Abstract
Chinese yam (Dioscorea opposita) is an important tuberous crop owing to its dual use as a food as well as a medicine. Tissue culture techniques allow the rapid multiplication of virus-free plant materials. The use of microtubers offers an attractive alternative to in vitro-grown plantlets for the micropropagation and exchange of healthy Chinese yam materials. A protocol for the in vitro production of Chinese yam microtubers was developed in this study. Though we tested both one-step and two-step procedures, only the two-step procedure showed favorable results for tuberization. Media with 60 g L−1 sucrose yielded the highest microtuber index. We demonstrate that table sugar was an efficient and economical alternative to analytical grade sucrose for microtuber production. Using an orthogonal experimental design, we determined the optimal growth regulator combination for microtuber induction and development. The microtubers obtained from our protocol sprouted readily both in vitro and in soil.
Graphical Abstract
In Vitro Production of Microtubers in Chinese yam and Sprouting Test. A Protocol for Efficient Microtuber Production was Developed in This Study.
Chinese yam, a member of the family Dioscoreaceae, is widely cultivated in East and South Asia for its subterranean and aerial tubers. These tubers have long been used as both a nutritious food source and as a traditional medicine in China. Chinese yam has properties which make it one of the most important and commonly used materia medica in the Chinese medicinal repertoire.Citation1,Citation2) Modern science is now revealing that Chinese yam has great health benefits.Citation3−Citation7) Chinese yam production systems face the problem that the plants are very often infected with various viruses during long-term vegetative reproduction. As such, the production of virus-free plants is a desirable technological goal. However, the traditional methods of clonal propagation using in vitro plantlets are limited in their potential for implementation, due to plant susceptibility to environmental changes and poor field establishment. Further, there are specialized requirements for germplasm exchange and the maintenance of in vitro plantlets.
Microtubers are small tubers originating from plant tissues in vitro. Microtubers offer several advantages over in vitro-grown plants. They are more tolerant to light and temperature variations than plantlets, they do not have the requirement of frequent subculturing as is needed for plantlet maintenance, and they are easier to handle and ship than plantlets. Of additional benefit, microtubers can be stored for a long period of time without losing their viability. Therefore, microtubers may represent a very attractive alternative to plantlets as a means of commercialization and international exchange of virus-free Chinese yam germplasm.
The conditions for microtuber induction have already been studied in a number of Dioscorea species, including D. alata,Citation8) D. composita,Citation9) D. rotundata,Citation10) D. nipponica,Citation11) the D. cayenensis–D. rotundata complex,Citation12) and D. fordii.Citation13) Factors known to influence microtuberization in vitro include carbohydrate supply, light, photoperiod, temperature, and phytohormone concentration.Citation8−Citation13) Investigations of microtuberization in Chinese yam have not yet been described. The optimal conditions for in vitro microtuberization with the reported species were different. As such, examination of a case-by-case basis is required for establishment of an optimal protocol for microtuberization in Chinese yam. In this paper, we report the results of a series of experiments carried out to determine the cultural conditions required to maximize in vitro production of microtubers in Chinese yam.
Materials and methods
Plant material and culture media
The D. opposita cv. No. B (originating from Henan Province) was used for the establishment of an in vitro microtuberization protocol. Four more cultivars of Chinese yam originating from Shanxi Province (Shanxiyongji and Wenxishangguan), Shandong Province (Jiaxiangxichangmao), and Japan (Baiyu) were evaluated for use with this protocol. We also evaluated these cultivars for traits including tuber size, tuber dry matter, DNA polymorphisms, etc. though we do not present this data here (Mingjun Li, unpublished results). Nodal segments were obtained from germ-free plants in flasks (500 mL) containing 50 mL Murashige and Skoog (MS) basal mediumCitation14) supplemented with 0.005 mg L−1 thidiazuron and 30 g L−1 sucrose. Subculturing was carried out every 30 days. The pH of the culture medium was adjusted to 5.6–5.8 prior autoclaving for 20 min at 121 °C. Cultures were maintained at 60 mE·s−1·m−2, 25 ± 2 °C with a 14 h light/10 h dark photoperiod.
Culture procedures
Both a one-step protocol and a two-step protocol were compared for their effects on microtuber induction. In the one-step procedure, 2 cm single nodal segments were excised from plants as explants and incubated in 500 mL flasks containing 50 mL liquid induction medium (MS basal medium +60 g L−1 sucrose) for 90 days. In the initial step of the two-step procedure, 2 cm single nodal segments were cultured in 500 mL flasks with 50 mL liquid propagation medium [MS basal medium + 3 mg L−1 kinetin (KT) + 0.02 mg L−1 naphthalene acetic acid + 30 g L−1 sucrose] for 30 days. In the second stage, plantlets of uniform size were transferred into 500 mL flasks containing 50 mL liquid induction medium and incubated for an additional 60 d.
Carbohydrate supply evaluation
The effects of sucrose on tuberization were investigated in the second stage of the two-step procedure. The MS basal medium was supplemented with sucrose at concentrations of 0, 30, 60, 90, and 120 g L−1. Various carbon sources were compared. These included analytical grade sucrose (Dengke, Tianjin, China) and white table sugar (Nanning Sugar Manufacturing CO., Nanning, China).
Supplement of plant growth regulators
In the second stage of the two-step procedure, MS basal medium was supplemented with 60 g L−1 sucrose and plant growth regulators. An orthogonal experimental design [L9 (34)]Citation13) was used to investigate the effects of KT, 2,4-dichlorophenoxyacetic acid (2,4-D), and paclobutrazol (PP333) on tuberization. Various levels of KT, 2,4-D, and PP333 were used in nine treatments, arranged according to the design (Table ). The independent variables were KT at 0.5, 1.0, and 2.0 mg L−1, 2,4-D at 0.02, 0.1, and 0.5 mg L−1, and PP333 at 0.01, 0.1, and 1.0 mg L−1.
Table 1. Concentrations of KT, 2,4-D, and PP333 used in the orthogonal experimental design L9 (34).
Data analysis
Each treatment consisted of three flasks with three explants per flask. Three replications were performed for each treatment. For this study, microtubers larger than 3 mm in diameter were defined as valid microtubers. At the time of harvest, the numbers of valid microtubers per plant, the fresh weight (FW, g), and the diameter (mm) of the microtubers were recorded. The production of microtubers was evaluated by the microtuber index [microtuber index = the number of valid microtuber per flask × mean diameter (mm) × mean FW (g)]. The significant differences (p < 0.05) were determined by t test for two correlated samples, and Fisher’s least significant difference (LSD) method was used for multiple comparisons. All statistical analyses were performed by using Statistical Product and Service Solutions (SPSS) software (version 13.0).Citation15)
Results and discussion
We evaluated the relative effects of cultural procedures and various culture media components on the induction of microtubers in D. opposite.
Comparison of the one-step and two-step culture procedures
Our results showed that the two-step protocol yielded a higher frequency of microtuber formation as compared to the one-step procedure (Table ). When using the one-step procedure, no shoot buds were regenerated from the nodal segments, and the segments turned yellow around day 20, eventually turning brown and dying. No microtubers were formed in the one-step procedure (data not shown).
Table 2. Comparison between the one-step and the two-step procedures for tuberization of Dioscorea. opposita cv. No. B.
In stage one of the two-step procedure, shoots were regenerated from the nodal segments in the propagation medium, and plantlets were well established at the end of this stage (30 days). In the second (induction) stage, the plants showed strong vegetative growth, and many shoot buds were regenerated. The formation of microtubers was observed within 10 days of incubation in the induction medium. The average microtuber number per plantlet was 1.7 by the end of the two-step procedure (Table ).
Optimization of carbon source
Sucrose is an important factor that supports the development of microtubers by facilitating carbohydrate supply.Citation16) In general, high sucrose concentrations have been shown to induce high frequencies of microtuber formation and development. This is the case for studies with D. cayenensis (40 g L−1),Citation17) D. composita (80 or 100 g L−1),Citation9) and D. fordii (75 g L−1).Citation18) However, contradictory results were reported for studies with D. fordii,Citation13) D. bulbifera,Citation16) and the D. cayenensis–D. rotundata complex.Citation12) With these materials, high sucrose concentrations inhibited tuberization rates. In our study, we tested a broad range of concentrations of sucrose (0, 30, 60, 90, and 120 g L−1) in the second step of the two-step protocol. Although high average numbers of valid microtubers per plantlet were observed with both 30 and 60 g L−1 sucrose concentrations, the highest microtuber index was obtained with 60 g L−1 sucrose (Table ). As such, we concluded that the optimal sucrose concentration for microtuber production in D. opposita cv. No. B was 60 g L−1.
Table 3. Optimization of carbon source and carbon concentration for tuberization of D. opposita cv. No. B.
We evaluated the efficiency of using white table sugar purchased from the local market as an alternative low-cost medium component for producing Chinese yam microtubers. Our result showed that there was no significant difference in the efficiency of white table sugar vs. analytical grade sucrose (Table ). We therefore concluded that white table sugar was a cheap and satisfactory carbon source for microtuber production.
Screening of highly efficient plant growth regulator combinations
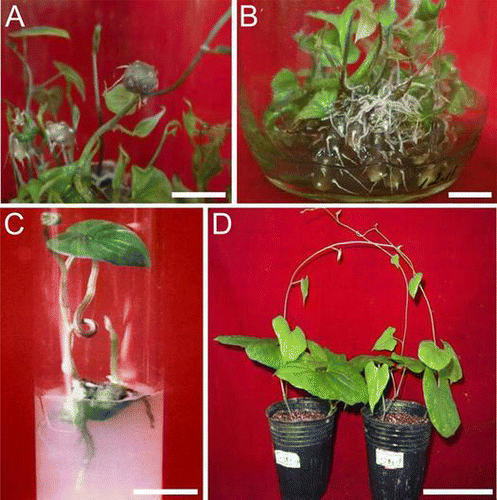
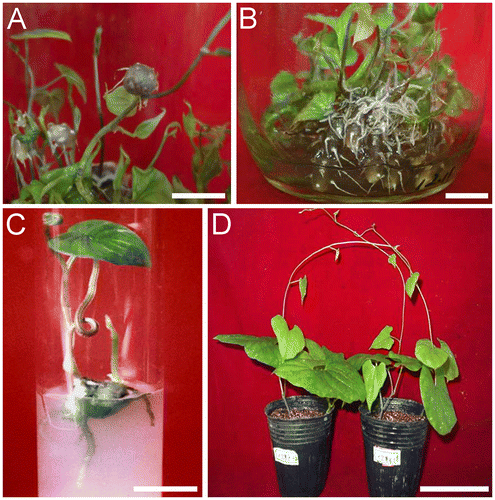
We conducted experiments in an attempt to exploit the individual effects of various plant growth regulators on tuberization in Chinese yam. We identified a positive role of 2,4-D (0.1 mg L−1) for increasing the number of valid microtubers, the mean microtuber diameter, and the microtuber index.Citation19) In addition, KT (0.5 mg L−1) was found to increase the mean diameter and the mean fresh weight of microtubers,Citation19) and PP333 (0.01 mg L−1) was found to increase the number of valid microtubers (Supplemental Table 1; see http://dx.doi.org/10.1080/09168451.2014.912119). In order to evaluate the optimal combinations of these regulators for microtuber production, an orthogonal experiment was carried out in which the interaction of these factors was investigated (Table ). The results (Table ) indicated that, of all the treatments, treatment number 2 (MS basal medium + 0.5 mg L−1 KT + 0.1 mg L−1 2,4-D + 0.1 mg L−1 PP333 + 60 g L−1 sucrose) yielded the highest mean number of valid microtubers per plantlet, had the highest microtuber index, and had high fresh weights. Further, the efficiency of this treatment was much higher than supplementation of the media with a single regulator. Similar results have been observed in other studies. For example, cytokinins were shown to have strong enhancing effects on microtuber induction when used in conjunction with auxin in D. nipponica.Citation18) Both aerial and submerged microtubers were formed when grown in the treatment number 2 media (Fig. (A) and (B)).
Table 4. Comparisons of D. opposita cv. No. B tuberization with various combinations of KT, 2,4-D, and PP333.
Fig. 1. Microtuber formation in plantlet cultures of D. opposita cv. No. B.
Notes: (A) Aerial microtubers that formed above the surface of the liquid medium. (B) Basal microtubers that formed under the surface of the liquid medium. (C) In vitro sprouted microtuber after transfer into culture tubes for 20 days. (D) Plant produced from microtubers after being sown in soil for approximately two months. Bars = 1 cm in A–C. Bar = 10 cm in D.
Effects of genotype on tuberization
The tuberization of four more Chinese yam cultivars was analyzed in the optimal medium (MS basal medium + 0.5 mg L−1 KT + 0.1 mg L−1 2,4-D + 0.1 mg L−1 PP333 + 60 g L−1 sucrose) using the two-step protocol. It appeared that the tuberization responses to our protocol were cultivar dependent. The preferred protocol was suitable for microtuber induction and development for Jiaxiangxichangmao, Baiyu, and Wenxishangguan, but not for Shanxiyongji (Table ).
Table 5. Influence of genotype on the induction of Chinese Yam microtubers.
Sprouting test
Microtubers produced with our optimal medium sprouted readily in solid tissue culture media as well as in soil. About 85% microtubers of the D. opposita cv. No. B sprouted in solid medium (MS basal medium + 30 g L−1 sucrose +8 g L−1 agar) 20 days after transfer from the induction medium (Fig. (C)). After removal from culture flasks and washing with tap water, 76% of valid microtubers sprouted and developed into young plantlets in soil (Fig. (D)).
Conclusion
The two-step procedure was more efficient than the one-step procedure in producing microtubers. The optimal concentration of sucrose to support microtuber induction and development was 60 g L−1, and table sugar was found to be an efficient and economical carbon source for microtuber production. The optimal medium tested for D. opposita cv. No. B for the production of microtubers was MS basal medium supplemented with 0.5 mg L−1 KT, 0.1 mg L−1 2,4-D, 0.1 mg L−1 PP333, and 60 g L−1 sucrose. This protocol could produce 2–3 microtubers per plantlet, and was demonstrated to work with several Chinese yam cultivars.
Supplemental material
The supplemental material for this paper is available at http://dx.doi.org/10.1080/09168451.2014.912119
Supplemental Fig. 1 caption
Download MS Word (19 KB)Supplemental Table 1
Download PDF (9.8 KB)Supplemental Fig. 1
Download MS Power Point (2 MB)Acknowledgments
We thank Tianliang Wang (Institute of Agricultural Sciences, Wenxian, China) for providing Chinese yam cultivars (Jiaxiangxichangmao, Baiyu, Wenxishangguan, and Shanxiyongji). The authors would like to thank Dr. Kang Chong and Dr. Yunyuan Xu (Institute of Botany, Chinese Academy of Sciences, Beijing, China) for comments on the project. This research was funded by the Plan for Scientific Innovation Talent of Henan Province (114200510013), the National Nature Science Foundation of China (81274019), the Program for Innovative Research Teams (in Science and Technology) of the University of Henan Province (IRTSTHN, 13IRTSTHN009), and the Innovation Scientists and Technicians Troop Construction Projects of Henan Province.
Notes
Abbreviations: 2,4-D, 2,4-dichlorophenoxyacetic acid; FW, fresh weight; KT, kinetin; LSD, Fisher’s least significant difference; MS, Murashige and Skoog (medium); PP333, paclobutrazol.
References
- Zuo Y, Tang D. Science of Chinese materia medica. Shanghai: Shanghai University of Traditional Chinese Medicine Press; 2003. p. 301–303.
- Bensky D, Clavey S, Gamble A, Stoger E, Bensky LL. Chinese herbal medicine: Materia Medica. Seattle, WA: Eastland Press; 2004, pp. 723.
- Chan YS, Ng TB. A lectin with highly potent inhibitory activity toward breast cancer cells from edible tubers of Dioscorea opposita cv. Nagaimo. PLoS One. 2013;8:e54212.10.1371/journal.pone.0054212
- Nagai T, Nagashima T. Functional properties of dioscorin, a soluble viscous protein from Japanese yam (Dioscorea opposita Thunb.) tuber mucilage tororo. Z. Naturforsch. C. 2006;61:792–798.
- Nishimura N, Tanabe H, Yamamoto T, Fukushima M. Raw Chinese yam (Dioscorea opposita) promotes cecal fermentation and reduces plasma non-HDL cholesterol concentration in rats. J. Nutr. Sci. Vitaminol. 2011;57:340–347.10.3177/jnsv.57.340
- Yang MH, Chin YW, Yoon KD, Kim J. Phenolic compounds with pancreatic lipase inhibitory activity from Korean yam (Dioscorea opposita). J. Enzyme Inhib. Med. Chem. 2014;29:1–6.10.3109/14756366.2012.742517
- Yang MH, Yoon KD, Chin YW, Park JH, Kim SH, Kim YC, Kim J. Neuroprotective effects of Dioscorea opposita on scopolamine-induced memory impairment in in vivo behavioral tests and in vitro assays. J. Ethnopharmacol. 2009;121:130–134.10.1016/j.jep.2008.10.010
- John JL, Courtney WH, Decoteau DR. The influence of plant growth regulators and light on microtuber induction and formation in Dioscorea alata L. cultures. Plant Cell Tiss. Org. Cult. 1993;34:245–252.10.1007/BF00029713
- Alizadeh S, Mantell SH, MariaViana A. In vitro shoot culture and microtuber induction in the steroid yam Dioscorea composita Hemsl. Plant Cell Tiss. Org. Cult. 1998;53:107–112.10.1023/A:1006036324474
- Balogun M, Fawole I, Ng S, Ng N, Shiwachi H, Kikuno H. Interaction among cultural factors in microtuberization of white yam (Dioscorea rotundata). Trop. Sci. 2006;46:55–59.10.1002/(ISSN)1556-9179
- Chen FQ, Fu Y, Wang DL, Gao X, Wang L. The effect of plant growth regulators and sucrose on the micropropagation and microtuberization of Dioscorea nipponica Makino. J. Plant Growth Regul. 2007;26:38–45.10.1007/s00344-005-0147-2
- Ovono PO, Kevers C, Dommes J. Axillary proliferation and tuberisation of Dioscorea cayenensis–D. rotundata complex. Plant Cell Tiss. Org. Cult. 2007;91:107–114.10.1007/s11240-007-9238-z
- Yan H, Yang L, Li Y. Axillary shoot proliferation and tuberization of Dioscorea fordii Prain et Burk. Plant Cell Tiss. Org. Cult. 2011;104:193–198.10.1007/s11240-010-9818-1
- Murashige T, Skoog F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962;15:473–497.10.1111/ppl.1962.15.issue-3
- Li J, Han Y, Zhao Q, Li C, Xie Q, Chong K, Xu Y. The E3 ligase AtRDUF1 positively regulates salt stress responses in Arabidopsis thaliana. PLoS One. 2013;8:e71078.10.1371/journal.pone.0071078
- Mantell SH, Hugo SA. Effects of photoperiod, mineral medium strength, inorganic ammonium, sucrose and cytokinin on root, shoot and microtuber development in shoot cultures of Dioscorea alata L. and D. bulbifera L. yams. Plant Cell Tiss. Org. Cult. 1989;16:23–37.10.1007/BF00044069
- Jasik J, Mantell S. Effects of jasmonic acid and its methylester on in vitro microtuberisation of three food yam (Dioscorea) species. Plant Cell Rep. 2000;19:863–867.10.1007/s002990000207
- Feng F, Ye C, Li Y, Xu W. Effects of growth regulators, carbon sources and photoperiod on in vitro formation and growth and development of microtubers of Dioscorea fordii Prain et Burk. Plant Physiol. Commun. 2007;43:1045–1049.
- Li M, Deng L, Liu X, Zhao X, Zhang X, Liu W. Effects of auxin and cytokinin on the induction of microtuber of Disocorea opposita Thunb. J. Henan Agric. Sci. 2008;11:102–106.
