Abstract
Apo-cytochomes c without heme are usually unstructured. Here we showed that apo-form of thermophilic Hydrogenophilus thermoluteolus cytochrome c’ (PHCP) was a monomeric protein with high helix content. Apo-PHCP was thermally stable, possibly due to the hydrophobic residues and ion pairs. PHCP is the first example of a structured apo-cytochrome c’, which will expand our view of hemoprotein structure formation.
Cytochrome c’ usually forms a homo dimeric structure,Citation1) and its single subunit is a four-helix bundle protein consisting of approximately 130 amino acid residues with a pentacoordinate heme. Cytochrome c’ binds diatomic gasses such as NO and CO, but not O2, through the heme, suggesting its putative roles in the dealing with these gases in cells. These structural and functional features distinctively differ from those of well-known class I spherical and monomeric cytochromes c that are components of electron transport chains.
Recently, we purified thermophilic Hydrogenophilus thermoluteolus cytochrome c’ (PHCP), which exhibits several unique features. For example, PHCP can be heterologously synthesized in Escherichia coli cells even with an imperfect System I,Citation2) which is unusual for general cytochrome c biogenesis. In addition, PHCP appears to be homotrimeric unlike other usual dimeric cytochromes c’, and is more stable than its mesophilic counterpart, Allochromatium vinosum cytochrome c’ (AVCP), exhibiting 55% sequence identity.Citation3) These unique features of PHCP were found as the holo-protein that covalently binds heme.
Here we investigated whether the stability of apo-PHCP without heme differs from that of apo-AVCP. In general, cytochromes c are unstructured in their apo-forms,Citation4) and heme binding leads to protein folding,Citation5,6) followed by stable structure formation allowing biological functions. So far apo-cytochromes c’ have not been investigated yet in such a way.
The authentic holo-PHCP and recombinant holo-AVCP proteins used in this study were prepared as described previously.Citation3) The apo-forms of PHCP and AVCP were prepared by the modified method of Fisher et al.Citation5) which had been applied to class I cytochromes c, as described previously.Citation7) Ten mg of Ag2SO4 in 0.1 mL of ultrapure water and 80 μL of acetic acid were added to 0.8 mL aliquots of 0.3–0.5 mM purified holo-PHCP (in the presence of 8 M urea) and holo-AVCP (without urea) solutions. The solutions were incubated in the dark for 4 h at 50 °C and then centrifuged for 20 min at 20,000 × g to remove heme aggregates. The supernatants were loaded onto a PD-10 desalting column (GE Healthcare, Tokyo) equilibrated with 25 mM sodium acetate buffer (pH 5.0), and the protein fractions were eluted with the same buffer. To dissociate their silver mercaptide bonds, the proteins were denatured with 6 M guanidine hydrochloride (GdnHCl), and treated with 1 M dithiothreitol (DTT). The solutions were incubated in the dark for 2 h at 25 °C and then centrifuged for 20 min at 20,000 × g to remove the silver mercaptide of DTT. The clear supernatants were loaded onto a PD-10 column equilibrated with 25 mM sodium acetate buffer (pH 5.0), and apo-proteins were eluted with the same buffer.
The resulting apo-protein solutions were extensively dialyzed against 20 mM potassium phosphate (pH 7.0) in order to completely remove the denaturant and other chemicals before the following measurements. Confirmation of the absence of residual holo-proteins was achieved by means of sodium dodecyl sulfate polyacrylamide gel electrophoresis followed by heme staining. Protein concentrations were determined spectrophotometrically using the extinction coefficient at 205 nm.
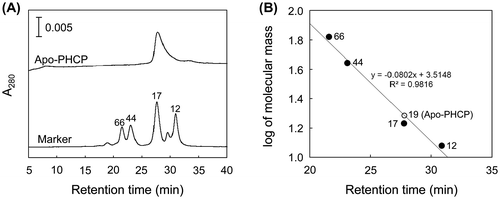
The native apo-PHCP molecular mass was estimated by a gel filtration TSK Gel SW3000 column (Tosoh, Tokyo) equilibrated with 10 mM Tris–HCl (pH 7.5) supplemented with 150 mM NaCl at a flow rate of 0.5 mL/min. The molecular markers used for calibration were horse cytochrome c (12 kDa), myoglobin (17 kDa), ovalbumin (44 kDa), and bovine serum albumin (66 kDa). A single peak of apo-PHCP was observed (Fig. (A)) with apparent molecular mass of 19 kDa (Fig. (B)). Since the calculated molecular mass of apo-PHCP is 14,829 Da, the present result indicates that apo-PHCP forms monomeric structure. Thus, multimeric structure observed in the holo-PHCP protein should be formed after the heme binding.
Fig. 1. Gel Filtration Analysis for apo-PHCP.
Note: (A) Gel filtration profile of the apo-PHCP. The elution profile is shown with that of the molecular markers. The values for molecular masses are also indicated in kDa. Protein was detected by the absorption at 280 nm. (B) Estimation of molecular mass as determined by gel filtration chromatography. Logarithm of marker molecular mass is plotted as a function of retention time (closed circles). Linear least-square fitting curve for the markers is shown with the equation and correlation coefficient. Retention time of apo-PHCP is plotted on the curve (open circle) with the value for the estimated molecular mass.
Circular dichroism (CD) spectra (190–260 nm) of the holo- and apo-forms of PHCP and AVCP (20 μM) in 20 mM potassium phosphate (pH 7.0) were obtained with a J-820 CD spectrometer (Jasco, Tokyo) in a cuvette of 1 mm path length at 25 °C to examine the secondary structures. From the ellipticity peak height of PHCP at 222 nm (Fig. (A)), which represents the helix structure, the helix content of holo-PHCP was estimated to be 65.6%. Even upon the removal of the heme, the peak at 222 nm remained, and the helix content was 54.8%. This value was far higher than that obtained for the thermally denatured holo-PHCP (13.7%). In addition, a peak at 208 nm, also representing a helix structure, was observed for both the holo- and apo-PHCP, whereas this peak disappeared for the denatured holo-PHCP; instead, a peak at 202 nm derived from the random coil structure appeared. These results indicate that the apo-PHCP is refolded after the denaturation with 6 M GdnHCl that was once added but removed during the protein preparation.
Fig. 2. CD Spectral Analysis.
Note: A and B, CD spectra of PHCP (A) and AVCP (B). The holo-proteins (black solid lines), apo-proteins (gray solid lines), and thermally denatured holo-proteins (black dashed lines) are shown in each panel. The spectra of the denatured holo-proteins were obtained at 95 °C for PHCP and 75 °C for AVCP. The vertical dotted lines indicate wavelength 222 nm. (C) Thermal Denaturation of Apo-PHCP. Representative normalized data for fraction of denatured apo-PHCP (closed circles) are plotted as a function of temperature, and a non-linear least-square fitting curve (solid line) is also shown. Midpoint of thermal denaturation (indicated by the vertical dashed line in panel C) was obtained from three independent measurements as described previouslyCitation3).
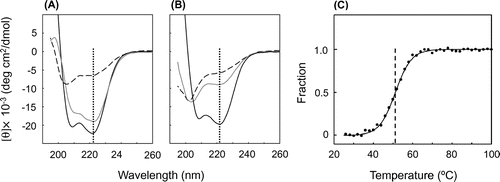
In contrast, although the helix content of holo-AVCP was estimated to be 60.4% from the CD 222 nm peak (Fig. (B)), apo-AVCP exhibited a helix content of only 21.7%. This value was close to that obtained with the thermally denatured holo-AVCP (11.1%). In addition, the random coil structure was formed in the apo-AVCP and thermally denatured holo-AVCP, as the spectra exhibited the peak at 202 nm.
Taken together, the higher helix content of apo-PHCP than that of apo-AVCP indicated that the PHCP helix structure tends to be formed without heme and that AVCP requires heme for the formation of its structure similar to in the case of general cytochromes c.Citation5,6) PHCP is the first example of a structured apo-cytochrome c’. The structured apo-PHCP protein is analogous to another four-helix bundle heme protein, E. coli cytochrome b562, which spontaneously acquires free heme in its structured apo-form.Citation8) Therefore, PHCP may be produced through DsbD (also known as DipZ, functioning as a reductant for cytochrome c heme-binding Cys residuesCitation9))-independent biosynthesis in E. coli.Citation2) Consistently, similar DsbD-independent biosynthesis has been observed exceptionally for hyperthermophilic Aquifex aeolicus class I cytochrome c555 (AA cytc555),Citation10,11) which is also structured as an apo-protein.Citation12)
Thermal stability of the apo-PHCP protein (20 μm) in 20 mm potassium phosphate (pH 7.0) was then measured by the temperature-dependent CD ellipticity change at 222 nm. The CD value was recorded from 25 to 100 °C at a heating rate of 1.0 °C/min. The raw CD data for the thermal denaturation of apo-PHCP could be normalized and subjected to nonlinear least-squares fitting (Fig. (C)) as described previously for the holo-protein,Citation3) which showed midpoint of thermal denaturation of 50.3 ± 1.7 °C. This value was lower than that of holo-PHCP (87.1 °C), but close to that obtained for the holo-AVCP protein under the same conditions (52.4 °C).Citation3) These results indicate that heme contributes to the PHCP stability, as observed for AA cytc555,Citation12) but that apo-PHCP is stable enough against thermal denaturation, the level being similar to that of mesophilic holo-AVCP.
The AVCP tertiary structure together with the PHCP primary sequence indicated that PHCP is more advantageous as to stability due to the hydrophobic core interior of the single subunit. In the AVCP region from Ala-28 to Tyr-37 (residue numbering of the PHCP system is adopted), Asn-30 and Tyr-37 were replaced by more hydrophobic Val and Phe, respectively, in PHCP (Fig. (A)). The gaps in this AVCP region were occupied by Met-29, Met-35, and Pro-36 in PHCP (Fig. (A)), which may be related to the better packing in the corresponding region in PHCP (Fig. (B)). In addition, Val-113 in the AVCP protein was replaced by more hydrophobic Ile in PHCP. These residues are located around one edge of the single subunit (Fig. (B)), and may contribute to tertiary structure formation of apo-PHCP, thus leading to the higher stability of apo-PHCP than that of apo-AVCP.
Fig. 3. Sequence Comparison and Tertiary Structure of the AVCP Protein.
Note: (A) Sequence comparison. Gaps in the alignment are indicated by dashes. Identical residues in PHCP and AVCP are indicated by asterisks between the sequences. Helix regions in AVCP are underlined. Residue numbers are indicated above the PHCP sequence. (B) Whole tertiary structure of AVCP (PDB ID:1BBH). The main chain backbone and heme are depicted as ribbon and stick models, respectively. As the root mean square deviation for the main chain folding of two subunits in AVCP is less than 0.5 Å, only one subunit structure is shown. The positions of α carbon atoms of relevant residues mentioned in the text are shown by closed circles. (C) A part of the tertiary structure of AVCP, in which ion pairs appear to be formed in PHCP.
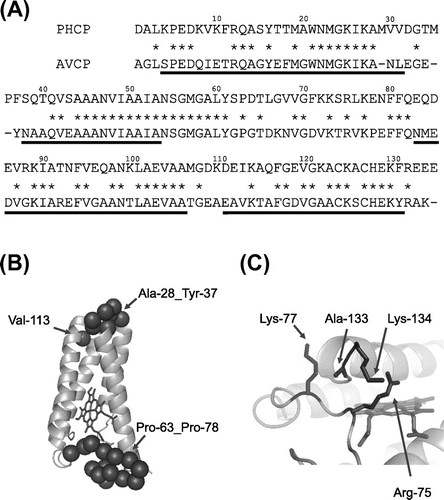
Another edge of the single subunit also differed between PHCP and AVCP (Fig. (A) and (B)). This region is located in the middle of the second and third helix regions of AVCP (from Pro-63 to Pro-78). Asp-66, Asn-68, Asp-71, and Val-76 in AVCP are less hydrophobic than the corresponding residues (Leu, Val, Phe, and Leu, respectively) in PHCP, which may hydrophobically contribute to the stability. In addition, this edge in PHCP is also advantageous as to stability; Arg-75 and Lys-77 in this region of PHCP may have a chance to form ion pairs with two successive unique Glu residues at positions 133 and 134 (Ala-133 and Lys-134 in the case of AVCP) (Fig. (C)).
In this study, we showed that monomeric apo-PHCP has higher helix content than apo-AVCP. Next, apo-PHCP exhibits a thermal denaturation profile. From the primary sequence comparison between PHCP and AVCP, and tertiary structure model of AVCP, two parts in PHCP were found to be advantageous due to its high stability. PHCP is the first example of a structured apo-cytochrome c’, which will expand our view of hemoprotein structure formation.
Acknowledgments
This work was performed under the Cooperative Research Program of the “Network Joint Research Center for Materials and Devices.”
Funding
This work was supported by a Grant-in-Aid for Scientific Research on Innovative Areas [grant number 20118005] from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Notes
Abbreviations: AA cytc555, Aquifex aeolicus cytochrome c555; AVCP, Allochromatium vinosum cytochrome c’; PHCP, Hydrogenophilus thermoluteolus cytochrome c’.
References
- Ren Z, Meyer T, McRee DE. Atomic structure of a cytochrome c’ with an unusual ligand-controlled dimer dissociation at 1.8 Å resolution. J. Mol. Biol. 1993;234:433–445.10.1006/jmbi.1993.1597
- Inoue H, Wakai S, Nishihara H, Sambongi Y. Heterologous synthesis of cytochrome c’ by Escherichia coli is not dependent on the System I cytochrome c biogenesis machinery. FEBS J. 2011;278:2341–2348.10.1111/j.1742-4658.2011.08155.x
- Fujii S, Masanari M, Inoue H, Yamanaka M, Wakai S, Sambongi Y. High thermal stability and unique trimer formation of cytochrome c’ from thermophilic Hydrogenophilus thermoluteolus. Biosci. Biotechnol. Biochem. 2013;77:1677–1681.10.1271/bbb.130226
- Stellwagen E, Rysavy R, Babul G. The conformation of horse heart apocytochrome c. J. Biol. Chem. 1972;247:8074–8077.
- Fisher WR, Taniuchi H, Anfinsen CB. On the role of heme in the formation of the structure of cytochrome c. J. Biol. Chem. 1973;248:3188–3195.
- Daltrop O, Allen JW, Willis AC, Ferguson SJ. In vitro formation of a c-type cytochrome. Proc. Nat. Acad. Sci. 2002;99:7872–7876.10.1073/pnas.132259099
- Yamanaka M, Masanari M, Sambongi Y. Conferment of folding ability to a naturally unfolded apocytochrome c through introduction of hydrophobic amino acid residues. Biochemistry. 2011;50:2313–2320.10.1021/bi101646 m
- Allen JW, Barker PD, Ferguson SJ. A cytochrome b562 variant with a c-type cytochrome CXXCH heme-binding motif as a probe of the Escherichia coli cytochrome c maturation system. J. Biol. Chem. 2003;278:52075–52083.10.1074/jbc.M307196200
- Sambongi Y, Ferguson SJ. Specific thiol compounds complement deficiency in c-type cytochrome biogenesis in Escherichia coli carrying a mutation in a membrane-bound disulphide isomerase-like protein. FEBS Lett. 1994;353:235–238.10.1016/0014-5793(94)01053-6
- Kojima N, Yamanaka M, Ichiki S, Sambongi Y. Unexpected elevated production of Aquifex aeolicus cytochrome c555 in Escherichia coli cells lacking disulfide oxidoreductases. Biosci. Biotechnol. Biochem. 2005;69:1418–1421.10.1271/bbb.69.1418
- Obuchi M, Kawahara K, Motooka D, Nakamura S, Yamanaka M, Takeda T, Uchiyama S, Kobayashi Y, Ohkubo T, Sambongi Y. Hyperstability and crystal structure of cytochrome c555 from hyperthermophilic Aquifex aeolicus. Acta Crystallogr., Sect D: Biol. Crystallogr. 2009;65:804–813.10.1107/S0907444909017314
- Yamanaka M, Mita H, Yamamoto Y, Sambongi Y. Heme is not required for Aquifex aeolicus cytochrome c555 polypeptide folding. Biosci. Biotechnol. Biochem. 2009;73:2022–2025.10.1271/bbb.90220
