Abstract
This study showed that both water extracts (WAF-W) and ethanol extracts (EAF-W) of Auricularia fuscosuccinea (Montagne) Farlow, white strain (AF-W) demonstrated significantly stronger antioxidative effects than did commercially available Tremella fuciformis sporocarp extracts (WSK; with the exception of EAF-W in terms of superoxide radical scavenging activity levels). The moisture retention capacity of WAF-W is as potent as that of sodium hyaluronate (SHA), but less than that of WSK. No corrugation or fissures were observed in WAF-W film; only the SHA and WSK films demonstrated such effects in low-moisture conditions. The WAF-W solution also exhibited stable viscosity at high temperatures, indicating that the WAF-W film was more stable compared with the SHA and WSK films. WAF-W induced no adverse effects when a hen’s egg test was performed on the chorioallantoic membrane (CAM). This study demonstrated that WAF-W exhibits excellent potential as a topical material for skin moisturizing and anti-aging effects.
Graphical Abstract
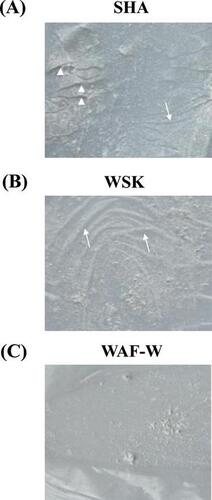
(A) Corrugation (arrow) and fissures (arrow head) were observed on the SHA film; (B) Only corrugation (arrow) on the WSK film; (C) no corrugation or fissures on WAF-W film.
Oxidative stress is a major cause of age-related diseases.Citation1) Oxidative stress can cause collagen reduction, reducing the skin’s elasticity and damaging the skin barrier.Citation2Citation–Citation4) As a result, the water content of the skin decreases, becoming susceptible to dryness.Citation5) Numerous natural extracts have been reported to possess potent antioxidative effects such as grapes, blue raspberries, and mushrooms.Citation6Citation–Citation8) These natural ingredients have also been shown to substantially improve skin complexion, elasticity, and moisture retention.Citation9Citation–Citation11)
Fungal extracts have been widely applied in food and topical formulationsCitation12−Citation14) and used as raw materials in cosmetics, in which they support skin hydration.Citation15−Citation17) The fruit bodies of Tremella fuciformis are popular fungi in Taiwanese and Chinese cuisines.Citation18) Various studies have reported that T. fuciformis can reduce wrinkles and smooth fine lines by increasing the presence of superoxide dismutase (SOD) or by acting as a potent antioxidant in the body, particularly in the skin.Citation19)
The fungus used in this study, Auricularia fuscosuccinea (Montagne) Farlow, white strain (AF-W), is a white mutation variety of Auricularia fuscosuccinea (Montagne) Farlow. The phenotype was selected from the wild native Auricularia fuscosuccinea (AF) (Montagne) Farlow by the Taiwan Agricultural Research Institute, Council of Agriculture in 1990.Citation20) The cultivation technique was successfully transferred to local farmers in 1992. However, AF-W remains unpopular in foods, or topical and biomedical applications because of its high price. It has been suggested that AF contains high levels of polysaccharides and is a film-forming polymer, similar to sodium hyaluronate (SHA).Citation17) A high-quality film polymer should demonstrate excellent aesthetic characteristics, exhibiting a clear, smooth film, and a stable viscosity for use in pharmaceutical formulations.Citation21Citation–Citation25)
This study involved investigating the biological activity of AF-W and its possible applications in topical formulations. The antioxidative and moisture retention capacity of AF-W water extracts (WAF-W) and ethanol extracts (EAF-W) were examined and compared to those of SHA and the Tremella fuciformis sporocarp extract (WSK). In addition, the film formation, film contraction, viscosity stability, and irritation scores (ISs) of the WAF-W extracts were also evaluated.
Materials and methods
Fungal material
The AF-W was provided by Mr Chen, a farmer from Nantou County, Taiwan, who cultivated the AF-W based on the guidance of the Taiwanese Agricultural Research Institute. The WSK extraction mean molecule was >1,000,000 (Kalin Enterprise Co., Ltd., Taichung, Taiwan).
Chemicals
The 1, 1-diphenyl-2-picrylhydrazyl (DPPH), phosphate buffered saline (pH = 6.6), vitamin C (ascorbic acid), nicotiamide adenine dinucleotide (NADH), ethylenediaminetetraacetatic acid (EDTA-2Na), trichloroacetic acid, potassium ferricyanide (K3Fe(SCN)6), ferrous sulfate (FeSO4·7H2O), phenazine methosulphate (PMS), and nitro blue tetrazolium (NBT) were purchased from Sigma-Aldrich Co. Ltd. (St. Louis, MO, USA.). The iron chloride (III) was purchased from Acros Co., Ltd. (Japan) and the sodium hydroxide (NaOH) was purchased from Showa Co. Ltd. (Japan).
EAF-W, WAF-W, and WSK sample preparation
EAF-W preparation: fruiting bodies of AF-W were weighed and sliced into small pieces. The sample was subsequently agitated in 50% ethyl alcohol (sample to ethyl alcohol ratio, 1:10) at 50 °C for 3 h. The samples were collected, filtered through Whatman No. 1 paper, and concentrated in vacuum under reduced pressure. The sample was then dried in dessiccator and stored at −80 °C.
WAF-W preparation: fruiting bodies of AF-W were washed, weighed, and ground into small pieces. The samples were subsequently immersed in double-distilled water (sample to water ratio, 1:10 by weight), boiled at 100 °C, and maintained at 80 °C for 60 min. After boiling, the samples were filtered through Whatman No.1 paper using vacuum assistance. The WAF-W was then freeze-dried (Fd-20L-6S, Kingmech, Co. Ltd., Taipei, Taiwan) and stored at −20 °C. Prior to being used, the samples were dissolved in double-distilled water at a concentration of 50 mg/mL to prepare a stock solution.
WSK preparation: WSK is an acidic hetero-polysaccharide extracted from the edible fruit bodies of the Chinese “silver ear” mushroom. WSK powder was purchased and dissolved in double-distilled water at a concentration of 50 mg/mL to prepare a stock solution.
DPPH free radical scavenging assay
The scavenging activity levels of DPPH radicals was determined using a previously described method.Citation26) Various concentrations (0.1, 1, 2, 5, and 10 mg/mL) of WAF-W, EAF-W, or WSK were prepared in 50 μL samples and mixed with 150 μL of freshly prepared 0.1 mM DPPH in ethanol. Double-distilled water was used as a vehicle control, and ascorbic acid was used as a positive control. The mixture remained in darkness for 30 min. Subsequently, the DPPH absorbance was measured at 517 nm using an ELISA reader (Tecan, Austria). The percent activity was calculated using the following equation: % activity = (1 − (ASample/ABlank) × 100. The EC50 value, which is the sample concentration required for 50% inhibitory activity, was determined using interpolation. Each test was performed in triplicate.
Reducing power assay
The reducing power of the WAF-W, EAF-W, and WSK were determined using a previously described method.Citation27) Briefly, 100 μL samples at various concentrations (in double-distilled water) were mixed with PBS (100 μL, 2 M, pH 6.6) and K3Fe(CN)6 (100 μL, 1% w/v). Double-distilled water was used as the negative control, and vitamin C was used as the positive control. The mixture was incubated at 50 °C for 20 min in a water bath. Trichloroacetic acid (10% w/v, 100 μL) was added, and the resulting mixture was centrifuged at 3000 rpm for 10 min. The supernatant (100 μL) was combined with distilled water (100 μL) and a FeCl3 solution (20 μL, 0.1 % w/v). The absorbance was then measured at 700 nm using a V630 UV–Vis Spectrophotometer (JASCO Co. Ltd., Japan). An increased level of absorbance in the reaction mixture indicated an increased level of reducing power. Interpolating the linear regression analysis of Huang demonstrated an absorbance of 0.5 at a reducing power of EC50.Citation28)
Ferrous-chelating capacity assay
The ferrous-chelating capacities of WAF-W, EAF-W, and WSK were determined using the method of Dinis.Citation29) Briefly, 25 μL aliquots of a WAF-W, EAF-W, and WSK samples at various concentrations (0.1–1 mg/mL) were prepared using stock solution and mixed with 175 μL of methanol, 25 μL of 400 μM FeCl2 4H2O, and 25 μL of 2 mM ferrozine. The mixture rested for 10 min and the absorbance was subsequently measured at 562 nm using an ELISA reader. EDTA was used as the positive control and the test was conducted in triplicate.
Superoxide-radical scavenging assay
The superoxide anion-scavenging ability levels were measured using a previously described method.Citation30) The PMS-NADH system generates superoxide radicals that reduce NBT to a purple-colored diformazan compound. Reaction solutions that contained various concentrations of WAF-W, EAF-W, and WSK (50 μL, 0.1, to 1 mg/mL) were mixed with PMS (80 μM), NADH (1248 μM), and NBT (200 μM) in PBS (0.1 M, pH 7.4) and incubated at room temperature for 5 min. Double-distilled water was used as the negative control, and vitamin C was used as the positive control. The color was assessed at 560 nm against blank samples. The superoxide anion radical-scavenging percentage was calculated using the following equation: scavenging effect (%) = 1 − (ASample 560 nm/AControl 560 nm) × 100.
Total phenols
The total phenolic content was determined by Liao’s method27. A volume of 0.3 mL of samples at a concentration of 0.5 mg/mL was mixed with 2.4 mL of distilled water and 0.3 mL Folin–Ciocalteu reagent. Sodium carbonate (20%, 0.6 mL) was added to the reaction mixture and allowed to stand for 30 min. The absorbance at 730 nm was measured and compared to a gallic acid calibration curve and expressed as mg of gallic acid equivalent (GAE) per gram of sample.
Moisture retention capacity assay
The moisture retention capacity of WAF-W, WSK, and SHA was measured using a modified version of the method proposed by Li et al.Citation31) To summarize, 0.01 g (10 mg) of 2 mg/mL WAF-W, WSK, and SHA were added to a 9-cm Whatman No.1 paper at 24 °C and 68% relative humidity (RH) for 25 min. The weight change of the filter paper was recorded every minute to estimate the kinetic aspect of water loss over time.
Film formation and film contraction assay
Film formation assay: 1 g of 2 mg/mL WAF-W, WSK, or SHA solution was evenly spread on separate overhead projector sheets (30 cm) at 25 °C and 48% RH for 4 h. After film formation, TiO2 powder was sprinkled on the film to observe structural surface changes.
Film contraction assay: the film contraction was measured using a modified version of the method proposed by Li et al.Citation31) To summarize, 2 mg/mL of WAF-W, WSK, or SHA solution was sprayed on separate 15 cm × 15 cm Teflon plates. The plates were exposed to 30 °C and 88% RH conditions for 24 h for film formation and subsequently underwent a dewetting process at 25 °C and 23% RH for 4 h. The film area was expressed as a percentage of the film area at 88% RH.
Measure viscosity changes with temperature
To measure viscosity changes, 100 mL of 1, 3, and 5 mg/mL water solutions were prepared using WAF-W, WSK, and SHA, and heated to 60, 80, and 100 °C, respectively. Viscosity was measured using a viscometer (Brookfield DV-E Viscometer, USA), No. 62 spindle code, and a scrolling speed that ranged from 5 to 100 rpms. Viscosity changes were recorded for each sample at various temperatures.
Hen’s egg test-chorioallantoic membrane (HET-CAM)
The HET-CAM test protocol was modified from the method of Wilson and Steck.Citation32) Fresh (not older than 7 d), clean, and fertile 50–60 g chicken eggs were selected and placed in an incubator with a rotating tray. The eggs were incubated at 37.5 ± 0.5 °C and 55 ± 5% RH. The eggs were subsequently assessed using candle light, and any nonviable or defective eggs were discarded. On day 8, the large end of each egg was positioned upward for 1 d and their air cells were marked. These carefully marked sections were cut using a rotating dental saw blade and pared away. The inner membranes were moistened using 300 μL of 0.9% NaCl and the eggs were placed into the incubator for 30 min at maximum. The eggs were subsequently removed from the incubator and decanted in 0.9% NaCl solution. Their inner membranes were carefully removed using forceps, ensuring that these remained intact. Subsequently, 0.3 mL of test substances was directly applied to the CAM surface. The reactions of the CAM were observed for 300 s. At 0.5, 2, and 5 min, the following observations should be made: hyperplasia, hemorrhage, and coagulation (maximal value of 21).Citation33) Regarding the IS, 0–0.9 represents no irritation, 1–4.9 represents slight irritation, 5–8.9 represents moderate irritation, and 9–21 represents serious irritation.Citation34) A 0.1 M NaOH solution was used as the positive control group.
Statistical analysis
Three samples were prepared for each assay. The results were expressed as means and standard deviations. The antioxidant data analysis included a one or two ways analysis of variance, followed by a Tukey test (p < 0.05) and a correlation test using the SigmaStat 3.5 program.
Results and discussion
EAF-W and WAF-W yields
The EAF-W and WAF-W yields obtained using alcohol and hot water extraction were 2.09 ± 0.24% and 1.99 ± 0.26%, respectively. No significant difference was observed between the yields of the two extraction methods.
DPPH free radical scavenging activity
The DPPH free radical scavenging activity levels of EAF-W and WAF-W were significantly stronger than were those of WSK (Fig. ). The DPPH scavenging activity levels of extracts are dose dependent. The EC50 of the scavenging activity of EAF-W, WAF-W, and WSK were 1.15, 1.31 mg/mL, and not determined (>10 mg/mL), respectively. The scavenging activity levels at 1 mg/mL of EAF-W, WAF-W, and WSK were 55, 47.15, and 5.69%, respectively. The DPPH scavenging activity levels of EAF-W and WAF-W at concentrations greater than 2 mg/mL were extremely similar (Fig. ).
Reducing power
The reducing powers of EAF-W and WAF-W were significantly greater than were those of WSK in a dose-dependent manner (Fig. ). The EC50 of the reducing power of EAF-W and WAF-W were 1.22 and 1.71 mg/mL, respectively (Fig. ). The reducing power of WSK was the weakest of the three samples and its EC50 could not be determined. At 10 mg/mL of WSK, the reducing power was only 12.96% (Fig. ). Vitamin C served as the positive control (EC50: 0.11 mg/mL; Fig. ).
Ferrous-chelating capacity assay
The ferrous-chelating capacities of EAF-W and WAF-W were extremely potent in a dose-dependent manner (Fig. ). The EC50 of EAF-W and WAF-W were 5.66 and 3.88 mg/mL, respectively (Fig. ). The Fe2+-chelating capacities of 5 and 10 mg/mL WSK were only 22.43 and 33.94%, respectively (Fig. ). This experiment involved using EDTA as the positive control, yielding an EC50 value of 0.42 mg/mL.
Fig. 3. Ferrous ion chelating capacity of the EAF-W, WAF-W, and WSK samples.
Notes: EDTA-2Na served as the positive control. The values are expressed as means ± SD (n = 3). The lowercase letters above the bars of the four samples at the same concentration indicate a significant difference (p < 0.05).
The results are consistent with those of Zhao, which maintained that numerous auricularia-class alcohol extracts demonstrated potent ferrous-chelating capacities.Citation35) When iron is combined with hydrogen peroxide, it induces lipid oxidation, deteriorating the nutrition and safety of foods.Citation36) Therefore, the strong ferrous-chelating capacity of auricularia-class extracts can potentially prevent the oxidation of metallic ions.
Superoxide-radical scavenging assay
Of the samples, WAF-W demonstrated the highest levels of superoxide-radical scavenging activity in a dose-dependent manner. The 5 mg/mL WAF-W superoxide-radical scavenging activity was 63.12% and the EC50 was 1.87 mg/mL (Fig. ). The superoxide-radical scavenging activity levels of 5 mg/mL EAF-W and WSK were 19.05 and 11.35%, respectively (Fig. ). At 10 mg/mL, the superoxide-radical scavenging activity levels of EAF-W and WSK remained below 50% (Fig. ). Therefore, the EC50 could not be determined. The EC50 of vitamin C, which served as the positive control, was 0.26 mg/mL (Fig. ).
Fig. 4. Scavenging activity levels of the superoxide radicals of the EAF-W, WAF-W, and WSK samples.
Notes: Vitamin C served as the positive control. The values are expressed as means ± SD (n = 3). The lowercase letters above the bars of the four samples at the same concentration indicate a significant difference (p < 0.05).
Total phenols
Naturally occurring antioxidant components polyphenols with antioxidant capacity could scavenge reactive chemical species as well as reduce oxidative damage. Total phenols of WAF-W and EAF-W are 35.9 mg GAE/g sample and 195.5 mg GAE/g sample, respectively. The result indicated that EAF-W may exhibit much more potent antioxidant capacity than WAF-W did.
Moisture retention capacity test
Fig. (A) shows the kinetic moisture retention capacity curves of the samples and water between 0 and 25 min. The moisture retention capacity and temperature demonstrated strong negative relationships (r2: 0.987–0.995; Fig. (A)). The time required for 50% evaporation of WAF, SHA, WAF-W, and water were 16.16, 14.45, 14.19, and 12.03 min, respectively (Fig. (B)).The WSK demonstrated the strongest moisture retention capacity, followed by WAF-W and sodium SHA. The moisture retention capacity of WAF-W was nearly equal to that of SHA (Fig. (B)).
Fig. 5. Moisture-retention and evaporation rates of SHA, WSK, WAF-W solutions, and water.
Notes: (A) Kinetic moisture-retention capacity of the 2 mg/mL SHA, WSK, WAF-W solutions, and water at 25 min; and (B) the time required for 50% of sample to evaporate. The values are expressed as means ± SD (n = 3). The samples with different lowercase letters indicate a significant difference (p < 0.05).
SHA is a polysaccharide composed of repeating disaccharide units of D-glycuronic acid and N-acetyl glucosamine.Citation37) SHA is currently recognized an optimal moisture-retention ingredients.Citation38) Therefore, the results indicate that both WAF-W and WSK demonstrate potent moisture retention capacities which may result from high content of polysaccharidesCitation39).
Film formation and film contraction assay
At identical temperature, humidity, and time conditions, the samples demonstrated varying film appearances. Corrugation and fissures were observed on the SHA film (Fig. (A)). No corrugation was evidenced, however, fissures were observed on the WSK film (Fig. (B)). The WAF-W film lacked both corrugation and fissures (Fig. (C)); this crack-free appearance indicates that the WAF-W demonstrated the strongest film formation capacity of the samples.
Fig. 6. Surface Morphologies of the Forming SHA, WSK, and WAF-W films at 25 °C and 48% RH for 4 h.
Notes: (A) Corrugation (arrow) and fissures (arrow head) were observed on the SHA film; (B) Only corrugation (arrow) was observed on the WSK film; and (C) no corrugation or fissures were observed on the WAF-W film.
In Fig. , the film volumes of the WAF-W, WSK, and SHA films at 23% RH shrunk to 28, 11, and 11%, respectively, at 88% RH. The WAF-W film exhibited the lowest film contraction rate in low-moisture conditions, demonstrating that the WAF-W film has a smooth, crack-free appearance that may decrease feelings of discomfort or constraint following its application.
Fig. 7. Film contraction assay of the forming SHA, WSK, and WAF-W films.
Notes: The film areas at RH 88% were recognized as 100% (empty bars). The relative film contraction areas were measured at RH 23% (gray bars). The values are expressed as means ± SD (n = 3). The lowercase letters above the bars indicate a significant difference (p < 0.05).
Measure viscosity changes with temperature
Controlling viscosity, heating conditions, and the thermal expansion of the film and substrate are critical factors when preparing thin films. Fig. shows that increasing the temperature from 25 to 100 °C reduced the viscosity of the samples (Fig. (A)–(C)). WSK was the most viscous fluid followed by SHA and WAF-W (Fig. (A)–(C)). Although the viscosities of 5 mg/mL SHA, and 3 and 5 mg/mL WSK are greater than 1000 cP (Fig. (A) and (B)), at 100 °C the loss in solution viscosity compared with that at 25 °C was 44, 71, and 67%, respectively (Fig. (D), and (E)). The results demonstrate that the viscosities of SHA and WSK could not prevent high-temperature induced viscosity instability. By contrast, the viscosity of WAF-W is less than 20 cP (Fig. (C)) and it demonstrates high-temperature stability levels (from 60 to 100 °C), losing less than 30% viscosity (Fig. (F)); this may prevent film sagging during the curing process and could explain why no corrugation or fissures were observed on the WAF-W film (Fig. ). The results are consistent with the results of previous studies; the strongest morphologic characteristics were obtained using crack-free BBT films, which were deposited from a solution of 20 cP viscosity.Citation40)
Fig. 8 Effects of temperature and concentrations on the viscosities of the SHA, WSK, and WAF-W fluids.
Notes: The viscosities of the 1 mg/mL (empty), 3 mg/mL (gray), and 5 mg/mL (black) SHA, WSK, and WAF-W fluids were measured when the temperature was raised from 25 to 60, 80, and 100 °C ((A)–(C)). The values are expressed as means ± SD (n = 3). The viscosity loss was determined by comparing with the viscosity at 25 °C ((D)–(F)).
Hen’s egg test-chorioallantoic membrane (HET-CAM)
At concentrations of 1 and 5 mg/mL, the WAF-W induced no hyperemia, hemorrhage, or coagulation in the CAM (Table ). The IS of WAF-W was 0, which was the same as that of the 0.9% NaCl control (Table ), indicating that WAF-W causes no skin irritation. The positive control comprised 0.1 NaOH, which can induce hyperplasia and hemorrhage in 0.5 M min and induce coagulation in 2 min. The IS value of NaOH was 19, indicating that NaOH could induce severe irritation (Table ).
Table 1. ISs and 5-min images of WAF-W in HET-CAM assay.
In general, this study demonstrates that WAF-W exhibits the higher ferrous chelating ability and superoxide scavenging activity levels compared with the EAF-W and WSK; flavonoids, polysaccharides, and active SOD may cause these potent abilities.Citation41–Citation43) The WAF-W exhibited less DPPH free-radical scavenging activity and reducing power than did EAF-W, possibly because of the high correlation between DPPH radical-scavenging activity and total phenols.Citation44–46) In this study, the total phenols of EAF-W is much higher than of WAF-W and the result is consistent with previous reports. The reducing power is associated with reductones, which usually are strong, acidic reducing agents that contain enediols derived from oxidized sugars such as ascorbic acid.Citation47) The results indicated that EAF-W contains more total phenols and ascorbic acid than WAF-W dose. The WSK exhibited the lowest activity level among 4 antioxidant assays, potentially because the WSK extract contained the lowest level of antioxidant contents.
In recent studies, methanol extracts of AF-W exhibited more effective DPPH free-radical scavenging activity, reducing power, and ferrous chelating ability compared with the methanol extracts of Auricularia polytricha and T. fuciformis because the AF-W extracts contained high levels of total phenols [7.88 mg gallic acid equivalents (GAE)/g], SOD activity (2.10 U/mg)total sugars [44.73 mg dextrose equivalents (DEX)/g] and flavonoids [1.60 mg quercetin equivalents (QE)/g].Citation42) In Mau (2001) reported methanol extracts of AF-W demonstrated superior reducing power among five evaluated ear mushrooms;Citation48) however, the moisture retention capacity and antioxidative activities of WAF-W have yet to be reported. The potent moisture retention capacity and antioxidative activities of WAF-W should facilitate preventing skin dryness and reducing the elastin and collagen breakdown caused by excess oxidative stress.
Supplemental material
The supplemental material for this paper is available at http://dx.doi.org/10.1080/09168451.2014.912113.
Supplemental Fig. 1
Download MS Power Point (286.5 KB)Supplemental Fig 1. caption
Download MS Word (11.8 KB)Acknowledgments
The authors thank the National Science Council, Taiwan, ROC for its financial support (NSC 102-2622-E-241-006-CC3).
Notes
Abbreviation: AF, Auricularia fuscosuccinea (Montagne) Farlow; AF-W, Auricularia fuscosuccinea (Montagne) Farlow, white strain; WAF-W, water extracts of Auricularia fuscosuccinea (Montagne) Farlow, white strain; EAF-W, ethanol extracts of Auricularia fuscosuccinea (Montagne) Farlow, white strain; WSK, Tremella fuciformis sporocarp extracts; SHA, sodium hyaluronate; HET, hen’s egg test; CAM, chorioallantoic membrane; DPPH, 1, 1-diphenyl-2-picrylhydrazyl;NADH, nicotiamide adenine dinucleotide; EDTA-2Na, ethylenediaminetetraacetatic acid; K3Fe(SCN)6, potassium ferricyanide; FeSO4·7H2O, ferrous sulfate; PMS, phenazine methosulphate; NBT, nitro blue tetrazolium; NaOH, sodium hydroxide; ANOVA, analysis of variance; SD, Standard deviation; OHP, overhead projector; EC50, effective concentration required for 50% inhibitory activity; IS, irritation score; SOD, superoxide dismutase; GAE, gallic acid equivalents; DEX, dextrose equivalents; QE, quercetin equivalents.
References
- Khansari N, Shakiba Y, Mahmoudi M. Chronic inflammation and oxidative stress as a major cause of age-related diseases and cancer. Recent Pat. Inflamm. Allergy Drug Discov. 2009;3:73–80.10.2174/187221309787158371
- Yaar M, Gilchrest BA. Photoageing: mechanism, prevention and therapy. Br. J. Dermatol. 2007;157:874–887.10.1111/bjd.2007.157.issue-5
- Massudi H, Grant R, Braidy N, Guest J, Farnsworth B, Guillemin GJ. Age-associated changes in oxidative stress and NAD+ metabolism in human tissue. PLoS One. 2012;7: e42357, 1–9.10.1371/journal.pone.0042357
- Baek JO, Byamba D, Wu WH, Kim TG, Lee MG. Assessment of an imiquimod-induced psoriatic mouse model in relation to oxidative stress. Arch. Dermatol. Res. 2012;304:699–706.10.1007/s00403-012-1272-y
- Poljsak B, Dahmane RG, Godic A. Intrinsic skin aging: the role of oxidative stress. Acta Dermatovenerol. Alp. Panonica Adriat. 2012;21:33–36.
- Ndiaye M, Philippe C, Mukhtar H, Ahmad N. The grape antioxidant resveratrol for skin disorders: promise, prospects, and challenges. Arch. Biochem. Biophys. 2011;508:164–170.10.1016/j.abb.2010.12.030
- Kim MY, Seguin P, Ahn JK, Kim JJ, Chun SC, Kim EH, Seo SH, Kang EY, Kim SL, Park YJ, Ro HM, Chung IM. Phenolic compound concentration and antioxidant activities of edible and medicinal mushrooms from Korea. J. Agric. Food Chem. 2008;56:7265–7270.10.1021/jf8008553
- Koponen JM, Happonen AM, Mattila PH, Törrönen AR. Contents of anthocyanins and ellagitannins in selected foods consumed in Finland. J. Agric. Food Chem. 2007;55:1612–1619.10.1021/jf062897a
- Gianeti MD, Mercurio DG, Maia Campos PM. The use of green tea extract in cosmetic formulations: not only an antioxidant active ingredient. Dermatol. Ther. 2013;26:267–271.10.1111/dth.2013.26.issue-3
- Freedman BM. Topical antioxidant application augments the effects of intense pulsed light therapy. J. Cosmet. Dermatol. 2009;8:254–259.10.1111/jcd.2009.8.issue-4
- Berson DS. Natural antioxidants. J. Drugs Dermatol. 2008;7:s7–12.
- Peter-Valence F, Llarena-Hernandez C, Largeteau M, Savoie JM, Ruaudel F, Ziarelli F, Ferré E, Farnet AM. Chemical characterization of the biomass of an edible medicinal mushroom, Agaricus subrufescens, via solid-state 13C NMR. J. Agric. Food Chem. 2011;59:8939–8943.10.1021/jf2017622
- Ukawa Y, Izumi Y, Ohbuchi T, Takahashi T, Ikemizu S, Kojima Y. Oral administration of the extract from Hatakeshimeji (Lyophyllum decastes sing.) mushroom inhibits the development of atopic dermatitis-like skin lesions in NC/Nga mice. J. Nutr. Sci. Vitaminol. 2007;53:293–296.10.3177/jnsv.53.293
- Han BQ. Experimental pharmacological study on polysaccharide of Tremella fuciformis spores. Zhong Yao Tong Bao. 1982;7:35–38.
- Adachi Y, Okazaki M, Ohno N, Yadomae T. Enhancement of cytokine production by macrophages stimulated with (1→3)-beta-D-glucan, grifolan (GRN), isolated from Grifola frondosa. Biol. Pharm. Bull. 1994;17:1554–1560.10.1248/bpb.17.1554
- Wu Y, Shan L, Yang S, Ma A. Identification and antioxidant activity of melanin isolated from Hypoxylon archeri, a companion fungus of Tremella fuciformis. J. Basic Microbiol. 2008;48:217–221.10.1002/(ISSN)1521-4028
- Khamlue, R, Naksupan, N, Ounaroon, A, Saelim, N. Skin wound healing promoting effect of polysaccharides extracts from Tremella fuciformis and Auricularia auricula on the ex-vivo porcine skin wound healing model. In ICCBEE. Singapore: IACSIT Press; 2012. p. 93–98.
- Reshetnikov SV, Wasser SP, Duckman I, Tsukor K. Medicinal Value of the Genus Tremella Pers. (Heterobasidiomycetes) (Review). Int. J. Med. Mushrooms. 2000;2:169–194.
- Tsai CH, Chang RC, Chiou JF, Liu TZ. Improved superoxide-generating system suitable for the assessment of the superoxide-scavenging ability of aqueous extracts of food constituents using ultraweak chemiluminescence. J. Agric. Food Chem. 2003;51:58–62.10.1021/jf020799t
- Annual Report of Taiwan Agricultural Research Institute. Edible mushroom. Taiwan: TARI; 2009.
- Loginova Y, Shah V, Allen G, Macchio R, Farer A. Approaches to polymer selection for mascara formulation. J. Cosmet. Sci. 2009;60:125–133.
- Vijayendra SV, Shamala TR. Film forming microbial biopolymers for commercial applications-A review. Crit. Rev. Biotechnol. 2013. doi:10.3109/07388551.2013.798254.
- Felton LA. Mechanisms of polymeric film formation. Int. J. Pharm. 2013;457:423–427.
- Lippold BC, Monells Pages R. Film formation, reproducibility of production and curing with respect to release stability of functional coatings from aqueous polymer dispersions. Pharmazie. 2001;56:5–17.
- Zurdo Schroeder I, Franke P, Schaefer UF, Lehr CM. Development and characterization of film forming polymeric solutions for skin drug delivery. Eur. J. Pharm. Biopharm. 2007;65:111–121.10.1016/j.ejpb.2006.07.015
- Abdelwahab SI, Mohan S, Mohamed Elhassan M, Al-Mekhlafi N, Mariod AA, Abdul AB, Abdulla MA, Alkharfy KM. Antiapoptotic and Antioxidant Properties of Orthosiphon stamineus Benth (Cat's Whiskers): Intervention in the Bcl-2-Mediated Apoptotic Pathway. Evid. Based Complement Alternat. Med. 2011. doi:10.1155/2011/156765.
- Liao WC, Lai YC, Yuan MC, Hsu YL, Chan CF. Antioxidative activity of water extract of sweet potato leaves in Taiwan. Food Chem. 2011;127:1224–1228.10.1016/j.foodchem.2011.01.131
- Huang YC, Chang YH, Shao YY. Effects of genotype and treatment on the antioxidant activity of sweet potato in Taiwan. Food Chem. 2005;98:529–538.
- Dinis TC, Madeira VM, Almeida LM. Action of phenolic derivatives (acetaminophen, salicylate, and 5-aminosalicylate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch. Biochem. Biophys. 1994;315:161–169.10.1006/abbi.1994.1485
- Nishikimi M, Appaji Rao N, Yagi K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem. Biophys. Res. Commun. 1972;46:849–854.10.1016/S0006-291X(72)80218-3
- Li H, Xu J, Liu Y, Ai S, Qin F, Li Z, Zhang H, Huang Z. Antioxidant and moisture-retention activities of the polysaccharide from Nostoc commune. Carbohyd. Polym. 2011;83:1821–1827.10.1016/j.carbpol.2010.10.046
- Wilson TD, Steck WF. A modified HET-CAM assay approach to the assessment of anti-irritant properties of plant extracts. Food. Chem. Toxicol. 2000;38:867–872.10.1016/S0278-6915(00)00091-0
- Luepke NP. Hen’s egg chorioallantoic membrane test for irritation potential. Food Chem. Toxicol. 1985;23:287–291.10.1016/0278-6915(85)90030-4
- Schrage A, Gamer AO, van Ravenzwaay B, Landsiedel R. Experience with the HET-CAM method in the routine testing of a broad variety of chemicals and formulations. Atla-Altern. Lab. Anim. 2010;38:39–52.
- Chao, G-R. Antioxidant properties and polysaccharide physicochemical analysis of ear mushrooms. [M.S., Master]. National Chung Hsing University; Taichung, 2001.
- Yamauchi R, Tatsumi Y, Asano M, Kato K. Effect of metal salts and fructose on the autoxidation of methyl linoleate in emulsions. Agric. Biol. Chem. 1988;52:849–850.10.1271/bbb1961.52.849
- Fouissac E, Milas M, Rinaudo M. Influence of the ionic strength on the dimensions of sodium hyaluronate. Macromolecules. 1992;25:5613–5617.10.1021/ma00047a009
- Davies A, Gormally J, Wyn-Jones E, Wedlock DJ, Phillips GO. Depolymerization of sodium hyaluronate during freeze drying. Int. J. Biol. Macromol. 1982;4:436–438.10.1016/0141-8130(82)90091-5
- Li H, Xu J, Liu Y, Ai S, Qin F, Li Z, Zhang H, Huang Z. Antioxidant and moisture-retention activities of the polysaccharide from Nostoc commune. Carbohyd. Polym. 2011;83:1821–1827.10.1016/j.carbpol.2010.10.046
- Costa GCd, Simões AZ, Gasparotto G, Zaghette MA, Stojanovic B, Cilense M, Varela JA. Effect of viscosity and temperature on the microstructure of BBT thin films. Mater. Res. 2003;6:347–351.10.1590/S1516-14392003000300008
- Ribeiro B, Rangel J, Valentão P, Baptista P, Seabra RM, Andrade PB. Contents of carboxylic acids and two phenolics and antioxidant activity of dried portuguese wild edible mushrooms. J. Agric. Food Chem. 2006;54:8530–8537.10.1021/jf061890q
- Lin W-Y, Yang MJ, Hung L-T, Lin L-C. Antioxidant properties of methanol extract of a new commercial gelatinous mushrooms (white variety of Auricularia fuscosuccinea) of Taiwan. Afr. J. Biotechnol. 2013;12:6210–6221.
- Jia Z, Tang M, Wu J. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999;64:555–559.
- Liu F, Ng TB. Antioxidative and free radical scavenging activities of selected medicinal herbs. Life Sci. 2000;66:725–735.10.1016/S0024-3205(99)00643-8
- Siriwardhana N, Lee K, Jeon Y-J, Kim S-H, Haw J-W. Antioxidant activity of Hizikia fusiformis on reactive oxygen species scavenging and lipid peroxidation inhibition. Food Sci. Technol. Int. 2003;9:339–346.10.1177/1082013203039014
- Kumar K, Ganesan K. Antioxidant potential of solvent extracts of Kappaphycus alvarezii (Doty) Doty - An edible seaweed. Food Chem. 2008;107:289–295.10.1016/j.foodchem.2007.08.016
- Duh P. Antioxidant activity of Budrock (Arctium lappa Linné):Its scavenging effect on free radical and active oxygen. J. Am. Oil Chem. Soc. 1998;75:455–461.10.1007/s11746-998-0248-8
- Mau J, Chao G, Wu K. Antioxidant properties of methanol extracts from several ear mushrooms. J. Agric. Food Chem. 2001;49:5461–5467.10.1021/jf010637h
