Abstract
Long-chain N-vanillyl-acylamides (LCNVAs) were generated from plant oils and vanillylamine (VA) by nucleophilic amidation without any catalytic reagents. The resulting LCNVAs varied according to the fatty acid composition of the plant oil used. Therefore, the LCNVAs contained in Capsicum oleoresins were products that were spontaneously generated from the oleoresin during storage.
N-vanillyl-acylamides (NVAs) are a group of compounds known as capsaicinoids, which are natural ingredients specifically derived from Capsicum fruits. Capsaicin, the most abundant NVA in capsaicinoids, causes a pungent sensation and possesses various physiological and biological activities.Citation1) NVAs with an acyl chain length of approximately nine carbons, such as capsaicin, cause the strongest sensation of pungency, whereas those with longer or shorter acyl chain than that of capsaicin’s are less pungent.Citation2) Although NVAs with a chain length of more than 18 carbons do not generate any stimulus, some capsaicin-like useful activities remain in these long acyl chain NVAs (LCNVAs). Among the LCNVAs developed as synthetic capsaicinoids, olvanil, which contains an oleic (C18:1) acyl moiety, has received much attention because of its high capsaicin-like potency.Citation3) Other LCNVAs having acyl moieties of naturally occurring type, such as myristic (C14:0), palmitic (C16:0), stearic (C18:0), linoleic (C18:2), and α-linolenic (C18:3) acids have been developed as myrvanil, palvanil, stevanil, livanil, and linvanil, respectively.Citation4)
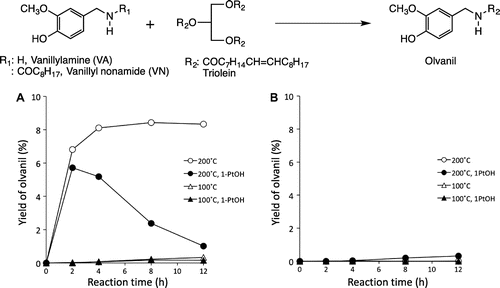
In our previous study, we identified these LCNVAs in a Capsicum oleoresin commonly used as a seasoning.Citation5) Because the oleoresin contained more amounts of LCNVAs than that in the fresh chili pepper fruits and because the composition of acyl moieties in LCNVAs in the oleoresin was closely correlated to that in the oil fraction (triacylglyceride) of the oleoresin, we speculate that the LCNVAs were generated in Capsicum oleoresin by spontaneous transacylation of triacylglyceride and capsaicinoid or vanillylamine (VA) during storage. In this study, we performed a model reaction by using purified reagents, triolein, VA, and vanillyl nonamide (VN) that mimics the chemical structure of capsaicin, to generate olvanil (Fig. ). Furthermore, by using some plant oils instead of triolein, we attempted to generate LCNVAs that contain acyl moieties of plant oils.
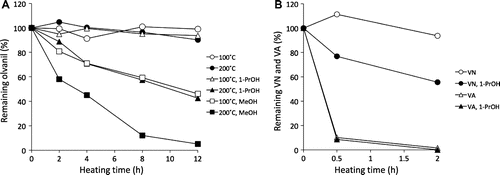
Fig. 1. Time course of olvanil converted from triolein and (A) vanillylamine (VA) or (B) vanillyl nonamide (VN).
VA hydrochloride (Sigma, St. Louis, MO, USA) or VN (Sigma) (1 mM) in 1 mL of triolein (Sigma) (1 M) was sealed using N2 gas. When 1-propanol (1-PrOH) was used as an additive to dissolve VA into triolein, the concentration of 1-PrOH was 20% of that of the mixture. After heating at various conditions, 0.1 mL of the mixture was extracted five times by using 0.5 mL of methanol (MeOH). The combined MeOH solution was dried in vacuo. The residue was dissolved with MeOH to a volume of 5 mL for HPLC analysis. HPLC analysis was performed using a LC-10A system (Shimadzu, Kyoto, Japan) with the following specifications: column, Wakosil-II 5C18 AR 4.6 × 150 mm (Wako, Osaka, Japan); solvent, 95–100%MeOH (0–10 min), 100% MeOH (10–18 min); flow rate, 1 mL/min; fluorescence detection, Ex 280 nm and Em 320 nm. Authentic olvanil was purchased from Sigma.
The time-course yield in olvanil synthesized from VA and triolein is shown in Fig. (A). Significant olvanil production up to 5.8 or 8.4% yield was observed at 200 °C with or without 1-PrOH. However, no further increase in yield was observed over the next 4 h, and instead, a gradual decrease occurred when using 1-PrOH. Only a small yield of olvanil (less than 0.4%) was generated at 100 °C without 1-PrOH. On the other hand, a six-month reaction without using any alcohol at 25 °C resulted in olvanil accumulation (0.8% yield). When VN was used instead of VA, a 0.3% olvanil production was achieved only in the conditions with 1-PrOH at 200 °C for 12 h (Fig. (B)). Fig. shows the stabilities of olvanil, VN, and VA in triolein with or without alcohol. Although olvanil was stable at 100 °C with or without alcohol and at 200 °C without 1-PrOH, it gradually underwent decomposition in the presence of alcohol, especially with MeOH (Fig. (A)). The same trend was also observed with VN at 200 °C with 1-PrOH. The remaining VA at 0.5 h after heating to 200 °C was only around 10% regardless of the presence or absence of 1-PrOH (Fig. (B)).
In the model reaction as mentioned above, olvanil was significantly produced from triolein and VA at higher temperatures (200 °C). Olvanil production was also observed at ambient temperature, although this occurred after an extended period. Because the amine (i.e. VA) showed a higher olvanil yield than the amide (i.e. VN), the model reaction probably occurred through a nucleophilic amidation, where VA acted as a stronger nucleophile by attacking a carbonyl carbon of the acyl group than VN. We had expected high yield of olvanil from VA by using excess triolein (1000-fold mole of VA). However, the solubility of VA in triolein was significantly low so the reaction had to be carried out using a heterogeneous system. We, therefore, added 1-PrOH into the model system to dissolve VA in triolein to generate a homogeneous solution. However, the addition of alcohol specifically resulted in the decomposition of the produced olvanil. This might be a reason for the decrease of olvanil accumulation after 2 h from VA at 200 °C using 1-PrOH. The low production of olvanil from VN with 1-PrOH might have resulted from the generated VA, which could be a decomposed product of VN when using alcohol. Furthermore, the low stability of VA might also be responsible for the low olvanil yield. Although VA might be a good material for LCNVA production, the amount of VA in Capsicum fruits seems to be extremely smaller than that of capsaicin in the fruits. For example, the chili pepper cultivar Peru contained only 14 μg/g dry weight fruit of VA, which accounted for about 1/150 of its total capsaicinoid content.Citation6) In our previous study, the highest LCNVA content (ca. 2400 μg/g) in Capsicum oleoresin was around 40% of its capsaicin content.Citation5) Therefore, the LCNVAs in the oleoresin were probably generated from the oil fraction of the oleoresin and VA that would have been decomposed from capsaicin over a long period.
For practical use, we conducted LCNVA production by using plant oils and VA, and the reaction was conducted using higher VA concentrations than that in the model reaction to supply much more effective VA based on its instability and insolubility. The production process was set at 180 °C, which is the temperature commonly used in deep frying food. Approximately 10 g of olive oil (Wako) or soybean oil (Wako) containing 5 mm of VA was heated at 180 °C for 1 h. Analytical samples of the generated LCNVAs were prepared using the previously described method. LCNVAs in the samples were qualified and quantified by LC-MS/MS, according to our previous study.Citation5)
The total yields of LCNVAs from VA in olive oil and soybean oil were 0.15 and 0.02%, respectively. The relative LCNVA yield by using the plant oils was similar to that of fatty acids that compose triacylglycerides of these oils (Fig. ). The lower LCNVA yield might be due to the slightly lower reaction temperature utilized in the assay compared to that of the model reaction previously described. The differences in yield between the oils might also be due to the variations in the solubility of VA in the oils.
Fig. 3. Comparison of the fatty acid composition of plant oilsCitation7) and relative content of LCNVAs generated from VA and plant oils.
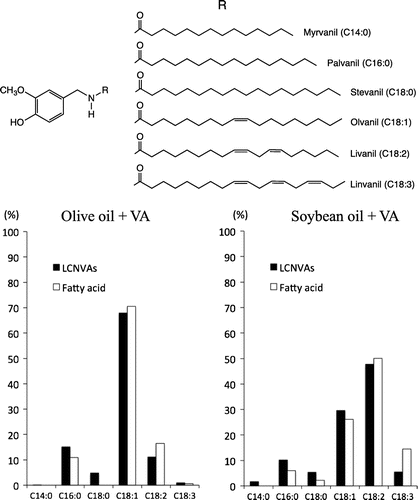
In this study, we demonstrated that LCNVAs could be generated from plant oils (triacylglycerides) and VA without the use of any catalytic reagents. This indicates that the LCNVAs contained in Capsicum oleoresins were spontaneously generated from the oil fraction of the oleoresin and VA, which originated from capsaicin over the course of time. Furthermore, the types of LCNVAs depended on the fatty acid composition of the plant oils used in the reaction, suggesting that blending plants oils could produce oils that contain desirable LCNVAs. Although the optimal material for LCNVA production was the stronger nucleophile VA, no studies have shown the use of VA from natural resources except for that from the Capsicum plants. Our research team is currently exploring a wide range of plants that harbor VA.
Notes
Abbreviations: LCNVA, long-chain N-vanillyl-acylamide; LC-MS/MS, liquid chromatography-tandem mass spectrometry; VA, vanillylamine; VN, vanillyl nonamide.
References
- Govindarajan VS, Sathyanarayana MN. Capsicum - production, technology, chemistry, and quality. Part V. Impact on physiology, pharmacology, nutrition, and metabolism; structure, pungency, pain, and desensitization sequences. CRC Crit. Rev. Food Sci. Nutr. 1991;29:435–474.10.1080/10408399109527536
- Watanabe T, Kawada T, Kato T, Harada T, Iwai K. Effects of capsaicin analogs on adrenal catecholamine secretion in rats. Life Sci. 1994;54:369–374.10.1016/0024-3205(94)00793-4
- Szallasi A, Di Marzo V. New perspectives on enigmatic vanilloid receptors. Trends Neurosci. 2000;23:491–497.10.1016/S0166-2236(00)01630-1
- Melck D, Bisogno T, De Petrocellis L, Chuang H, Julius D, Bifulco M, Di Marzo V. Unsaturated long-chain N-acyl-vanillyl -amides (N-AVAMS): vanilloid receptor ligands that inhibit anandamide-facilitated transport and bind to CB1 cannabinoid receptors. Biochem. Biophys. Res. Commun. 1999;262:275–284.10.1006/bbrc.1999.1105
- Kobata K, Saito K, Tate H, Nashimoto A, Okuda H, Takemura I, Miyakawa K, Takahashi M, Iwai K, Watanabe T. Long-chain N-vanillyl-acylamides from Capsicum oleoresin. J. Agric. Food Chem. 2010;58:3627–3631.10.1021/jf904280z
- Kobata K, Sugawara M, Mimura M, Yazawa S, Watanabe T. Potent production of capsaicinoids and capsinoids by Capsicum peppers. J. Agric. Food Chem. 2013;61:11127–11132.10.1021/jf403553w
- Ayorinde FO, Garvin K, Saeed K. Determination of the fatty acid composition of saponified vegetable oils using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 2000;14:608–615.10.1002/(ISSN)1097-0231