Abstract
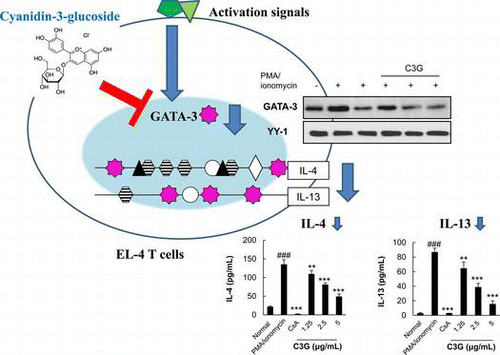
Allergic disease is dominated by Th2 immune responses. Interleukin (IL)-4 and IL-13, representative Th2 cytokines, play pivotal roles in the pathogenic activation of the Th2 immune response. In this study, we found that cyanidin-3-glucoside chloride (C3G), an anthocyanin suppressed IL-4 and IL-13 produced in activated EL-4 T cells but not Th1 cytokines including IL-2, interferon-γ, or IL-12. IL-4 and IL-13 mRNA levels and luciferase activation in cells transiently transfected with IL-4 and IL-13 promoter reporter plasmids were significantly inhibited by C3G, suggesting that suppression might be, at least in part, regulated at the transcriptional level. Data from western blot and reverse transcription-polymerase chain reaction analyses of transcription factors involved in cytokine expression suggested that expression of GATA-3, but not T-bet, was downregulated in the nucleus by C3G. Taken together, our data indicate that C3G may has potential as an anti-allergic agent suppressing Th2 activation by downregulating Th2 cytokines and the GATA3 transcription factor in allergies.
Graphical Abstract
Cyanidin-3-glucoside chloride, an anthocyanin, suppresses IL-4 and IL-13 expression via down regulation of GATA3 transcription factor in EL-4 T cells.
Th2 cytokines secreted from T helper 2 (Th2) cells play important roles in the pathogenesis of allergic disease.Citation1) Interleukin (IL)-4, a representative Th2 cytokine, initiates the development of Th2 cells from Th0 cells, resulting in an unbalanced Th2-skewed immune response,Citation2) that also promotes production of pro-inflammatory cytokines.Citation3) IL-13, another Th2 cytokine, contributes to allergic inflammation and hyper-reactivity and has various functions such as recruitment and activation of immune cells, B cell activation, smooth muscle cell proliferation, and mucus hypersecretion.Citation4) Furthermore, these cytokines are responsible for in situ release of inflammatory and toxic mediators, leading to cell damage and smooth muscle contraction.Citation5) Expression of IL-4 and IL-13 genes is coordinately regulated at the transcriptional level by several selective transcription factors, including activator protein 1 (AP-1), nuclear factor of activated T cells (NF-AT), nuclear factor kappa B (NF-κB), and GATA-binding proteins (GATAs) in T cells.Citation6) GATA-3, a transcription factor that is specifically expressed in Th2 cells but not in Th1 cells, is essential for IL-4 and IL-13 gene expression as well as for Th2 cell differentiation.Citation7)
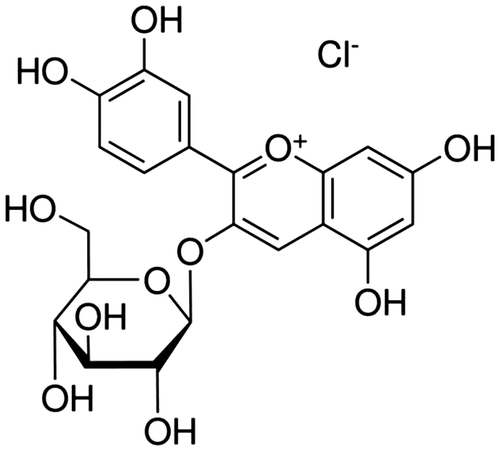
Anthocyanins are part of a widespread group of water-soluble phytochemicals known collectively as flavonoids.Citation8) They are naturally occurring phenolic compounds responsible for the coloring of many plants, flowers, fruits, and red wines. Cyanidin-3-glucoside chloride (C3G, Fig. ), principal type of anthocyanin, is probably the best known and most investigated.Citation9) Many studies have focused on the biological activities and effects of C3G, and several reports are available on its potential free radical-scavenging activities, ability to prevent low-density lipoprotein oxidation, and its beneficial effects on cardiovascular diseases, obesity, and inflammation.Citation10–13) In particular, oral administration of C3G has an anti-scratching effect in compound 48/80 or histamine-induced mice.Citation14) However, the cellular and molecular mechanisms of the C3G anti-allergic effects have rarely been examined.
In this study, we investigated the effects of C3G on PMA/ionomycin or anti-CD3/CD28-induced IL-4 and IL-13 production in EL-4 T cells and the molecular mechanisms involved in this process.
Materials and methods
Reagents and cell culture
C3G, PMA (phorbol 12-myristate 13-acetate), and ionomycin were purchased from Sigma (St. Louis, MO, USA). Anti-CD3 and anti-CD28 antibodies were purchased from BD Bioscience (San Diego, CA, USA). Cyclosporin A (CsA) was obtained from Calbiochem (La Jolla, CA, USA). EL-4 thymoma cells were purchased from the Korea Cell Bank (Seoul, Korea) and maintained in RPMI-1640 medium (Lonza, Walkersville, MD, USA) supplemented with 10% fetal bovine serum (Lonza) and 100 μg/mL penicillin-streptomycin (Lonza) at 37 °C in a 5% CO2-humidified air atmosphere.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium (MTT) assay
EL-4 T cells (1 × 106 cells/mL) were grown with 0.1–10 μg/mL C3G at 37 °C in a 5% CO2 incubator. After a 24 h incubation with C3G, viable cells were stained with 50 μL MTT (3 mg/mL) for 4 h at 37 °C. The medium was removed, and formazan crystals were dissolved in 50 μL dimethyl sulfoxide. Absorbance was measured at 540 nm using an enzyme-linked immunosorbent assay (ELISA) microplate reader.
Enzyme-linked immunosorbent assay
EL-4 T cells (1 × 106 cells/mL) were treated with PMA/ionomycin (50 ng/mL + 0.5 μM) for 16 h in the absence or presence of C3G. For TCR stimulation, EL-4 T cells treated with C3G were incubated in a plate bound with anti-CD3 (5 μg/mL) and anti-CD28 (10 μg/mL) antibodies for 24 h. The levels of IL-2, IL-4, IL-12, interferon (IFN)-γ (BD BioScience), and IL-13 (Invitrogen, Carlsbad, CA, USA) in the culture supernatant were determined using a commercially available ELISA kit according to the manufacturer’s instructions.
Reverse transcriptase-polymerase chain reaction
EL-4 T cells (1 × 106 cells/mL) were treated with PMA/ionomycin (50 ng/mL + 0.5 μM) for 6 h in the absence or presence of C3G. Total RNAs were isolated by the single-step method using TRIzol reagent (Invitrogen). Each sample was reverse-transcribed to cDNA for 60 min at 45 °C, followed by 5 min at 95 °C using a cDNA synthesis kit (iNtRON Biotechnology, Gyeonggi-do, Korea). The sequences for the PCR primers were as follows: Mouse IL-4 sense; 5′-ATG GGT CTC AAC CCC CAG C-3′, mouse IL-4 antisense; 5′-GCT CTT TAC GCT TTC CAG GAA GTC-3′, mouse IL-13 sense; 5′-GGA GCT GAG CAA CAT CAC ACA-3′, mouse IL-13 antisense; 5′-GGT CCT GTA GAT GGT GGC ATT GCA-3′, mouse GATA-3 sense; 5′-GAA GGC ATC CAG ACC CGA AAC-3′, mouse GATA-3 antisense; 5′-ACC CAT GGC GGT GAC CAT GC-3′, mouse β-actin sense; 5′- ACC GTG AAA AGA TGA CCC AG -3′, and mouse β-actin antisense; 5′- TCT CAG CTG TGG TGG TGA AG -3′. The PCR reaction was run for 32 cycles at 94 °C (30 s), 57 °C (30 s), and 72 °C (30 s). After amplification, the reverse transcriptase-polymerase chain reaction (RT-PCR) products were separated on 1.2% (w/v) agarose gels and stained with ethidium bromide. The sizes of the IL-4, IL-13, GATA-3, and β-actin PCR products were 397, 141, 254, and 272 bp, respectively.
Transient transfection and luciferase assay
EL-4 T cells (1 × 106 cells/mL) were transfected with pGL4.14-IL-4 or pGL4.14-IL-13 plasmids generated in our laboratoryCitation15,Citation16) using Lipofectamine 2000 (Invitrogen), according to the manufacturer’s recommendations. Following a 24 h incubation, the cells were treated with C3G for 1 h and stimulated with PMA/ionomycin (50 ng/mL + 0.5 μM) for 16 h. Subsequently, cells were washed three times with PBS, extracted with 50 μL lysis reagent (Promega, Madison, WI, USA) at 4 °C for 20 min, and centrifuged at 12,000 rpm at 4 °C for 1 min. The supernatants were mixed with 100 μL of luciferase substrate solution, and emitted light was measured using a Victor3 1420 multilabel counter (Perkin-Elmer, Waltham, MA, USA).
Western blot analysis
EL-4 T cells (1 × 106 cells/mL) were treated with PMA/ionomycin (50 ng/mL + 0.5 μM) for 20 min or 2 h in the absence or presence of C3G. Total cell lysates were prepared in 70 μL lysis buffer (RIPA buffer, protease inhibitor cocktail, 1 mM PMSF, phosphatase inhibitor). Nuclear extracts were obtained using a Nuclear Extract kit (Active Motif, Carlsbad, CA, USA), according to the manufacturer’s recommendations. Protein concentrations were determined with the BCA protein assay (Pierce Biotechnology, Rockford, IL, USA). Equal amounts (40 μg) of proteins were heated to 95 °C for 5 min in sample buffer, chilled on ice, and then separated on 10% sodium dodecyl sulfate-polyacrylamide gels. Proteins were transferred to polyvinylidene difluoride membranes (Millipore Corp., Bedford, MA, USA) and blocked with 2% skim milk in PBS/T buffer for 1 h. They were then probed overnight with primary antibodies specific to GATA-3 and T-bet (Santa Cruz Biotechnology, Santa Cruz, CA, USA). Blots were incubated with HRP-conjugated secondary antibodies. HRP was detected using a chemiluminescent HRP substrate (Millipore).
Statistical analysis
All data were expressed as means ± standard deviations. The data presented were one representative experiment of three independent experiments. One-way analysis of the variance (ANOVA; via SPSS) was used to analyze the differences between the control group and various experimental groups. Tukey’s multiple comparison test was used to compare the mean values of the treatments. p-values of less than 0.05 were considered to be statistically significant.
Result
Suppressive effects of C3G on IL-4 and IL-13 expression in activated T cells
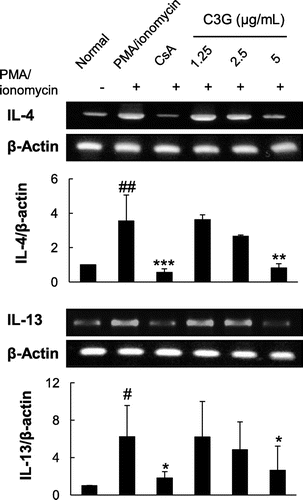
To test the effects of C3G on IL-4 and IL-13 production in T cells, EL-4 T cells were stimulated by PMA/ionomycin in the absence or presence of C3G. PMA/ionomycin are potent activator of cytokine expression,Citation17) and EL-4 T cells are widely used T cells, which can produce Th2 cytokine after stimulation with specific agents.Citation18) Cells stimulated by PMA/ionomycin exhibited a dramatic increase in IL-4 and IL-13 production (6.4- and 33-fold increases compared with normal cells, respectively; Fig. A and B). When the cells were treated with varying concentrations of C3G, PMA/ionomycin-induced production of IL-4 and IL-13 was suppressed in a dose-dependent manner (Fig. ). Since C3G did not affect cell viability at any concentration tested, the effects were not caused by cytotoxicity of the compound (data not shown). In the presence of 2.5 or 5 μg/mL C3G, IL-4 production was suppressed by 40.1 or 63.9%, respectively, as compared with PMA/ionomycin-treated cells. Similarly, IL-13 production was inhibited by 55.5 and 82.4%, respectively. We also tested whether C3G affected production of IL-2, IFN-γ, and IL-12 as representative Th1 cell cytokines.Citation19,Citation20) The levels of IL-2, IFN-γ and IL-12 increased following PMA/ionomycin stimulation. C3G further increased the levels of IL-2 but the levels of IFN-γ and IL-12 were not significantly changed in the presence of C3G. Furthermore, we activated EL-4 T cells by T cell receptor engagement. When EL-4 T cells were stimulated by anti-CD3 and anti-CD28 antibodies, the levels of IL-4 and IL-13 increased significantly, and C3G treatment suppressed IL-4 and IL-13 production in a dose-dependent manner. As shown in Fig. , C3G also increased the levels of IL-2 in these activated cells and the effects on IL-12 were similar to those shown for PMA/ionomycin-activated cells; However, IFN-γ decreased following C3G treatment. CsA (1 μM), a well-known immunosuppressive agent used as a positive control, almost completely inhibited PMA/ionomycin-induced IL-4 and IL-13 production, as reported previously.Citation15,Citation16) To determine whether C3G suppressed IL-4 and IL-13 gene expression at the transcriptional level, the mRNA levels of the cytokines were determined by RT-PCR. As shown in Fig. , PMA/ionomycin stimulation significantly increased IL-4 and IL-13 mRNA levels by 3.6- and 6-fold, respectively, compared with that in normal cells. However, PMA/ionomycin-induced expression of IL-4 and IL-13 mRNA was significantly inhibited in the presence of C3G. Following a 3 h treatment with 5 μg/mL C3G, the cells exhibited reductions in PMA/ionomycin-induced IL-4 and IL-13 mRNA expression of 76.9 and 55.5%, respectively. These results suggest that C3G suppresses IL-4 and IL-13 production and mRNA expression in activated EL-4 T cells, suggesting the suppression might be, at least in part, regulated at the transcriptional level.
Fig. 2. Effects of C3G on Cytokine Production in PMA/Ionomycin-Activated EL-4 T Cells.
Note: EL-4 T cells were pretreated with various concentrations of C3G or 1 μM CsA for 1 h and stimulated with PMA/ionomycin for 16 h. The levels of IL-4, IL-13, IL-2, IFN-γ, and IL-12 in EL-4 T cells were determined by ELISA. Values are means ± standard deviations from independent experiments. ##p < 0.01; ###p < 0.001 vs. normal group. *p < 0.05; **p < 0.01; ***p < 0.001 vs. PMA/ionomycin-treated group.
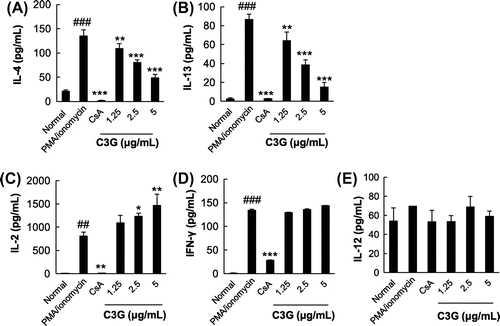
Fig. 3. Effects of C3G on Cytokine Production in CD3 and CD28-Activated EL-4 T Cells.
Note: EL-4 T cells were pretreated with various concentrations of C3G or 1 μM CsA for 1 h and stimulated with anti-CD3 and anti-CD28 for 16 h. The levels of IL-4, IL-13, IL-2, IFN-γ, and IL-12 in EL-4 T cells were determined by ELISA. Values are means ± standard deviations from independent experiments. #p < 0.05; ##p < 0.01; ###p < 0.001 vs. normal group. *p < 0.05; **p < 0.01; ***p < 0.001 vs. anti-CD3 and anti-CD28-treated groups.
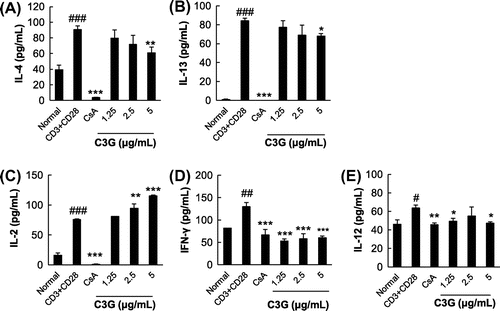
Fig. 4. Effects of C3G on IL-4 and IL-3 mRNA Expression in EL-4 T Cells.
Note: EL-4 T cells were pretreated with various concentrations of C3G or 1 μM CsA for 1 h and stimulated with PMA/ionomycin for 3 h. Total RNA was extracted from cells following specific treatments, and IL-4 and IL-13 mRNA expression in EL-4 T cells was analyzed by RT-PCR. Histograms represent quantification of mRNA expression. Data are representative of three independent experiments. #p < 0.05; ##p < 0.01 vs. normal group. *p < 0.05; **p < 0.01; ***p < 0.001 vs. PMA/ionomycin-treated group.
Suppressive effects of C3G on IL-4 and IL-13 promoters
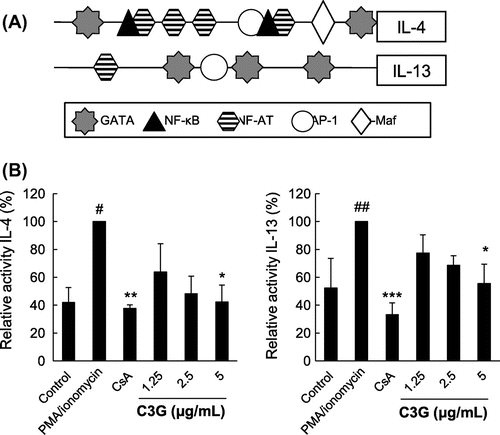
As IL-4 and IL-13 expression is regulated by proximal promoter activities (Fig. (A)) at the transcriptional level, the effects of C3G on these promoters were further examined. We used luciferase reporter plasmids containing the IL-4 and IL-13 promoter sequences previously generated in our laboratory.Citation15,Citation16) EL-4 T cells transiently transfected with these plasmids were stimulated with PMA/ionomycin in the absence or presence of C3G. As shown in Fig. (B), stimulation with PMA/ionomycin increased IL-4 promoter luciferase activity by up to 2.4-fold compared with that of cells transfected with control plasmid, whereas treatment with 5 μg/mL C3G suppressed this activation by 57.7%. Similarly, IL-13 luciferase activity increased up to 2-fold, and C3G significantly inhibited this effect by 44.5%. These results suggest that C3G has a suppressive effect on IL-4 and IL-13 promoter activation in EL-4 T cells.
Fig. 5. Effects of C3G on IL-4 and IL-13 Promoters.
Note: EL-4 T cells were transiently transfected with pGL4.14-IL-4 or pGL4.14-IL-13, generated by inserting the mouse IL-4 or IL-13 promoters into the pGL4.14 plasmid. (A) Overview of the regulatory regions in the IL-4 and IL-13 promoters. (B) EL-4 T cells were pretreated with C3G or 1 μM CsA for 1 h and stimulated with PMA/ionomycin for 16 h. Luciferase activities were measured using a luminometer. Activity was calculated as the luciferase activity of C3G- or CsA-treated group relative to that of the PI-treated group. #p < 0.05; ##p < 0.01 vs. normal group. *p < 0.05; **p < 0.01; ***p < 0.001 vs. PMA/ionomycin-treated group.
Effects of C3G on transcription factors associated with the IL-4 and IL-13proximal promoters
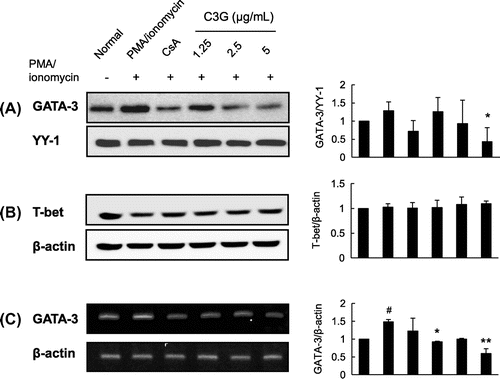
As the above data indicate that C3G suppresses IL-4 and IL-13 mRNA expression and promoter activities, we performed Western blot to examine whether C3G affected the activities and nuclear expression of transcription factors known to modulate the expression of IL-4 and IL-13. C3G did not affect AP-1 and NF-AT nuclear expression (data not shown). Further, we could not find any effects of C3G on NF-κB in EL-4 T cells (data not shown) although previousCitation21) study indicated C3G has inhibitory effects on this transcription factor in epithelial cells. However, GATA-3 protein and mRNA expression was dramatically suppressed in response to C3G treatment (Fig. (A) and (C)). When we examined T-bet regulating Th1 cell differentiation, no significant induction of T-bet was found following PMA/ionomycin stimulation, and the expression level was not affected by C3G (Fig. (B)). These data suggest that C3G has suppressive effects on the activation of GATA-3 transcription factors in EL-4 T cells.
Fig. 6. Effects of C3G on GATA-3 and T-bet Expression.
Note: EL-4 T cells were pretreated with C3G or 1 μM CsA for 1 h and stimulated with PMA/ionomycin for 30 min or 2 h. (A) Nuclear extracts were isolated and separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. GATA-3 expression was determined by Western blot analysis using GATA-3 antibody. (B) T-bet expression in total cell lysates, and (C) total RNA was extracted from cells following each treatment, and GATA-3 mRNA expression in EL-4 T cells was analyzed by RT-PCR. Histograms represent quantification of protein and mRNA expression. Data shown are representative of three independent experiments. #p < 0.05 vs. normal group. *p < 0.05; ***p < 0.001 vs. PMA/ionomycin-treated group.
Discussion
As the prevalence of allergic disease is rapidly increasing in all major developed countries, there is a great need to develop new anti-allergic agents. Increasing attention has been focused on studying the biological effects of phytochemicals in diverse plantsCitation22,Citation23) with safety and efficacy. Recent studies revealed that anthocyanin has various biological effects including antioxidant, anti-inflammatory, and anti-allergic effects.Citation14,24,Citation25) Further, oral administration of anthocyanin such as C3G had a beneficial effect on allergic status involving scratching behaviors in an in vivo studyCitation14) as well as it inhibit airway inflammation and hyper-responsiveness in murine asthma model.Citation26) However, the cellular and molecular mechanisms for the anti-allergic effects of C3G have rarely been examined.
In the present study, we showed that C3G had inhibitory effects on the expression of IL-4 and IL-13 in activated EL-4 T cells without affecting cell viability, while the effects on Th1 cytokines were variable, and C3G might suppress expression of these Th2 cytokines at the transcriptional level. It is widely accepted that imbalanced immune responses dominated by Th2 cells cause allergic diseases. IL-4 and IL-13, which are mainly produced by activated Th2 cells and inflammatory cells such as mast cells and basophils, play crucial roles in the pathogenesis of various allergic diseases such as atopic dermatitis, allergic rhinitis, and asthma.Citation27) During the allergic response, secretion of IL-4 and IL-13 from Th2 cells drives IgE isotype switching in B cells,Citation28) promoting mast cell activation and inflammatory mediator release. IL-13 production in the airways promotes survival and migration of eosinophils, activation of macrophages, and increase permeability and mucus production by airway epithelial cells.Citation29) Therefore, suppressing Th2 cytokine expression has been suggested as a potential therapeutic strategy for the control of allergic diseases.Citation30) Additionally, our data suggest that C3G regulates the GATA-3 transcription factor to suppress expression of these cytokines. The GATA family consists of six members (GATA-1–GATA-6) and is characterized by recognition of the W(A/T)GATAR(A/G) motif and their DNA-binding domains including highly conserved homologous zinc fingers Cys-X2-Cys-X17-Cys-X2-Cys. GATA-1 and GATA-2 are expressed in mast cells and basophils, whereas GATA-3 is expressed in T cells. Notably, GATA-3 plays a critical role regulating the expression of cytokines from Th2 cells based on the observation that conditional knockout of the GATA-3 gene in mice and CD4+ T cellsCitation31) reduces expression of Th2 cytokines.Citation32) Moreover, transgenic expression of a dominant-negative GATA-3 construct in T cells attenuates allergic inflammation in a murine model of asthma, with decreased eosinophilia, mucus production, and IgE levels associated with decreased IL-4, IL-5, and IL-13 production,Citation33) suggesting that GATA-3 is a key mediator of allergic disease. In contrast, T-bet is expressed predominantly in Th1 cells and exhibits reciprocal inhibitory effects with GATA-3 during T-cell differentiation.Citation34) Our data clearly showed that C3G suppressed not only mRNA levels but also nuclear expression of GATA-3 in T cells. Further, as it is possible C3G may affect T-bet activation to inhibit GATA-3 expression we tested whether it changed the levels of the transcription factor. We found that C3G treatment did not have any obvious effect on T-bet expression in EL-4 T cells. Furthermore, various transcription factors including AP-1, NF-AT, and NF-κB involved in the IL-4 and IL-13 expression were not affected by C3G (data not shown). Several flavonoids such as chrysin,Citation35) apigenin,Citation36) and genisteinCitation37) have been reported to regulate the Th1/Th2 cytokine balance and the GATA-3 transcription factor in vitro and in vivo in animal models of allergy. Further, increased consumption of natural products rich in flavonoid prevent development of allergies and reduce the symptoms of allergies in humans.Citation38) It is necessary to compare the potency of C3G as an anti-allergic agent with that of known flavonoids in animal models and in humans. Taken together, C3G may be a candidate anti-allergic agent with novel mechanisms controlling IL-4 and IL-13 rather than an immunosuppressive agent generally suppressing T cell activation. Our results indicate that C3G may have potential as a novel therapeutic targeting Th2 cytokines and GATA-3 for various allergic diseases.
Supplemental material
The supplemental material for this paper is available at http://dx.doi.org/10.1080/09168451.2014.912115.
Supplemental Fig. 1
Download MS Power Point (1.2 MB)Supplemental Fig. 1 caption
Download MS Word (13.4 KB)Funding
This work was supported by “Food Functionality Evaluation Program” under the Ministry of Agriculture, Food and Rural Affairs, The Traditional Korean Medicine R&D Project, Ministry of Health & Welfare, Republic of Korea (HI13C0493), The Korea Science and Engineering Foundation (KOSEF) funded by the Korea Government (MEST) [grant number 2010-0012505], and The RIC program of the MKE (Ministry of Knowledge Economy) of Daejeon University.
Notes
Abbreviations: C3G, cyanidin-3-glucoside chloride; CsA, cyclosporin A; GATA-3, GATA-binding protein 3; IL, interleukin; PMA, phorbol 12-myristate 13-acetate; RT-PCR, reverse transcriptase-polymerase chain reaction.
References
- Venkayya R, Lam M, Willkom M, Grünig G, Corry DB, Erle DJ. The Th2 lymphocyte products IL-4 and IL-13 rapidly induce airway hyperresponsiveness through direct effects on resident airway cells. Am. J. Respir. Cell Mol. Biol. 2002;26:202–208.10.1165/ajrcmb.26.2.4600
- O’Garra A. Cytokines induce the development of functionally heterogeneous T helper cell subsets. Immunity. 1998;8:275–283.10.1016/S1074-7613(00)80533-6
- Ricci M, Matucci A, Rossi O. IL-4 as a key factor influencing the development of allergen-specific Th2-like cells in atopic individuals. J. Investig. Allergol. Clin. Immunol. 1997;7:144–150.
- Hershey GK. IL-13 receptors and signaling pathways: an evolving web. J. Allergy Clin. Immunol. 2003;111: quiz 691, 677–690.10.1067/mai.2003.1333
- Mamessier E, Magnan A. Cytokines in atopic diseases: revisiting the Th2 dogma. Eur. J. Dermatol. 2006;16:103–113.
- Agnello D, Lankford CS, Bream J, Morinobu A, Gadina M, O’Shea JJ, Frucht DM. Cytokines and transcription factors that regulate T helper cell differentiation: new players and new insights. J. Clin. Immunol. 2003;23:147–161.10.1023/A:1023381027062
- Takemoto N, Arai K, Miyatake S. Cutting edge: the differential involvement of the N-finger of GATA-3 in chromatin remodeling and transactivation during Th2 development. J. Immunol. 2002;169:4103–4107.
- Moyer RA, Hummer KE, Finn CE, Frei B, Wrolstad RE. Anthocyanins, phenolics, and antioxidant capacity in diverse small fruits: vaccinium, rubus, and ribes. J. Agric. Food. Chem. 2002;50:519–525.10.1021/jf011062r
- Scalbert A, Williamson G. Dietary intake and bioavailability of polyphenols. J. Nutr. 2000;130:2073S–2085S.
- Amorini AM, Fazzina G, Lazzarino G, Tavazzi B, Di Pierro D, Santucci R, Sinibaldi F, Galvano F, Galvano G. Activity and mechanism of the antioxidant properties of cyanidin-3-O-β-glucopyranoside. Free Radic. Res. 2001;35:953–966.10.1080/10715760100301451
- Rossi A, Serraino I, Dugo P, Paola R, Mondello L, Genovese T, Morabito D, Dugo G, Sautebin L, Caputi AP, Cuzzocrea S. Protective effects of anthocyanins from blackberry in a rat model of acute lung inflammation. Free Radic. Res. 2003;37:891–900.10.1080/1071576031000112690
- Serraino I, Dugo L, Dugo P, Mondello L, Mazzon E, Dugo G, Caputi AP, Cuzzocrea S. Protective effects of cyanidin-3-O-glucoside from blackberry extract against peroxynitrite-induced endothelial dysfunction and vascular failure. Life Sci. 2003;73:1097–1114.10.1016/S0024-3205(03)00356-4
- Tsuda T, Horio F, Uchida K, Aoki H, Osawa T. Dietary cyanidin 3-O-beta-D-glucoside-rich purple corn color prevents obesity and ameliorates hyperglycemia in mice. J. Nutr. 2003;133:2125–2130.
- Han SJ, Ryu SN, Trinh HT, Joh EH, Jang SY, Han MJ, Kim DH. Metabolism of cyanidin-3-O-β-D-glucoside isolated from black colored rice and its antiscratching behavioral effect in mice. J. Food Sci. 2009;74:H253–H258.10.1111/jfds.2009.74.issue-8
- Choi JJ, Park BK, Song GY, Kim JS, Kim JH, Kim DH, Jin M. Establishment of an in vitro test system to evaluate the down-regulatory activities of natural products on IL-4. Arch. Pharm. Res. 2007;30:1102–1110.10.1007/BF02980244
- Choi JJ, Park BK, Park S, Yun CY, Kim DH, Kim JS, Hwang ES, Jin M. Development of an in vitro test system measuring transcriptional downregulatory activities on IL-13. J. Microbiol. Biotechnol. 2009;19:331–337.
- Hsu WH, Lee BH, Hsu YW, Pan TM. Inhibition of Th2 cytokine production in T cells by monascin via PPAR-γ activation. J. Agric. Food. Chem. 2013;61:8126–8133.10.1021/jf402373z
- Garaude J, Cherni S, Kaminski S, Delepine E, Chable-Bessia C, Benkirane M, Borges J, Pandiella A, Iniguez MA, Fresno M, Hipskind RA, Villalba M. ERK5 activates NF-kappaB in leukemic T cells and is essential for their growth in vivo. J. Immunol. 2006;177:7607–7617.
- Romagnani S. T-cell subsets (Th1 versus Th2). Ann. Allergy Asthma Immunol. 2000;85: quiz 18, 21, 9–21.10.1016/S1081-1206(10)62426-X
- Yang J, Murphy TL, Ouyang W, Murphy KM. Induction of interferon-γ production in Th1 CD4+ T cells: evidence for two distinct pathways for promoter activation. Eur. J. Immunol. 1999;29:548–555.10.1002/(ISSN)1521-4141
- Serra D, Paixão J, Nunes C, Dinis TC, Almeida LM. Cyanidin-3-glucoside suppresses cytokine-induced inflammatory response in human intestinal cells: comparison with 5-aminosalicylic acid. PLoS One. 2013;8:e73001.10.1371/journal.pone.0073001
- Grusak MA. Phytochemicals in plants: genomics-assisted plant improvement for nutritional and health benefits. Curr. Opin. Biotechnol. 2002;13:508–511.10.1016/S0958-1669(02)00364-6
- Palombo EA. Phytochemicals from traditional medicinal plants used in the treatment of diarrhoea: modes of action and effects on intestinal function. Phytother. Res. 2006;20:717–724.10.1002/(ISSN)1099-1573
- Acquaviva R, Russo A, Galvano F, Galvano G, Barcellona ML, Li Volti G, Vanella A. Cyanidin and cyanidin 3-O-beta-D-glucoside as DNA cleavage protectors and antioxidants. Cell Biol. Toxicol. 2003;19:243–252.10.1023/B:CBTO.0000003974.27349.4e
- Galvano F, La Fauci L, Lazzarino G, Fogliano V, Ritieni A, Ciappellano S, Battistini NC, Tavazzi B, Galvano G. Cyanidins: metabolism and biological properties. J. Nutr. Biochem. 2004;15:2–11.10.1016/j.jnutbio.2003.07.004
- Park SJ, Shin WH, Seo JW, Kim EJ. Anthocyanins inhibit airway inflammation and hyperresponsiveness in a murine asthma model. Food Chem. Toxicol.. 2007;45:1459–1467.10.1016/j.fct.2007.02.013
- Izuhara K, Arima K, Yasunaga S. IL-4 and IL-13: their pathological roles in allergic diseases and their potential in developing new therapies. Curr. Drug Targets Inflamm. Allergy. 2002;1:263–269.10.2174/1568010023344661
- Abbas AK, Murphy KM, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787–793.10.1038/383787a0
- Kumar RK, Herbert C, Yang M, Koskinen AM, McKenzie AN, Foster PS. Role of interleukin-13 in eosinophil accumulation and airway remodelling in a mouse model of chronic asthma. Clin. Exp. Allergy. 2002;32:1104–1111.10.1046/j.1365-2222.2002.01420.x
- Oh CK, Geba GP, Molfino N. Investigational therapeutics targeting the IL-4/IL-13/STAT-6 pathway for the treatment of asthma. Eur. Respir. Rev. 2010;19:46–54.10.1183/09059180.00007609
- Yamashita M, Ukai-Tadenuma M, Miyamoto T, Sugaya K, Hosokawa H, Hasegawa A, Kimura M, Taniguchi M, DeGregori J, Nakayama T. Essential role of GATA3 for the maintenance of type 2 helper T (Th2) cytokine production and chromatin remodeling at the Th2 cytokine gene loci. J. Biol. Chem. 2004;279:26983–26990.10.1074/jbc.M403688200
- Zhu J, Min B, Hu-Li J, Watson CJ, Grinberg A, Wang Q, Killeen N, Urban JF Jr, Guo L, Paul WE. Conditional deletion of Gata3 shows its essential function in TH1-TH2 responses. Nat. Immunol. 2004;5:1157–1165.10.1038/ni1128
- Zhang DH, Yang L, Cohn L, Parkyn L, Homer R, Ray P, Ray A. Inhibition of allergic inflammation in a murine model of asthma by expression of a dominant-negative mutant of GATA-3. Immunity. 1999;11:473–482.10.1016/S1074-7613(00)80122-3
- Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100:655–669.10.1016/S0092-8674(00)80702-3
- Du Q, Gu X, Cai J, Huang M, Su M. Chrysin attenuates allergic airway inflammation by modulating the transcription factors T-bet and GATA-3 in mice. Mol. Med. Rep. 2012;6:100–104.
- Choi JR, Lee CM, Jung ID, Lee JS, Jeong YI, Chang JH, Park HJ, Choi IW, Kim JS, Shin YK, Park SN, Park YM. Apigenin protects ovalbumin-induced asthma through the regulation of GATA-3 gene. Int. Immunopharmacol. 2009;9:918–924.10.1016/j.intimp.2009.03.018
- Gao F, Wei D, Bian T, Xie P, Zou J, Mu H, Zhang B, Zhou X. Genistein attenuated allergic airway inflammation by modulating the transcription factors T-bet, GATA-3 and STAT-6 in a murine model of asthma. Pharmacology. 2012;89:229–236.10.1159/000337180
- Tanaka T. Flavonoids For Allergic Diseases: Present Evidence And Future Perspective. Curr. Pharm. Des. 2013;20:879–885.