Abstract
N-methyl-2-pyrrolidone (NMP) is known for its multi-solvent properties. However, its biological, especially immunological significance still remains to be elucidated. In this study, we show for the first time that NMP stimulates the skin immune system by activating epidermal Langerhans cells (LCs). In contrast with the placebo tape, when the NMP-containing adhesive tape was applied on murine skin, LCs were stimulated immediately. Activated LCs not only exhibited enhanced expression of major histocompatibility complex class II and morphological changes, including the loss of dendrites, but also migrated effectively to draining lymph nodes. In addition, application of the tyrosine-related protein-2 peptide, which is the cytotoxic T lymphocyte (CTL) epitope against B16 melanoma, in combination with the NMP tape, resulted in explosive expansion of specific CTLs in mouse spleens. Taken together, these results demonstrate a novel role of NMP as an adjuvant in percutaneous peptide immunization.
Graphical Abstract
N-methyl-2-pyrrolidone (NMP) is known for its multi-solvent properties. In this study, we show for the first time that NMP stimulates the skin immune system by activating epidermal Langerhans cells.
Compared to widely used subcutaneous route for immunization, epidermal Langerhans cell (LC)-based percutaneous peptide immunization (PPI) is a safe and noninvasive method for inducing tumor-associated peptide-specific cytotoxic T lymphocytes (CTLs)Citation1–3) in immunotherapy against cancer. The method involves the following steps: (1) complete removal of stratum corneum (SC) by tape-stripping (TS) and painting of tumor-associated peptides dissolved in adequate solvents on the tape-stripped skin; (2) capture of applied peptides by epidermal LCs, migration of peptide-bound LCs into lymphatic vessels, and priming of peptide-specific naïve CD8+ T cells in draining lymph nodes (LN); and (3) immune surveillance and tumor attack by systemically circulating tumoricidal CTLs. PPI mainly targets epidermal LCs, which are professional antigen-presenting cells (APCs) capable of cross-priming for naïve CD8+ T cells, as well as antigenic peptide vectors. Previous animal and clinical studies on malignant melanoma revealed that PPI followed by three times of TS for complete SC removal resulted in the attenuation of tumor growth with moderate expansion of melanoma peptide-specific CTLs in vivo.Citation1–3) Meanwhile, however, insolubility of some synthetic peptides under aqueous conditions is still a controversial issue. To accomplish more effective clinical responses, the use of adjuvants has been considered to be necessary for activating skin APCs, since cancer patients are often in the state of immune suppression by treatment of radiation and chemical drugs.Citation4,5)
To overcome these problems, we decided to develop materials exerting adjuvant effects on epidermal LCs, in combination with an adhesive tape previously generated in our laboratory for complete SC removal.Citation6) Some adjuvants have been used to activate innate immunity for decades,Citation5) and ligands for APC-associated toll-like receptors (TLRs) are well known to modulate innate immunity.Citation5,7,8) For example, the TLR7 agonist imiquimod, widely used as Aldara cream, is capable of selectively activating intracellular TLR7.Citation7–9) Imiquimod has been shown to affect CTL-mediated antitumor immune responses via LC activationCitation9,10) and MHC class I upregulation in tumor cells.Citation11) Interestingly, a recent report showed that isostearic acid, another major component of Aldara cream, also activated the skin immune system in a TLR7-independent manner.Citation12)
N-Methyl-2-pyrrolidone (NMP; MW, 99.13; CAS No. 872-50-4), widely used as a multiple solvent with strong solubility,Citation13,14) has been reported to play a biological role in the inhibition of osteoclast differentiation.Citation15,16) In addition, NMP was demonstrated to interact with the acetylated lysine-binding pocket of the bromodomain of BET family proteins in vitro, and some of which are critical for inducing APC-mediated immunity.Citation17–21) These findings raise a possibility that NMP may be implicated in LC-mediated immunity via the bromodomain of BET proteins.
In this present study, we show that 24 h after an adhesive tape containing NMP was applied to murine skin, epidermal LCs were activated immediately, accompanied by the upregulation of surface MHC class II and morphological changes. With the use of the NMP tape, but not the placebo tape, in PPI, we also demonstrate efficient migration of skin APC-peptide complexes to draining LN, vigorous expansion of peptide-specific CD8+ T cells, as well as complete SC removal in vivo. Overall, our novel PPI using the NMP tape provides new insights into vaccination with antigenic peptides.
Materials and methods
Mice
C57BL/6 (B6; H-2b) female mice (8–10 weeks old) purchased from Charles River (Yokohama, Japan) were maintained under conventional conditions. The LINTEC Corporation Animal Experiment Committee approved the following animal experiments: 2011-01 (activation of LCs with the NMP tape; Fig. ), 2011-02 (protein detection, and skin permeation of NMP; Fig. ), 2011-04 (migration of fluorescein isothiocyanate (FITC) conjugated tyrosine-related protein-2 (TRP-2) peptides to draining LN; Fig. ), 2012-01 (TRP-2 peptide tetramer analysis; Fig. ), and 2012-02 (skin irritation analysis, see Supplementary materials). Back hairs of B6 mice were shaved with a razor (Natsume Seisakusyo Co., Ltd. Tokyo, Japan) before tape or peptide application.
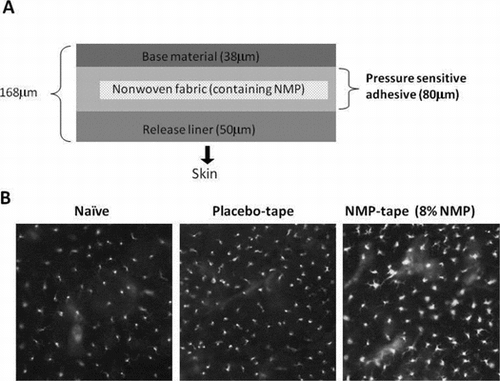
Fig. 1. Activation of epidermal LCs by the NMP tape.
Notes: (A) Diagram of the NMP-containing tape. NMP was dispensed on nonwoven fabric and laminated by base material and release liner. Tapes containing 0 (placebo), 4, 8, or 16% of NMP were prepared and applied to skin after removing release liner. (B) After the placebo or NMP tape was applied on the shaved back skin of B6 mice for 24 h, epidermal sheets were separated from dermis, stained with FITC-conjugated anti-MHC class II mAb, and visualized by fluorescence microscopy. Data shown are representative of 3 independent experiments. (C) The mean fluorescence intensity (MFI) of MHC class II (I-Ab) expression in I-Ab and CD45 double positive cells. Epidermal cell suspensions were prepared from placebo tape-treated or NMP (4 or 8%) tape-treated skin. After Fc blocking, the cells were dual stained with FITC-MHC class II mAb and APC-CD45 mAb and analyzed by flow cytometry. The values of the MFI of double positive cells are represented in the graph (n = 3).
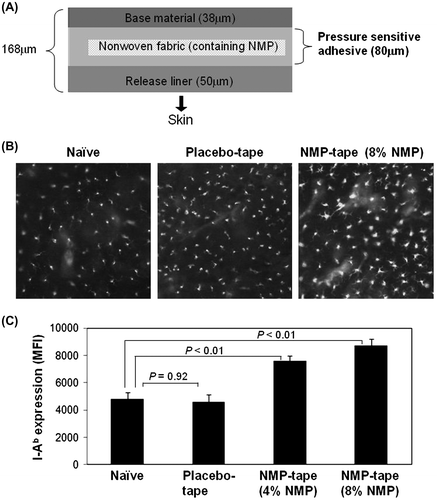
Fig. 2. Protein detection derived from each tape and NMP penetration into skin.
Notes: (A) The protein levels obtained from tapes described in Fig. were detected by the Lowry method. (B) After fixation of the shaved back skin of B6 mice on Franz diffusion cells, NMP tapes containing 4, 8, or 16% of NMP were applied. After 24 h, the concentration of NMP penetrating from each tape to PBS solution through skin was detected by gas chromatography. The bar graph indicates the concentration of NMP in PBS (mg/mL, left Y-axis) from each tape. The line graph shows the percentage of released NMP from each tape (%, right Y-axis). Data shown are representative of 3 (A) or 4 (B) independent experiments performed in triplicate.
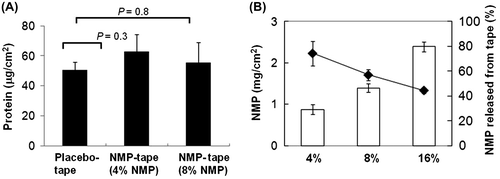
Fig. 3. Enhanced migration of peptide-bearing skin APCs by application of the NMP tape.
Notes: (A) The protocol of tape treatment and peptide painting. (B) The number of dual FITC-TRP-2+ and PE-MHC class II+ cells in inguinal LN determined by flow cytometry. Data shown are representative of 3 independent experiments performed on 3 mice.
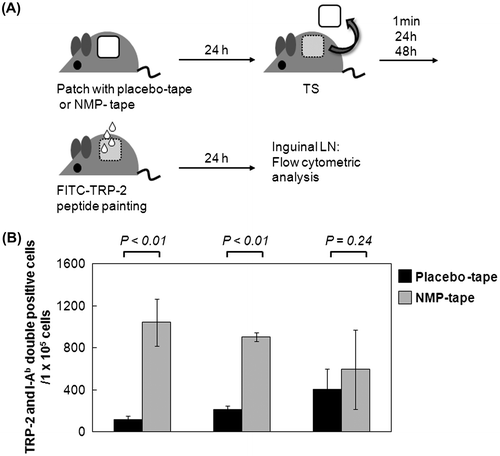
Fig. 4. Vigorous induction of peptide-specific CD8+ T cells in draining LN by PPI using the NMP tape.
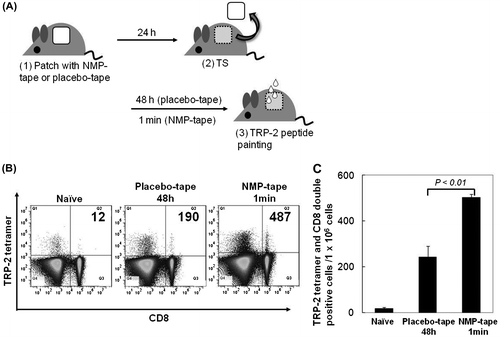
Notes: (A) The protocol of tape treatment and peptide painting includes the following steps: (1) patch with placebo or NMP tape, (2) TS, (3) peptide painting. Two weeks later, the steps (1)–(3) were performed again. Ten days after last immunization, mouse spleens were separated. (B) and (C) Splenocytes from immunized mice were subjected to Fc blocking, stained with FITC-CD8 mAb/PE-TRP-2-tetramer, and analyzed by flow cytometry. The representative of dot plot of flow cytometry, and the number of TRP2 and CD8 double positive cells is shown in (B). Data shown are representative of 2 independent experiments performed on 5 mice (C).
Synthetic peptides
Non-labeled or FITC-conjugated H-2Kb-restricted TRP-2 peptides (positions 180–188; SVYDFFVWL, MW, 1088.2; FITC-SVYDFFVWL, MW, 1446.6) were synthesized with a solid phase peptide synthesizer and purified by reverse phase HPLC at >99% purity (BEX Co., Ltd. Tokyo, Japan). Each peptide was dissolved in DMSO (Tokyo Kasei Co., Ltd. Tokyo, Japan) at a concentration of 10 mg/mL and stored at −80 °C until use. Stock solutions of peptides were diluted with oleic acid (DMSO/oleic acid, 3/97) and used for skin application.
Analysis of epidermal LCs by fluorescence microscopy
Placebo or NMP tapes prepared as described previouslyCitation6) were plastered on the shaved back skin of B6 mice. After 24 h, the skin was removed by scissors and treated with 20 mM EDTA/0.25% trypsin (Invitrogen, Tokyo, Japan) for 30 min at 37 °C. Epidermal sheets were separated from dermis and incubated with FITC-conjugated anti-I-Ab mAb (AF6-1201; BioLegend, Tokyo, Japan) for 30 min at room temperature. After three washes with PBS, samples were observed by fluorescence microscopy (ECLIPSE 80i; Nikon Co., Ltd. Tokyo, Japan).
Protein detection
Placebo or NMP-tapes were applied on the shaved back skin of B6 mice for 24 h.Citation19) For detecting SC-derived proteins, each tape after TS was treated with 1 M NaOH for 2 h at room temperature. After neutralization with 1 M HCl, protein detection was performed by the Lowry method using the DC™ protein assay system (Bio-Rad Co., Ltd. Tokyo, Japan).
Skin penetration of NMP
Shaved skin of B6 mice was fixed on Franz diffusion cells, and NMP tapes containing 4, 8, or 16% NMP were applied on the skin for 24 h. The rate of permeation of NMP into PBS solution from each tape was detected by gas chromatography. To determine the concentration of NMP, the standard curve based on N-ethyl-2-pyrrolidone was used.
Flow cytometry
Epidermal cells, inguinal cells, or splenocytes were pretreated with TruStain FcX™ (BioLegend, Tokyo, Japan) for 5 min to block Fc receptors (CD16/CD32). Epidermal cell suspensions were dual stained with phycoerythrin (PE)-conjugated anti-I-Ab mAb (AF6-1201) and allophycocyanin (APC)-conjugated anti-CD45 mAb (30-F11), with PE-mouse IgG2a mAb (MOPC-173) or APC-rat IgG2b mAb (RTK4530) used as isotype controls for anti-I-Ab or anti-CD45, respectively (all mAbs from BioLegend). Inguinal LN cell suspensions were PE-anti-I-Ab mAb/APC-anti-CD40 mAb (3/23), PE-anti-anti-I-Ab mAb/APC-anti-CD54 mAb (YN1/1.7.4), PE-anti-I-Ab mAb/APC-anti-CD80 mAb (16-10A1), or PE-anti-I-Ab mAb/APC-anti-CD86 mAb (GL-1), with PE-mouse IgG2a mAb (MOPC-173), APC-rat IgG2a (RTK2758), APC-rat IgG2b (RTK4530), or APC-Armenian hamster IgG (HTK888) used as isotype controls for anti-I-Ab, CD40/86, CD54, CD80, respectively (all mAbs from BioLegend). For detecting TRP-2 peptide-specific CD8+ T cells, splenocytes were hemolyzed with 0.9% ammonium chloride and stained with FITC-anti-CD8 mAb and PE-H-2Kb TRP-2 tetramer (KT15 and TS-5004-1C, respectively; Medical and Biological Laboratories Co., Ltd., Nagoya, Japan). Cells treated with FITC-rat IgG2a mAb (RTK2758) were used as the negative control. All samples were washed with reaction buffer (PBS containing 1% BSA and 0.02% NaN3), and 7-AAD (Beckman Coulter, Tokyo, Japan) was used for discriminating dead cells. Finally, samples were analyzed by BD Accuri™ C6 (BD Biosciences, Tokyo, Japan).
TS and application of FITC-conjugated peptides
Placebo or NMP tapes were applied on the shaved back skin of B6 mice for 24 h. At 1 min, 24, or 48 h after TS, FITC-conjugated TRP-2 peptides (20 μg/mouse) were applied on the same site. After 24 h, draining inguinal LN were separated, and single cell suspensions were prepared and analyzed by flow cytometry.
Vaccination of tumor-derived peptides with PPI after treatment with NMP tapes
To detect TRP-2 peptide-specific CD8+ T cells, placebo or NMP tapes were applied to the shaved back skin of B6 mice for 24 h. At 48 h (placebo) or 1 min (NMP) after TS, purified TRP-2 peptides (100 μg/mouse) were applied to the tape-stripped skin. After 14 days, the same procedure was performed. Ten days after the last immunization, mouse spleens were separated, and single cell suspensions were analyzed by flow cytometry.
Statistical analysis
Between-group differences were calculated using a student’s t-test (Figs. (B) and (C)) or Dunnett test (Figs. (C), (A), and Supplemental data 2(B)); statistical significance was set at p < 0.01.
Results and discussion
NMP acts as a novel adjuvant for stimulating epidermal LCs
We first explored whether the NMP tape stimulates skin immune responses. Since the maximum administration dose of NMP against human body is set as 8% in Japanese Pharmacopoeia, 8% NMP tape, or placebo tape without NMP (Fig. (A)) was prepared and applied on the shaved back skin of B6 mice, and the LC morphology was observed by fluorescence microscopy. The signs of LC activation, including elevated expression of MHC class II and the loss of dendrites, were detected upon application of the NMP tape (Fig. (B), right), indicating the sign of rapid migration to draining LN. In contrast, LC activation was not observed in the cells from placebo tape-treated intact mouse skin (Fig. (B), middle). In addition to the histological study using fluorescence microscopy, the flow cytometric analysis was performed to confirm NMP-dependent upregulation of the MHC class II expression on LCs. As shown in Fig. (C), the expression levels of MHC class II on I-Ab+ CD45+ LCs were enhanced after 24 h treatment with the NMP tape, but not the placebo tape. Since the degree of SC removal between the placebo and NMP tapes was comparable (Fig. (A)) and the ratio of NMP penetration through skin from the tape was dependent on the NMP concentrations (Fig. (B)), we propose that the increment of MHC II expression on epidermal LCs was caused by the adjuvant effects of NMP rather than the peeling effects. These results indicate crucial roles of NMP in the activation of LC-mediated skin immune responses.
NMP tape enhances the migration of skin-associated DCs including LCs
Based on our findings that NMP activated the skin immune system in vitro and in vivo, we further examined the efficaciousness of NMP in PPI. The experimental procedure is summarized in Fig. (A). Since other groups have reported that skin APCs, including LCs, are activated maximally at 24–48 h after TS,Citation1,2,22) we explored whether PPI using the NMP tape would also require 48 h to obtain functional APCs after TS. On a stripped site after 24 h sticking of the placebo or NMP tape on the shaved back skin of B6 mice, FITC-conjugated TRP-2 peptides were applied at indicated time points (1 min, 24, or 48 h). The cells from inguinal draining LN obtained 24 h after peptide application were subjected to the flow cytometric analysis. Compared to placebo tape treated mice, the number of TRP-2-expressing MHC class II+ skin APCs in inguinal LN derived from NMP-tape-treated mice was significantly increased (Fig. (B), placebo tape, black bar: 1 min, 116 ± 35; 24 h, 211 ± 38; 48 h, 403 ± 194, NMP-tape, gray bar: 1 min, 1042 ± 226; 24 h, 903 ± 41; 48 h, 593 ± 377 in counts/105 cells). In addition, the number of TRP-2- or co-stimulatory molecules (CD40/CD54/CD80/CD86) expressing APCs in inguinal LN reached a peak at 1 min after TS and decreased thereafter (24 or 48 h after TS, Fig. (B) and Supplemental material: see Biosci. Biotechnol. Biochem. Web site). These results strongly indicate that skin resident APCs that were activated by NMP released from the NMP tape migrated immediately from epidermis to draining LN through lymphatic vessels in dermis.
Vigorous expansion of peptide-specific CD8+ T cells in draining LN by PPI using the NMP tape
We next examined whether NMP tape treatment enhances the clonal expansion of peptide-specific CD8+ T cells in vivo in comparison with the placebo tape (Fig. (A)). Since the painting of TRP-2 peptides induced the migration of APC-peptide into draining LN most vigorously at 1 min after TS with the NMP tape (Fig. (B)), purified TRP-2 peptides were also applied at 1 min after TS in this vaccination experiment. Plastering of the placebo or NMP tape, as well as TRP2-peptide painting at 48 h (placebo tape) or 1 min (NMP tape) after TS, was performed twice at a 2-week interval. Ten days after last immunization, mouse spleens were separated. The flow cytometric analysis of TRP-2 peptide tetramer-treated splenocytes indicates that the induction of TRP-2 peptide-specific CD8+ T cells in mice treated with the NMP tape was more vigorous than placebo tape-treated animals (Fig. (B) and (C): naive, 17.7 ± 4.9; placebo tape, 242.3 ± 48.2; NMP tape, 502.7 ± 14.6). These results suggest that NMP may serve as an effective adjuvant for the expansion of peptide-specific CD8+ T cells in PPI.
Taken together, we demonstrate that the NMP tape is a powerful tool to shorten the amount of time of antigen painting after TS, whereas the placebo tape required about 48 h to induce maximal activation of skin APCs for peptide delivery. These results may support the possibility that LCs are activated by NMP through its binding to acetylated lysines scattered in histone protein, which are the targets of bromodomain of BET family proteins. The safety of NMP in the human body has been warranted by various kinds of tests, including skin irritation (Supplemental material: see Biosci. Biotechnol. Biochem. Web site), pharmacokinetics, and toxicity.Citation13,14) With high safety, our tape containing NMP is expected to be useful for removing SC as well as activating skin APCs in response to CTL-mediated treatments, such as cancer immunotherapy, in future clinical trials.
Supplemental material
The supplemental material for this paper is available at http://dx.doi.org/10.1080/09168451.2014.912114.
Supplemental Material 1 and 2
Download PDF (445.9 KB)Acknowledgments
We are grateful to Dr N. Fujiwara (Marianna University School of Medicine, Japan) and Dr N. Seo (Mie University, Japan) for their critical reading of the manuscript.
Notes
Abbreviations: APCs, antigen-presenting cells; BET, bromodomain and extra-terminal; CTLs, cytotoxic T lymphocytes; DCs, dendritic cells; LC, Langerhans cell; MFI, mean fluorescence intensity; MHC, major histocompatibility complex; SC, stratum corneum; TRP-2, tyrosine-related protein-2; TS, tape-stripping.
References
- Seo N, Tokura Y, Nishijima T, Hashizume H, Furukawa F, Takigawa M. Proc. Nat. Acad. Sci. USA. 2000;97:371–376.10.1073/pnas.97.1.371
- Yagi H, Hashizume H, Horibe T, Yoshinari Y, Hata M, et al. Cancer Res. 2006;66:10136–10144.10.1158/0008-5472.CAN-06-1029
- Seo N, Takigawa M. J. Dermatol. Sci. 2007;48:77–85.10.1016/j.jdermsci.2007.05.004
- Finn OJ. Ann. Oncol. 2012;23(Suppl. 8):viii6–viii9.
- Mesa C, Fernandez LE. Immunol. Cell Biol. 2004;82:644–650.10.1111/icb.2004.82.issue-6
- Miyazaki K, Tatsuno T, Miyata S. J. Adh. Soc. Jpn. 2011;47:465–470.
- Wagstaff AJ, Perry CM. Drugs. 2007;67:2187–2210.10.2165/00003495-200767150-00006
- Gaspari AA, Tyring SK, Rosen T. J. Drugs Dermatol. 2009;8:467–474.
- Palamara F, Meindl S, Holcmann M, Lührs P, Stingl G, Sibilia M. J. Immunol. 2004;173:3051–3061.
- Shackleton M, Davis ID, Hopkins W, Jackson H, Dimopoulos N, et al. Cancer Immun. 2004;4:9.
- Stary G, Bangert C, Tauber M, Strohal R, Kopp T, Stingl G. J. Exp. Med. 2007;204:1441–1451.10.1084/jem.20070021
- Walter A, Schäfer M, Cecconi V, Matter C, Urosevic-Maiwald M, et al. Nat. Commun. 2013;4:1560.10.1038/ncomms2566
- Jouyban A, Fakhree MA, Shayanfar A. J. Pharm. Pharm. Sci. 2010;13:524–535.
- Nishimura S, Yasui H, Miyauchi H, Kikuchi Y, Kondo N. Ind. Health. 2009;47:355–362.10.2486/indhealth.47.355
- Ghayor C, Correro RM, Lange K, Karfeld-Sulzer LS, Gratz KW, Weber FE. J. Biol. Chem. 2011;286:24458–24466.10.1074/jbc.M111.223297
- Miguel BS, Ghayor C, Ehrbar M, Jung RE, Zwahlen RA, Hortschansky P, Schmoekel HG, Weber FE. Tissue Eng. Part A. 2009;15:2955–2963.10.1089/ten.tea.2009.0009
- Philpott M, Yang J, Tumber T, Fedorov O, Uttarkar S. Mol. Biosyst. 2011;7:2899–2908.10.1039/c1mb05099k
- Hewings DS, Wang M, Philpott M, Fedorov O, Uttarkar S. J. Med. Chem. 2011;54:6761–6770.10.1021/jm200640v
- Jacobi U, Weigmann HJ, Ulrich J, Sterry W, Lademann J. Skin Res. Technol. 2005;11:91–96.10.1111/srt.2005.11.issue-2
- Ni Z, Hassan MAE, Xu Z, Yu T, Bremner R. Nat. Immunol. 2008;9:785–793.10.1038/ni.1619
- Zhang F, Boothby M. J. Exp. Med. 2006;203:1493–1505.10.1084/jem.20060066
- Nishijima T, Tokura Y, Imokawa G, Seo N, Furukawa F, Takigawa M. J. Invest. Dermatol. 1997;109:175–182.10.1111/jid.1997.109.issue-2
