβ. Abbreviation; E2: 17β-estradiol. Data are shown as the mean ± S.E.M. Means with a different lower case letter above them are significantly different (p < 0.05).
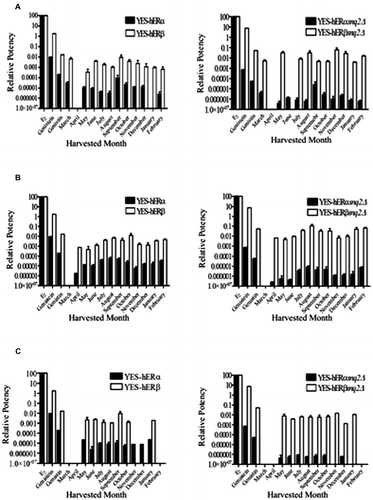
In some contrast to the PM-III cultivar, in the PM-IV cultivar, the highest estrogenic activity was detected in leaves harvested during August when assayed by three of the four yeast assay systems, but the YES-hERβ system gave a slightly higher estrogenic activity on leaves harvested during October than in September. Moreover, there was no clear monthly decline from this maximum over the 12-month period, but rather it tended to decline to a false minimum in November, rise to a smaller peak by February before then declining again to the minimum level at April, and then rise to the maximum level in September or October as above.
The highest estrogenic activity of the crude leaf-extracts from the PM-V cultivar was found in leaves which were harvested in January 2008 or May 2007 for the YES-hERα assay, and in September, July, and November for the YES-hERβ, YES-hERαΔsnq2, and YES-hERβΔsnq2 assays, respectively (Fig. ), but without any clear pattern of monthly decline. Indeed, the monthly variation in estrogen levels determined by both the YES-hERαΔsnq2 and YES-hERβΔsnq2 assays were slight, except for a minimum in December (Fig. ). However, the estrogenic activity of all plant samples was lower than that of E2, the positive control (Fig. ), and was not correlated with the isoflavonoid contents (analysis not shown), suggesting that there might be other isoflavonoids, or complex interactions between them, or as-yet-undetermined compounds responsible for this activity.
Discussion
The three cultivars of P. mirifica (PM-III, PM-IV, and PM-V) produced abundant numbers and masses of leaves for harvesting every month, except for the month(s) when there were no or too few leaves to harvest (April in the PM-III cultivar, March in the PM-IV cultivar, and February, March, and April in the PM-V cultivar). During February to April, the decrease in rainfall amount and increase in temperature might cause water-limited environment. Plants then become water stressed and dropped their leaves.Citation26)
HPLC analysis
The crude leaf-extracts derived from the three P. mirifica cultivars varied in their yield and relative composition of the isoflavonoids between the different cultivars and cultivation month during the 12-month period assayed. Whether this temporal monthly pattern is representative of typical years remains to be established. However, the study also confirmed the existence of a significant level of accumulated isoflavonoids in the plant leaves, including puerarin, daidzin, genistin, and daidzein. Determination of the isoflavonoid levels revealed significant cultivar-dependent difference, i.e. difference in the isoflavonoid levels among the three cultivars grown in the same field trial and harvested at the same time, as well as between each month. Daidzin and genistin were found to have the highest average annual isoflavonoid accumulation in the leaf samples (genistin for PM-III and daidzin for PM-IV and PM-V) with puerarin also being found in all analyzed plant samples (cultivars and harvest months), but at lower levels. In contrast, daidzein was not found in all three cultivars in March to August, plus not in cultivar PM-IV in October, PM-III in November and PM-V in November to January. Moreover, genistein was not found in any of the cultivars in any month of the year. Overall, the chemovariety at the isoflavonoid level in the three P. mirifica cultivars, which have distinct differences in their botanical characteristics, were confirmed. The net yield of total isoflavonoidin P. mirifica leaf (41.68 ± 4.01 mg/ 100 g powder) still lower than in tuber (80.67 ± 4.11 mg/100 g powder) about two times.Citation27) However, in terms of plant cultivation, leaves harvesting is more worthwhile than tuber collection because tubers spend at least three years to grow maturely whereas leaves need only three months and can be collected quarterly. For the comparison of isoflavonoid yield between tuber and leaf harvested per year in general, the isoflavonoid yield harvested in leaf is higher than tuber 6.20 times per year.
The isoflavonoid pattern in P. mirifica leaves over a 12-month cultivation period remains to be ascertained if this pattern holds for other years and so is a real trend or not. However, with that caveat in mind, the puerarin, daidzin, genistin, and daidzein levels in the crude leaf-extracts were found to be strongly, but differentially correlated with either the rainfall amount or the average temperature during the 12-month cultivation period assayed within the same plant cultivar in different months (seasons). In addition, while the plant genetics per se (as morphologically distinct cultivars in this study) also strongly correlated to the differences in the isoflavonoid types and levels found in the leaves over this 12-month assay period. The extraction method employed in this study was efficient to extract a broad range of polar isoflavones e.g. daidzin, daidzein, genistin, and genistein, and the isolated components showed 98–99% purity.Citation28) Therefore, the non-detectable genistein in leave crude extract was not from this extraction method. Interestingly, genistein, the isoflavonoid not detected in this study in the crude leaf-extracts, has been found previously in the tubers of these three P. mirifica cultivars, although in smaller amounts compared with that of puerarin, genistin, daidzin, and daidzein.Citation27) The differences in the synthesis and/or accumulation of isoflavonoids between leaf and tuberous tissues is probably that genistein is an aglycosidic compound synthesized in the leaf, which is not stable enough for transportation or storage in tuberous root, it is converted into genistin, (by glucose conjugation) to increase both its stability (through the protection of reactive nucleophilic groups) and water solubility.Citation29,30) It is possible that genistin is then cleaved to the aglycosidic form after being transported into the tubers, accounting for the detection of genistin in the leaves but genistein in the tubers. Indeed, glycoside hydrolase has been reported in roots of Medicago truncatula, another legume plant in the same family.Citation31) This enzyme can catalyze the hydrolysis of the glycosidic linkage in genistin to convert it to genistein. In contrast, a proteomic analysis of soybean leaves did not report the finding of any enzymes with glycoside hydrolase activity,Citation32) and so the actual case for P. mirifica remains to be resolved.
YES assay
Genistein and genistin showed a higher estrogenic activity in the YES-hERβ than the YES-hERα assay system, in agreement with that previously reported.Citation15) The sensitivity of all tested compounds determined by YES-hERαΔsnq2 and YES-hERβΔsnq2 was higher than the corresponding wildtype Y190 yeast strains (YES-hERα and YES-hERβ), which correlated well with a previous study.Citation33) In wildtype Y190 yeast strains, anti-estrogenic activity could be determined by using tamoxifen against both E2Citation34) and P. mirifica tuber crude extract.Citation15) Furthermore, the anti-estrogenic activity of medicinal plants was also demonstrated in difference yeast strains.Citation35) Snq2 is a member of yeast ATP-binding cassette (ABC) transporter family,Citation36) which is an exporter of multiple cytotoxic and steroid compounds in S. cerevisiae. Deletion of SNQ2 gene was shown to increase the intracellular steroid level.Citation37) Double deletion of PDR5, another member of the yeast ABC transporter family, and Snq2 increased the uptake of several compounds,Citation38) while deletion of three yeast ABC transporters (PDR5, SNQ2, and YOR1) further increased the detection sensitivity for phytoestrogens by hERα.Citation39) Therefore, deletion of the SNQ2 gene causes an increased sensitivity to a wide range of compounds because the Δsnq2 cells are unable or less able to efflux these compounds out of the cell. It is worthwhile to examine anti-estrogenic activity in plant leaves by using YES-hERαΔsnq2 and YES-hERβΔsnq2. Although overall investigation shown that isoflavonoid contents in some month were not correlated with estrogenic activity that not obviously difference in YES-hERβΔsnq2, it was probably that the estrogenic activity assay employed crude extract which might contain other compounds exerting estrogenic activity, for example, deoxymiroestrol that belong to phytoestrogen group as same as isoflavonoid.Citation40)
There are growing interests in at least two major classes of isoflavonoids, namely the glycoside and aglycone forms.Citation41) The latter have been demonstrated to be active in the MCF-7 cell activityCitation42) and YESCitation43) assays, as well as in correlation analysis with its antioxidant activity.Citation44) However, the glycoside isoflavonoids, including daidzin and genistin, can be metabolized into the more active aglycone form after oral ingestion from either normal intestinal floraCitation25) or within the liver from microsome-associated enzymes.Citation45) Natural daidzin and genistin could, therefore, be an indirect, yet rich, source of aglycones for human consumption.
The results of this study suggest that P. mirifica leaves might be a novel source for the industrial scale extraction of isoflavonoids. Although there has been an increasing interest in isoflavonoid production from plant tissue grown in vitro from soy,Citation11) P. lobataCitation10) and P. mirifica,Citation46) so as to make the production independent of the influence of variation from changes in the physical environment, this in vitro approach is often more expensive than a conventional large scale production method using farm grown plants, which for example, can produce an abundant mass of leaves.
Supplemental material
The supplemental material for this paper is available at http://dx.doi.org/10.1080/09168451.2014.910091.
Supplemental Table 1
Download PDF (59.4 KB)Acknowledgments
Wichai Cherdshewasart and Jutarmas Jungsukcharoen were supported by the Higher Education Research Promotion and National Research University Project of Thailand, Office of the Higher Education Commission (FW 0663I); The Thailand Research Fund (DBG5180025); the Science for Locale Project under Chulalongkorn University Centenary Academic Development Plan (2008-2012). Chuenchit Boonchird was partially supported by the Faculty of Science, Mahidol University. Binar Asrining Dhiani received support from DIKTI Overseas Scholarships Ministry of National Education Republic of Indonesia.
Notes
Abbreviations: RP-HPLC, reverse phase high-performance liquid chromatography; P. mirifica, Pueraria mirifica; YES, yeast estrogen screen; hER, human estrogen receptors; E2, 17β-estradiol; RT, room temperature; DMSO, dimethyl sulfoxide; SDA, medium supplemented with adenine; RP, Relative Potency; SNQ2, Sensitivity to 4-NitroQuinoline-N-oxide; oNPG, ortho-nitrophenyl-β-galactoside; SEM, standard error mean; LOD, limit of detection; NA, not applicable.
References
- Yoshiki Y, Takagi S, Watanabe M, Okubo K. Fractionation of soybean functional glycosides from soy-waste based on the chemical reaction of soyasaponin β g. Food Chem. 2005;93:591–597.10.1016/j.foodchem.2004.10.042
- Zhao SH, Zhang LP, Gao P, Shao ZY. Isolation and characterisation of the isoflavones from sprouted chickpea seeds. Food Chem. 2009;114:869–873.10.1016/j.foodchem.2008.10.026
- Kuhnle GGC, Dell’Aquila C, Aspinall SM, Runswick SA, Joosen AMCP, Mulligan AA, Bingham SA. Phytoestrogen content of fruits and vegetables commonly consumed in the UK based on LC-MS and 13C-labelled standards. Food Chem. 2009;116:542–554.10.1016/j.foodchem.2009.03.002
- Kuhnle GGC, Dell'Aquila C, Runswick SA, Bingham SA. Variability of phytoestrogen content in foods from different sources. Food Chem. 2008;113:1184–1187.
- Zafra-Gómez A, Garballo A, García-Ayuso LE, Morales JC. Improved sample treatment and chromatographic method for the determination of isoflavones in supplemented foods. Food Chem. 2010;123:872–877.10.1016/j.foodchem.2010.05.009
- Cornwell T, Cohick W, Raskin I. Dietary phytoestrogens and health. Phytochemistry. 2004;65:995–1016.10.1016/j.phytochem.2004.03.005
- Schwartz H, Sontag G, Plumb J. Inventory of phytoestrogen databases. Food Chem. 2009;113:736–747.10.1016/j.foodchem.2008.09.051
- Kim GN, Song JH, Kim ES, Choi HT, Jang HD. Isoflavone content and apoptotic effect in HT-29 cancer cells of a soy germ extract. Food Chem. 2012;130:404–407.10.1016/j.foodchem.2011.07.063
- Kirakosyan A, Kaufman PB, Warber S, Bolling S, Chang SC, Duke JA. Quantification of major isoflavonoids and L-canavanine in several organs of kudzu vine (Pueraria montana) and in starch samples derived from kudzu roots. Plant. Sci. 2003;164:883–888.10.1016/S0168-9452(03)00077-3
- Matkowski A. In vitro isoflavonoid production in callus from different organs of Pueraria lobata (Wild.) Ohwi. J. Plant Physiol. 2004;161:343–346.10.1078/0176-1617-01145
- Gueven A, Knorr D. Isoflavonoid production by soy plant callus suspension culture. J. Food Eng. 2011;103:237–243.10.1016/j.jfoodeng.2010.10.019
- Cherdshewasart W, Traisup V, Picha P. Determination of the estrogenic activity of wild phytoestrogen-rich Pueraria mirifica by MCF-7 proliferation assay. J. Reprod. Develop. 2008;54:63–67.10.1262/jrd.19002
- Cherdshewasart W, Kitsamai Y, Malaivijitnond S. Evaluation of the estrogenic activity of the wild Pueraria mirifica by vaginal cornification assay. J. Reprod. Develop. 2007;53:385–393.10.1262/jrd.18065
- Cherdshewasart W, Sriwatcharakul S, Malaivijitnond S. Variance of estrogenic activity of the phytoestrogen-rich plant. Maturitas. 2008;61:350–357.10.1016/j.maturitas.2008.09.017
- Boonchird C, Mahapanichkul T, Cherdshewasart W. Differential binding with ERα and ERβ of the phytoestrogen-rich plant Pueraria mirifica. Braz. J. Med. Biol. Res. 2010;43:195–200.10.1590/S0100-879X2009007500026
- Wong KH, Li GQ, Li KM, Razmovski-Naumovski V, Chan K. Kudzu root: Traditional uses and potential medicinal benefits in diabetes and cardiovascular diseases. J. Ethnopharmacol. 2011;134:584–607.10.1016/j.jep.2011.02.001
- Wang LZ, Yang B, Du XQ, Yi C. Optimisation of supercritical fluid extraction of flavonoids from Pueraria lobata. Food Chem. 2008;108:737–741.10.1016/j.foodchem.2007.11.031
- Manonai J, Chittacharoen A, Theppisai U, Theppisai H. Effect of Pueraria mirifica on vaginal health. Menopause. 2007;14:919–924.10.1097/gme.0b013e3180399486
- Manonai J, Chittacharoen A, Udomsubpayakul U, Theppisai H, Theppisai U. Effects and safety of Pueraria mirifica on lipid profiles and biochemical markers of bone turnover rates in healthy postmenopausal women. Menopause. 2008;15:530–535.10.1097/gme.0b013e31815c5fd8
- Kim HY, Hong JH, Kim DS, Kang KJ, Han SB, Lee EJ, Chung RW, Song KH, Sho KA, Kwack SJ, Kim SS, Park KL, Lee SK, Kim MC, Kim CM, Song IS. Isoflavone content and estrogen activity in arrowroot Puerariae Radix. Food Sci. Biotechnol. 2003;12:29–35.
- Malaivijitnond S, Chansri K, Kijkuokul P, Urasopon N, Cherdshewasart W. Using vaginal cytology to assess the estrogenic activity of phytoestrogen-rich herb. J. Ethnopharmacol. 2006;107:354–360.10.1016/j.jep.2006.03.026
- Cherdshewasart W, Subtang S, Dahlan W. Major isoflavonoid contents of the phytoestrogen rich-herb Pueraria mirifica in comparison with Pueraria lobata. J. Pharm. Biomed. Anal. 2007;43:428–434.10.1016/j.jpba.2006.07.013
- Cherdshewasart W, Sriwatcharakul S. Major isoflavonoid contents of the 1-year-cultivated phytoestrogen-rich herb, Pueraria mirifica. Biosci. Biotechnol. Biochem. 2007;71:2527–2533.10.1271/bbb.70316
- Malaivijitnond S, Chansri K, Cherdshewasart W, Jareonporn S, Trisomboon H. Effects of Pueraria mirifica, an herb containing phytoestrogens, on reproduction in rodents and monkeys. J. Exp. Zool. Comp. Exp. Biol. 2006;305:152–152.
- Villeneuve DL, Blankenship AL, Giesy JP. Derivation and application of relative potency estimates based on in vitro bioassay results. Environ. Toxicol. Chem. 2000;19:2835–2843.10.1002/etc.v19:11
- Taiz L, Zeiger E, editors. Plant Physiology. 3rd Ed. Sunderland: Sinauer Associates; 2002. p. 591–594.
- Cherdshewasart W, Subtang S, Dahlan W. Major isoflavonoid contents of the phytoestrogen rich-herb Pueraria mirifica in comparison with Pueraria lobata. J. Pharm. Biomed. Anal. 2007;43:428–434.10.1016/j.jpba.2006.07.013
- Yang F, Ma Y, Ito Y. Separation and purification of isoflavones from a crude soybean extract by high-speed counter-current chromatography. J. Chromatogr. A. 2001;928:163–170.10.1016/S0021-9673(01)01144-X
- Gachon CMM, Langlois-Meurinne M, Saindrenan P. Plant secondary metabolism glycosyltransferases: the emerging functional analysis. Trends Plant Sci. 2005;10:542–549.10.1016/j.tplants.2005.09.007
- Malaivijitnond S. Medical applications of phytoestrogens from the Thai herb Pueraria mirifica. Front Med. 2012;6(1):8–21.10.1007/s11684-012-0184-8
- Schenkluhn L, Hohnjec N, Niehaus K, Schmitz U, Colditz F. J. Proteomics. 2010;79:759–768.
- Xu C, Garrett WM, Sullivan J, Caperna TJ, Natarajan S. Separation and identification of soybean leaf proteins by two-dimensional gel electrophoresis and mass spectrometry. Phytochemistry. 2006;67:2431–2440.10.1016/j.phytochem.2006.09.002
- Sievernich A, Wildt L, Lichtenberg-Fraté H. In vitro bioactivity of 17β-estradiol. J. Steroid Biochem. 2004;92:455–463.10.1016/j.jsbmb.2004.09.004
- Mahapanichkul T. Evaluation of estrogenic activity of Kwao Krua plants using yeast estrogen screen (YES) method [Master Thesis]. Mahidol University; 2006. p. 67–69. ISBN 974-04-7491-8.
- Kim IG, Kang SC, Kim KC, Choung ES, Zee OP. Screening of estrogenic and antiestrogenic activities from medicinal plants. Environ. Toxicol. Pharmacol. 2008;25:75–82.10.1016/j.etap.2007.09.002
- Decottignies A, Lambert L, Catty P, Degand H, Epping EA, Moye-Rowley WS, Balzi E, Goffeau A. Identification and characterization of SNQ2, a new multidrug ATP binding cassette transporter of the yeast plasma-membrane. J. Biol. Chem. 1995;270:18150–18157.
- Mahé Y, Parle-McDermott A, Nourani A, Delahodde A, Lamprecht A, Kuchler K. The ATP-binding cassette multidrug transporter Snq2 of Saccharomyces cerevisiae: A novel target for the transcription factors Pdr1 and Pdr3. Mol. Microbiol. 1996;20:109–117.10.1111/mmi.1996.20.issue-1
- Mitterbauer R, Weindorfer H, Safaie N, Krska R, Lemmens M, Ruckenbauer P, Kuchler K, Adam G. A sensitive and inexpensive yeast bioassay for the mycotoxin zearalenone and other compounds with estrogenic activity. Appl. Environ. Microb. 2003;69:805–811.10.1128/AEM.69.2.805-811.2003
- Hasenbrink G, Wildt L, Ludwig J, Lichtenberg-Frate H. Estrogenic effects of natural and synthetic compounds assessed in Saccharomyces cerevisiae. Febs J. 2006;273:80–80.
- Chansakaow S, Ishikawa T, Seki H, Sekine K, Okada M, Chaichantipyuth C. Identification of deoxymiroestrol as the actual rejuvenating principle of “Kwao Keur”, Pueraria mirifica. The known miroestrol may be an artifact. J. Nat. Prod. 2000;63:173–175.10.1021/np990547v
- Haron H, Ismail A, Azlan A, Shahar S. Daidzein and genestein contents in tempeh and selected soy products. Food Chem. 2009;115:1350–1356.10.1016/j.foodchem.2009.01.053
- Matsumura A, Ghosh A, Pope GS, Darbre PD. Comparative study of oestrogenic properties of eight phytoestrogens in MCF7 human breast cancer cells. J. Steroid Biochem. 2005;94:431–443.10.1016/j.jsbmb.2004.12.041
- Lin CC, Tsai YL, Ho CT, Teng SC. Determination of the differential estrogenicity of isoflavonoids by E2-ER-ERE-dependent gene expression in recombinant yeast and MCF-7 human breast cancer cells. Food Chem. 2008;108:719–726.10.1016/j.foodchem.2007.11.020
- Cherdshewasart W, Sutjit W. Correlation of antioxidant activity and major isoflavonoid contents of the phytoestrogen-rich Pueraria mirifica and Pueraria lobata tubers. Phytomedicine. 2008;15:38–43.10.1016/j.phymed.2007.07.058
- Cherdshewasart W, Sriwatcharakul S. Metabolic activation promotes estrogenic activity of the phytoestrogen-rich plant. Maturitas. 2008;59:128–136.10.1016/j.maturitas.2008.01.002
- Udomsuk L, Jarukamjorn K, Tanaka H, Putalun W. Improved isoflavonoid production in Pueraria candollei hairy root cultures using elicitation. Biotechnol. Lett. 2011;33:369–374.10.1007/s10529-010-0417-3
