Abstract
To investigate the involvement of Allium roylei metabolites in the plant’s defenses, a comprehensive analysis of the content of cysteine sulfoxides, flavonols, polyphenols, ascorbic acid, and saponins was carried out in the various organs of this species. Metabolomics high performance liquid chromatography (HPLC), spectral-based analysis, and histochemcial studies have given important insight to the validity of saponins as a key component involved in plant protection. The root-basal stem, bulb, and leaf extracts exhibited 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity with inhibition concentration (IC50) ranging from 0.649 to 0.757 mg/mL. The antimicrobial properties of the saponin and flavonoid crude extracts were evaluated. The saponin extracts demonstrated significant antifungal activity depending on the applied concentration, and the growth inhibition rate of the tested fungal pathogens ranged from 1.07 to 47.76%. No appreciable antibacterial activity was recorded in the same sample.
Graphical Abstract
A comprehensive phytochemical analysis in the various organs of A. roylei was carried out. Saponin was the key component involved in plant protection against pathogen.
The need for disease- and pest-resistant germplasm in onion (Allium cepa L.) breeding has promoted studies of the biosystematic relationships between edible and wild Allium species of the section Cepa.Citation1) Domestication of wild Allium species started millennia ago, followed by extensive dissemination of the flavoring condiment all over the world.Citation2) Many wild Allium species with the characteristic onion or garlic aroma are used as spices or vegetables by various folks.Citation3) The wild Allium species may serve as a potential source for Allium crop improvement, and it may comprise genes for desired traits, as in Allium roylei Stearn.Citation4) A. roylei has proven to be a crucial bridge species, enabling introgressions of genetic resources from other related species of section Cepa into the onion, allowing disease resistance and other valuable characteristics to be introgressed into the onion.Citation5) The prospect of exploiting A. roylei as a source of disease resistance in onion breeding is very promising, as this wild species has proven to be completely resistant to downy mildew (Perenospora destructor), and partially resistant to leaf blight disease caused by Botrytis squamosa and basal rot disease caused by Fusarium oxysporum f.sp. cepae.Citation6)
Although edible Allium species are rich in numerous phytonutrients and bioactive metabolites (cysteine sulfoxides (ACSO), flavonoids, polyphenols, saponins, etc.) which reportedly have many functional properties such as antimicrobial, anticancer, and antioxidant,Citation7,8) little effort has been invested in evaluating the wild Allium species as potential sources for these bioactive metabolites with therapeutic and disease resistance properties.
There is no available information concerning the distribution and localization of these bioactive metabolites in different organs of A. roylei and their role as a part of the defensive characteristics of the plant against F. oxysporum f.sp cepae. Therefore, understanding the distribution and functions of the defense material may elucidate new molecular and chemical markers so that better genotype selection with higher disease resistance may be introgressed into the onion-breeding program.
Our recent study addresses the phytochemical investigation of the Allium species and their functional compounds as a chemical barrier to pathogenic attack.Citation9) In continuation of our previous research, Citation10) a comprehensive quantitative and qualitative analysis of cysteine sulfoxides, flavonols, polyphenols, ascorbic acid, and saponins within different organs of A. roylei was conducted. Localization of saponins and flavonoids in A. roylei organs was explored for the first time in Allium species together with an assay of antioxidant activity. Furthermore, the antifungal activity of the saponin and flavonoid crude extracts was tested against different fungal and bacterial phytopathogens.
Materials and methods
Materials
A. roylei accessions 95002, 95004, and 95005 were grown in a greenhouse at Yamaguchi University, Japan. Plants were harvested in June 2011.
Chemical and reagent
Analytical grade chemicals were purchased either from Nacalai Tesque, Inc. (Kyoto, Japan), Wako Pure Chemical Industries Ltd., (Osaka, Japan), Somatech Center House Food Corporation (Tokyo, Japan), or Merck (Darmstadt, Germany). Millipore-grade water was used for all experiments.
Extraction and analysis of S-alk(en)yl-L-cysteine sulfoxides
A. roylei leaves, bulbs, and roots-basal stems, with a fresh weight 5 g, were microwaved for 2 min, water extracted, and homogenized. The homogenate was centrifuged at 4000 rpm for 10 min at room temperature. The supernatant was collected and kept at −20 °C. An additional 300 μL was taken in an Eppendorf tube and centrifuged at 15000 rpm for 2 min. The supernatant was diluted 10X using 0.005% Trifluoroacetic acid (TFA) buffer and filtrated using a 0.45 μm syringe-type filter (HCl-Disk3, Kanto Chemical Co., Inc., Tokyo, Japan). A 60 μL filtered sample was injected into high performance liquid chromatography (HPLC) system and quantified. The HPLC system included a pump, a degasser, a column oven, a diode array detector set to 220 nm, a data collection system (EZchrom EliteTM, Hitachi High-Technologies Corporation, Tokyo, Japan), and an AQUASIL SS-1251-120 column (4.6 mm i.d. ×250 mm long, Senshu Scientific Co., Ltd, Tokyo, Japan). The solvent was 0.005% TFA and flowed for 15 min at a flow rate of 0.6 mL/min. A series of standards were dissolved in distilled water and analyzed. In order to obtain the mean values of respective accessions, all chemical extractions consisted of three replications. Each extraction was applied to chemical determination two times.
Extraction and analysis of flavonols
A 3 g fresh weight of leaves, bulbs, and root-basal stem tissues were cut and extracted separately using 70% ethanol (EtOH). Extracted materials were filtered by using 0.45 μm syringe-type filters. A 20 μL filtered sample was injected into the HPLC system (Hitachi L-2130) and quantified. The following HPLC conditions were applied: UV detector (360 nm), data collector (Hitachi D-2520 GPC Integrator), and Mightysil RP C18 Aqua 250–4.6 (5 μm) column (Kanto Chemical. Co., Ltd, Tokyo, Japan). Samples were eluted using gradient system with a constant flow rate of 1 mL/min (A, formic acid 5%; B, methanol); gradient isocratic 85–65% A for 15 min; 65–60% A for 10 min; 60–20% A for 15 min; and 20–85% A for 20 min. Quercetin, quercetin 3,4′-O-diglucoside, and quercetin 4′-O-glucoside standards were dissolved in 70% EtOH and analyzed. In order to obtain the mean values of respective accessions, all chemical extractions consisted of four replications. Each extraction was applied to chemical determinations three times.
Extraction and analysis of total phenols
Two grams of leaves, bulbs, and root-basal stem tissues were extracted using 70% EtOH. Extracted materials were adequately diluted with water, and total phenolic compound content was determined by the Folin–Ciocalteu method.Citation11) One mL of the diluted extract was mixed with 1 mL of Folin–Ciocalteu’s reagent. After 3 min, 1 mL of 10% sodium carbonate aqueous solution was added and mixed thoroughly, and the mixture was incubated for 60 min at room temperature. The polyphenol content was quantified spectrophotometrically at 530 nm (Hitachi, Model U-2001, Tokyo, Japan). Quantification was achieved by comparison of catechol calibration curve. In order to obtain the mean values of respective accessions, all chemical extractions consisted of three replications and each extraction was applied to chemical determinations three times.
Extraction and analysis of total saponins
The leaves, bulbs, and root-basal stems of A. roylei were freeze-dried (Taitec VC-360), until a final dry weight (2 g) was obtained. The dry materials were extracted according to the procedure described by Mostafa et al.Citation9) Briefly, the dry weight was exhaustively extracted at room temperature with the solvents n-hexane and 80% methanol (MeOH). Each solvent extraction step was conducted for one day and repeated three times with 30 min of sonication and filtration. The MeOH extract was taken to dryness in a rotary evaporator with vacuum pump v-700 (Büchi, Rotavapor® R-3) under reduced pressure at 50 ºC and then partitioned between butanol (BuOH) and H2O (1:1). The BuOH layer was filtered and then concentrated under vacuum giving a saponins crude extract. Total saponin content was determined spectrophotometrically at 473 nm.Citation12) Saponin concentrations were calculated based on the average value of absorbance at each concentration of disogenin standard. In order to obtain the mean values of respective accessions, all chemical extractions consisted of three replications. Each extraction was applied to chemical determinations three times.
Extraction and analysis of ascorbic acid
Total ascorbic acid content in A. roylei leaves, bulbs, and root-basal stems were determined as described by Roe and OesterlingCitation13) with little modification. Fresh plant material (2.5 g) was extracted with 5 mL of 10% metaphosphoric acid and 5 mL of water in an icebox using a mortar and pestle, and the extract was filtered and filled with water up to 12.5 mL. One mL of filtrate was transferred into test tubes, and two drops of indophenol were added. One mL of 2% metaphosphoric acid was added followed by 1 mL of 2% thiourea, and 0.5 mL of 2,4-dinitrophenylhydrazine was added. All the tubes were incubated in a water path at 50 °C for 70 min. The tubes were kept in ice for cooling, and 2.5 mL 85% H2SO4 was added and kept at room temperature for 30 min. Ascorbic acid content was detected spectrophotometrically at 530 nm. Quantification was achieved by comparison with the ascorbic acid calibration curve. In order to obtain the mean values of respective accessions, all chemical extractions consisted of three replications. Each extraction was applied to chemical determinations three times.
Histochemcial analysis
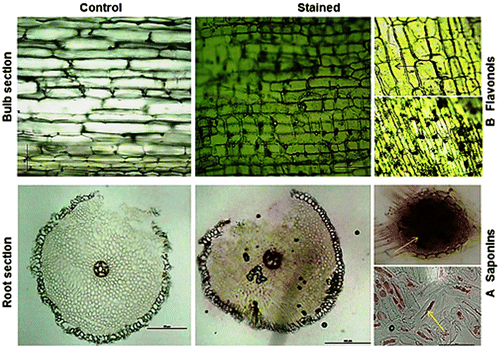
To explore histochemical analysis of saponins, sections of fresh root were cut using a Leica CM1850 cryostat microtome at −19 °C and stained with freshly prepared 5% vanillin–glacial acetic acid–perchloric acid solution.Citation14) Flavonoid localization was carried out using outer skin layers of fresh bulbs and stained with a 1% aluminum chloride EtOH solution. The sections were observed and photographed under a light microscope (Nikon-Eclipse E100).
Antioxidant activity
The radical scavenging activity of the extracts against 2,2-diphenyl-1-picrylhydrazyl (DPPH) radicals was measured using the method of Brand-Williams et al.Citation15) with a slight modification: Fresh plant materials were extracted with 70% ethanol, homogenized and filtered. A series of ethanol extract dilutions were added to freshly prepared DPPH solution (200 mM MES buffer pH 6; 30% EtOH; 400 μM DPPH (1:1:1, v/v/v), and the mixture was shaken gently and left to stand at room temperature in the dark for 20 min. Thereafter, the absorbance was read at 520 nm. The effective concentration having 50% radical inhibition activity (IC50) expressed as mg extract/mL, was determined from the graph of the free radical scavenging activity. In order to obtain the mean values of respective accessions, all chemical extractions consisted of four replications. Each extraction was applied to chemical determinations three times. Quercetin aglycon (1.7 × 10−2 mg/mL) and quercetin 4′-O-glucoside (0.5 mg/mL) were tested for their radical scavenging activity as described above.
Antimicrobial activity
Antifungal activity of the saponin crude extract isolated from root-basal stems and the flavonoid crude extracts isolated from bulb outer skin was tested on five soilborne pathogens (F. oxysporum f.sp. cepae 17, F. oxysporum f.sp. cepae TK, F. oxysporum f.sp. cepae TKN, F. oxysporum f.sp. cepae 12, and F. oxysporum f.sp. cepae 13) as a specific pathogen for Allium species and one airborne pathogen (Colletotrichum gloeosporioides) (Table ). Antifungal activity was assessed by in vitro agar diffusion test. Briefly, 3.2 cm Plates of Potato Dextrose Agar (PDA) mixed with saponin and flavonoid crude extracts at three different concentrations (100, 500, and 1000 ppm) were inoculated with a 5 mm plug containing the fungi. Plates were incubated at 25 °C, and the fungi’s radical growth was measured after seven days. Antibacterial properties of the saponin and flavonoid crude extracts were assessed for five bacterial strains (Agrobacterium tumefaciens, Agrobacterium rhizogenes, Burkholderia glumae, Clavibacter michiganensis, and Ralstonia solani) (Table ). The antibacterial activity was assessed by disk diffusion test on Nutrient Agar (NA) medium. One mL of the tested organism suspension was spread on the NA medium plate using a glass rod. The Petri dishes were left at room temperature for 1 h. Using a cork borer, a 10 mm disk of the NA medium was removed, and three different concentrations (100, 500, and 1000 ppm) of the saponin and flavonoid extracts were loaded on the inoculated plates. After 30 min, the plates were incubated at 37 °C for 48 h. The definite zone of inhibition was measured and expressed in millimeters. All microbes were obtained from the Department of Biological and Environmental Science, Molecular Plant Pathology, Faculty of Agriculture, Yamaguchi University, Japan.
Table 1. Fungal and bacterial isolates used in this study.
Statistics
Values are expressed as the mean ± standard error (SE). The analysis of variance carried out using SPSS, Inc., 11.5. The significant effects were determined by the magnitude of the F-value (p < 0.05). The different metabolite means were separated by the Tukey’s Honestly Significant Difference (HSD) test, and the antimicrobial activity was analyzed by the Dunnett’s comparison test.
Results and discussion
Cysteine sulfoxides
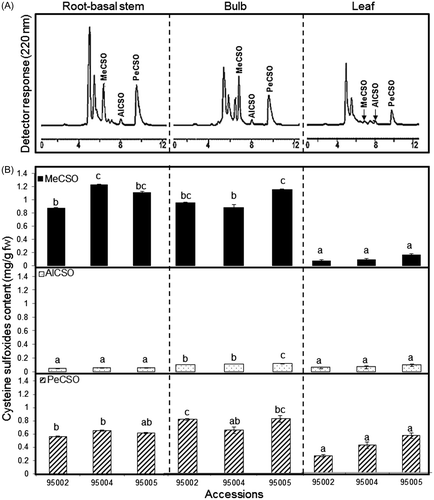
Determination of total cysteine sulfoxide (ACSO) content was carried out by means of HPLC. Methiin (MeCSO) was first separated at a retention time of 6.4 min, followed by alliin (AlCSO) with a retention time of 7.4 min and isoaliin (PeCSO) with a retention time of 9.4 min (Fig. (A)). A significant difference (p > 0.05) was detected in the ACSO content (mg/g fw) of A. roylei bulbs ranging from 1.59 to 2.03, of root-basal stems ranging from 1.48 to 1.93 and of leaves ranging from 0.37 to 0.79 (Fig. (B)). Since there is no available data regarding ACSO content in A. roylei, we compared our results with other Allium species. Our findings revealed substantially higher ACSO content (mg/g fw) within different organs of A. roylei than that reported in common onion bulbs, roots, and leaves (0.39, 0.39, and 0.40, respectively).Citation16) Our findings were fairly consistent with the ACSO levels in shallot (A. cepa L. Aggregatum group) bulbs as reported previously.Citation17,18) However, previous reports didn’t consider all three organs considered in this study. MeCSO, AlCSO, and PeCSO (mg/g fw) were detected in the three organs of A. roylei investigated. MeCSO content was the highest, followed by PeCSO and AlCSO content, but their distribution within the different organs showed significant differences (p > 0.05). MeCSO exhibited the highest concentrations in the bulbs, root-basal stem organs averaged 1.02, and the leaves had the lowest average at 0.11. However, PeCSO, predominant in the leaves, averaged 0.40. AlCSO was detected in trace amounts in the all plant organs, ranging between 0.04 and 0.11. The ACSO distribution pattern in A. roylei organs reveals a domination of MeCSO with detectable amounts of PeCSO in the leaves. This result is different from the ACSO pattern of the common onion, where PeCSO is the characteristic compound in the different organs.Citation19) MeCSO is ubiquitous in the genus Allium, and the ratio of MeCSO in combination with other CSOs is variable within the different species and is mainly correlated with their usage as vegetables or spices.Citation20) In the present study, A. roylei expressed high MeCSO, which is associated with a hot pungent taste and strong sulfur smell; this could yield an important insight to onion breeders for taste and aroma development. In addition to the critical role of ACSO in determining the characteristic smell and taste of Allium species, these substances are physiologically active and used as antibiotic and antitumor agents.Citation21) A. roylei leaves and inflorescences were reported to be used as a condiment, whereas bulbs are used to relieve headaches by local populations in India.Citation22)
Flavonol
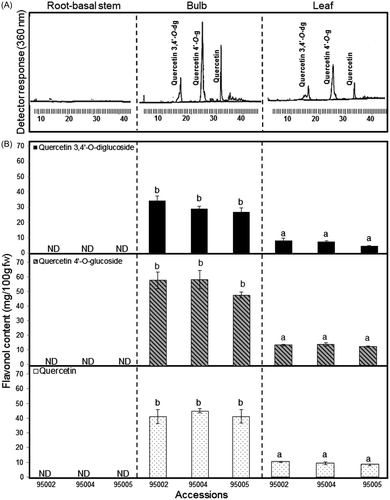
Significant differences (p > 0.05) have been found in the total flavonol content within the different organs of A. roylei accessions. Higher levels of total flavonol were detected in the outer layers (second, first, and dry skin) of the bulbs (133.92 mg/100 g fw) than in the leaves (30.36 mg/100 g fw); however, there was no trace of any flavonol in the root-basal stems (Fig. (B)). The observed results can be explained by the high activity of phenylalanine ammonia-lyase, responsible for flavonol synthesis, which is allocated to the outer layers of the bulb.Citation23) Quantitatively speaking, previous literature has reported that the total amount of flavonol in onion bulbs is strongly associated with their color; the richest in these metabolites is the red onion, and the lowest is the white onion.Citation24) The value of total flavonol in the A. roylei bulb was higher than that reported in different onion cultivars, which ranged between 5 and 18.6 mg/100 g fw,Citation25,26) 12.21 and 52.43 mg/100 g fw,Citation27) 116.7, 102.3 and 67.3 mg/100 g fw in the French shallot, Italian shallot, and red onion, respectively,Citation28) and between 16 and 149.75 mg/100 g dw in A. odorum and A. fistulosum leaves, respectively. Citation29) In general, flavonol levels reported in this study were higher than the values reported earlier. The procedural differences in the extractions and analyses as well as the characteristics of the species examined may explain these differences. We found two quercetin derivatives: quercetin 3,4′-O-diglucoside, quercetin 4′-O-glucoside, and quercetin aglycone (Fig. (A)). The quercetin glucoside and quercetin aglycone content varied significantly between organs, ranging from threefold to fourfold differences. Statistically significant higher levels of quercetin 3,4′-O-diglucoside, quercetin 4′-O-glucoside, and quercetin aglycone in A. roylei accessions were detected in the bulbs (ranges: 29.02–36.9, 48.12–58.85, and 44.96–49.31 mg/100 g fw, respectively). However, these values were lower in the leaves (ranges: 4.54–8.31, 12.52–14.1, and 9.1–11.23 mg/100 g fw, respectively). The dominant flavonol in the bulb and leaf was ranked as follows: quercetin 4′-O-glucoside representing 41.12–42.66% > qurecetin 34.7–33.62% > quercetin 3,4′-O-diglucoside 24.16–22.16%. Our querectin glucoside and quercetin aglycone values were twofold to threefold higher than those reported in yellow and red onion cultivars.Citation30) In our opinion, this high level of flavonol, especially quercetin aglycone, can be considered a characteristic feature of A. roylei which had a red-brownish bulb color that can be correlated with other red and yellow onions that show the same high levels of quercetin, ranging from 5.4 to 28.6 and from 11.7 to 20.2 mg/100 g fw, respectively.Citation31) Qualitatively speaking, the accumulation of flavonol in the bulb outer layer and the leaf rather than in the root can be linked to the role of these metabolites in the defense mechanism, in addition to their role in protecting plant cells against UVB radiation.Citation32)
Polyphenol
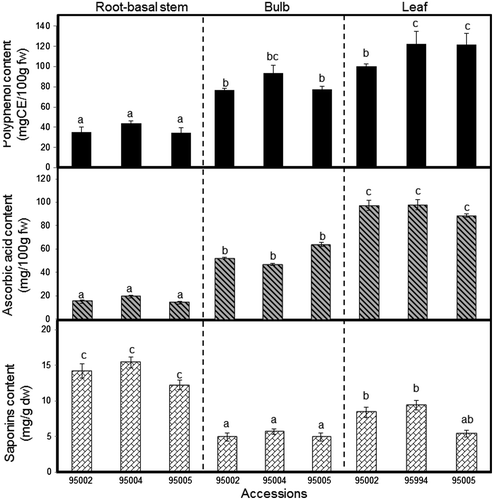
The total phenol content determined by the Folin–Ciocalteu method expressed as catechol equivalents is presented in Fig. . The highest phenol content (mg/100 g fw) in A. roylei accessions was recorded in the leaves, ranging between 97.99 and 119.72; then in the bulbs, ranging between 80.57 and 97.82; and the lowest in the root-basal stem, ranging from 34.82 to 44.16. The results obtained reveal a significant (p > 0.05) difference of total phenol content within different organs of A. roylei; this could be associated with the physiological and morphological importance of these metabolites in the plant. Phenolic compounds may act as phytoalexines,Citation33) antifeedants, attractants for pollinators, or as plant pigmentation and protection against UV lightCitation34,35); the high accumulation of phenol content in the bulbs and leaves as compared with the root-basal stem strongly supports this assumption. This organ-dependent repartition of the total phenol content has recently been reported for A. roseum with the highest content in the flowers, bulbs, and leaves (135, 44.3, and 55.9 mg GAE/100 g fw, respectively).Citation36) Our total phenol levels are slightly higher than the values reported in different commercial onion cultivars, which ranged between 25.2 and 75.9 mg/100 g fw,Citation37) and between 34.2 and 62.4 mg/100 g fw.Citation38) However, it was considerably lower than the values reported in yellow onion cultivars, which ranged between 73.3 and 180.8 mg GAE/100 g fw.Citation27) Total phenolic levels in different species vary considerably and mainly depend on the plant species/cultivars, the extraction procedure, or the growing region.Citation39)
Ascorbic acid
In the present study, A. roylei accessions have particularly abundant ascorbic acid content within different organs, ranging from 14.59 to 112.22 mg/100 g fw. The highest accumulation of ascorbic acid (mg/100 g fw) was detected in the leaves, ranging from 85.32 to 112.22, then in the bulbs, ranging from 45.22 to 69.74; considerably less content was found in the root-basal stem, ranging from 14.95 to 22.09 (Fig. ). The high level of ascorbic acid in the leaves suggests that ascorbic acid plays an essential role in the plant’s antioxidant response toward radiation exposure. Previous studies have pointed out that ascorbic acid is capable of reacting with radicals generated during radiation exposure.Citation40) The ascorbic acid levels in A. roylei were higher than those recorded in the white shaft and green leaves of leek cultivars (A. ampeloprasum var. porrum), which varied from 0.89 to 3.55 mg/g dw,Citation41) and in A. schoenoprasum leaves, 24.59 mg/100 g fw.Citation42) The high ascorbic acid content in A. roylei has important relevance, from a nutritional point of view, that makes this plant promising for nutritional enhancement of other edible Alliums through breeding programs.
Saponins
Despite much research that has addressed the role of saponins as a remarkable antifungal metabolites against different pathogens,Citation43) few investigations have considered characterizing the distribution of total saponins within the different organs in Allium species. This information is very important, especially in the vegetable crops research, for obtaining the most valuable plant material with optimum nutritional components for proper harvesting or breeding strategies.Citation44,45) Our results have revealed the highest accumulation of total saponin content (mg/g dw) in the root-basal stem, ranging between 12.26 and 15.42, followed by that in the leaf, ranging from 5.10 to 8.97, and the lowest detected in the bulb, ranging from 4.6 to 5.33 (Fig. ). These findings agree with our previous report,Citation9) where saponins were highly detected in the roots of A. nigrum as compared with other organs. The same tendency was reported in Medicago truncatula, with high accumulations of saponins, 5.924 mg/g dw in the root and 1.064 mg/g dw in the leaf.Citation46) From a biological point of view, the massive accumulation of saponins in the root tissue as compared with other tissues suggests that saponins are responsible for protecting plants against many soilborne pathogens underground where the root-basal stem is mainly found. Further, the quantitative variance of saponins and their translocation from the root into the bulb, leaf, or flowers could be associated with the developmental growth stage and external factors, including interaction with insects and plant pathogens.Citation47) The future prospects of saponin compounds as chemical markers for resistance against fungal pathogens would be an interesting point of research for onion breeders.
Histochemcial observations of roots and bulb skin
In the root section, cells of the epidermis, endodermis, and primary xylem did not show any color reaction, whereas a light to dark pinkish-brown color appeared in the cortex and phloem (Fig. (A)). The outer skin section of the A. roylei bulb showed a massive accumulation of flavonol in the epidermal cells, particularly quercetin, which reacts with the reagent, giving a fluorescent greenish-yellow color (Fig. (B)).
Antioxidant activity
The three ethanol extracts of the root-basal stem, the bulb, and the leaf were assayed for antioxidant activity against the DPPH radical (Tables and ). Free radical scavenging activity was expressed in terms of IC50 (mg/mL). There was no significant variation of IC50 among the three ethanol extracts, ranging from 0.649 to 0.757. The bulb extract was the most powerful scavenger for reducing the DPPH radical with IC50 (0.649–0.662) of the root-basal stem (0.710–0.715) and of the leaf (0.747–0.757). This result contradicts with A. roseum and A. neapolitanum flowers and leaves as being the most powerful antioxidant in the DPPH test in respect to bulbs.Citation34,36) However, their DPPH activity was not correlated with the total phenol content in these organs and it was mainly attributed to flavonoid content. In our study, A. roylei showed a considerable amount of flavonol in the bulb as compared to the leaf. The DPPH scavenging activity using flavonol standards revealed a remarkable radical scavenging power for quercetin aglycon (Q) with IC50 (8.2 × 10−3 mg/mL), which is approximately 67 times greater than quercetin 4′-O-glucoside (Q4′G) IC50 (5.5 × 10−1 mg/mL). However, quercetin 3,4′-O-diglucoside did not showed any scavenging activity. These results indicated that the flavonol antioxidant activity is mainly due to Q contribution which is considerably high in A. roylei bulb organ. In addition, we calculated the contribution percentages (CP) of Q and Q4′G in the DPPH scavenging IC50 (Q-CP = Estimated Q content in the plant extraction IC50/Q standard IC50 × 100; Q4′G-CP = Estimated Q4′G content in the plant extraction IC50/Q4′G standard IC50 × 100). The 69.4% of the CP was observed in Q and 1.2 % in Q4′G in the bulb organs. While, these CPs were very low in the leaf with 18.5 for Q and 0.3% for Q4′G. Generally speaking, our data revealed a high reducing power of A. roylei extracts as compared with previous IC50 levels reported in different onion cultivars, ranging from 1.44 to 4.20 mg/mL in the red onion and from 1.84 to 4.67 mg/mL in the violet onion.Citation7) However, it was lower than values reported previously in methanol extracts of five Allium species (A. nevsehirense, A. sivasicum, A. dictyoprosum, A. scrodoprosum, and A. atroviolaceum) which showed IC50 ranging between 0.079 and 0.104 mg/mL.Citation47) The most probable reason for this variation in antioxidant activity in A. roylei as compared with other species might be due to variation in the quantities of flavonols especially quercetin, phenolic acid, and ascorbic acid.Citation48,49) This hypothesis was supported with our HPLC and spectral analyses of these metabolites in each organ which revealed a high quercetin, phenolic acid, and ascorbic acid content in the bulb and leaf organs. On the other hand, these metabolites were not detected or were detected only in small amounts in the root-basal stem organ. In our opinion, the powerful antioxidant activity of the root-basal stem extract could be encountered with the high ACSO and saponin content. Similar reports have attributed the antioxidant activity of some Allium species to organosulfur compounds and their precursors.Citation50,51) Crude saponin extract from the root-basal stem organ revealed a strong antioxidant activity in the DPPH test (data not shown). The role of saponin compounds as an antioxidant agent has not yet been reported and is still unclear; future studies are needed to confirm this new finding.
Table 2. DPPH scavenging activities IC50 (mg/mL) of different A. roylei organs extracts.
Table 3. Analysis of variance for DPPH in different organs and accessions of A. roylei.
Antimicrobial activity
The crude saponin extract from the root-basal stem and the crude flavonoid extract from the bulb outer skin were tested for their antimicrobial activity against a number of fungal and bacterial pathogens. All extracts showed significant antifungal activity depending on their concentration and the tested fungal pathogen (Fig. ). The obtained results reveal a potent antifungal activity of the crude saponin extract against C. gloeosporioides and F. oxysporum f.sp. cepae isolates at 1000 ppm. Maximum inhibition of fungal growth (47.76%) was recorded with C. gloeosporioides. However, this activity was variable among F. oxysporum f.sp. cepae isolates. F. oxysporum f.sp. cepae 12 and 13 were the isolates most sensitive to the crude saponin with 41.33% growth inhibition. F. oxysporum f.sp. cepae TK and TKN were more resistant, showing 10.43 and 9.11% growth inhibition, respectively. Our results are in agreement with previous studies, which pointed out different responses of F. oxysporum strains against saponin compounds. Lanzotti et al.Citation8) reported that F. oxysporum f.sp. lycopersici was not affected by saponin compounds isolated from A. cepa. However, moderate to high responses of F. oxysporum and F. oxysporum f.sp. lycopersici toward saponin compounds isolated from A. minutiflorum were reported.Citation43) The variability of A. roylei crude saponin activity against F. oxysporum f.sp. cepae could be explained by the ability of some fungal pathogens to produce enzymes that degrade saponin into nontoxic molecules and the nature of the aglycone structure.Citation8,52) All the crude saponin activity showed a statistically significant difference as compared with the disogenin standard within the same tested fungal pathogens. The crude saponin extract did not show any antibacterial activity toward the examined bacteria (Table ). Previous studies have reported that bacteria in general are less sensitive to saponin as compared with fungi.Citation53) The crude flavonoid extract was not effective against most of the tested fungal pathogens at 1000 ppm (Fig. ). F. oxysporum f.sp. cepae isolates showed high (4.37–9.11%) to complete resistance to flavonoid doses. Furthermore, the highest inhibition (11.65%) was recorded with C. gloeosporioides. This result indicates that flavonoids’ role in defending the plant against F. oxysporum f.sp. cepae is limited as compared with that of saponins, which seem to be the major metabolites involved in plant protection. Similarly, fair to low antifungal activity of flavonoid compounds was recorded against Aspergillus niger and Candida albicans.Citation54) However, our results contradicts those of Skerget et al.Citation55) who showed high antifungal activity of the onion skin extract against A. niger and T. viride (35.6 and 63.0%, respectively). The crude flavonoid extracts exhibited different antibacterial activity among test bacterial pathogens, ranging between 5.6 and 22 mm (Table ). High affinity of the flavonoids’ antibacterial activity was recorded with R. solani, A. tumefaciens, and B. glumae (22, 16 and 5.6 mm, respectively). However, A. rhizogenes and C. michiganensis were highly resistant. The same tendency of considerable antibacterial activity was recorded in the flavonoid extract from the yellow onion (cv. GO) against gram-positive bacteria as compared with the flavonoid extract from the white onion (cv. FE).Citation56) The observed results demonstrate that the antibacterial activity of the crude flavonoid extract was generally higher than their antifungal activity.
Fig. 5. Fungal growth inhibition (%) of Saponin and Flavonoid crude extracts at three different concentration (100, 500 and 1000 ppm).
Notes: Data are expressed as mean ± SE (n = 3). Asterisks denote significant differences between treatments and disogenin standard determined by Dunnett’s test (NS = not significant; *, p < 0.05; **, p < 0.01; ***, p < 0.005). ND = not detected.
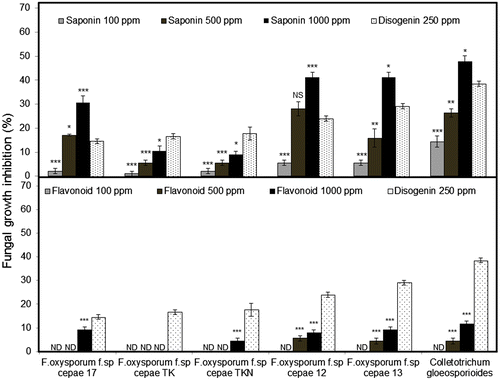
Table 4. Antibacterial activity of saponin and flavonoid crude extracts.
Conclusion
A. roylei ACSO can be implemented in the taste and aroma development with other edible Allium species. The prospects of A. roylei saponin compounds as chemical markers for resistance against fungal pathogens would be an interesting point of research for onion breeders.
Supplemental material
The Supplemental material for this paper is available at http://dx.doi.org/10.1080/09168451.2014.915722.
Supplemental Fig. 1 caption
Download MS Word (19 KB)Supplemental Fig. 1
Download MS Power Point (285 KB)Acknowledgement
A part of this research was supported by Strategic International Research Cooperative Program, Japan Science and Technology Agency (JST).
Notes
Abbreviations: HPLC, High performance liquid chromatography; DPPH, 2,2-diphenyl-1-picrylhydrazyl; IC50, Inhibition concentration; TFA, Trifluoroacetic acid; PDA, Potato dextrose Agar; ACSO, cysteine sulfoxides; MeCSO, Methiin; AlCSO, Alliin; PeCSO, isoaliin.
References
- McCollum GD. Experimental hybrids between Allium fistulosum and Allium roylei. Bot. Gaz. 1982;143:238–242.
- Hanelt P. Onions and allied crops. In: Taxonomy, evolution and history. Rabinowitch HD, Brewster JL, editors. Boca Raton, FL: CRC Press; 1990. p.1–26.
- Kamenetsky R, Rabinowitch HD. The Genus Allium: A Developmental and Horticultural Analysis. Hort. Rev. 2006;32:329–337.
- De Vries JN, Wietsma WA, De Vries T. Introgression of leaf blight resistance from Allium roylei Stearn into onion (A. cepa L.). Euphytica. 1992;62:127–133.10.1007/BF00037938
- Kofoet A, Kik C, Wietsma WA, Vries JN. Inheritance of resistance to downy mildew (Peronospora destructor (Berk) Casp.) from Allium roylei Stearn in the backcross Allium cepa L. x (A. roylei x A. cepa). Plant Breed. 1990;105:144–149.10.1111/pbr.1990.105.issue-2
- De Vries JN, Weitsma WA, Jongerius R. The genus Allium-taxonomic problems and genetic resources. In: Introgression of characters from Allium roylei Stearn into A. cepa L. Hanelt P, Hammer K, Knupffer H, editors. Proc. Int. Symp.; June 11–13; Gatersleben, Germany; 1992. p. 321–325.
- Prakash D, Singh BN, Upadhyay G. Antioxidant and free radical scavenging of phenols from onion (Allium cepa). Food Chem. 2007;102:1389–1393.10.1016/j.foodchem.2006.06.063
- Lanzotti V, Romano A, Lanzuise S, Bonanomi G, Scala F. Antifungal saponins from bulbs of white onion, Allium cepa L. Phytochemistry. 2012;74:133–139.10.1016/j.phytochem.2011.11.008
- Mostafa A, Sudisha J, El-Sayed M, Ito SI, Ikeda T, Yamauchi N, Shigyo M. Aginsodie saponin, a potent antifungal compound, and secondary metabolite analyses from Allium nigrum L. Phytochem. Lett. 2013;6:274–280.10.1016/j.phytol.2013.03.001
- Vu HQ, Yoshimatsu Y, Khrustaleva LI, Yamauchi N, Shigyo M. Alien genes introgression and development of alien monosomic addition lines from a threatened species, Allium roylei Strean, to Allium cepa L. Theor. Appl. Genet. 2011;7:1241–1257.
- Folin O, Denis W. Colorimetric method for the determination of phenols and phenol derivatives in urine. J. Biol. Chem. 1915;22:305–308.
- Ebrahimzadeh H, Niknam V. A revised spectrophotometric method for determination of triterpenoid saponins. Indian Drugs. 1998;35:379–382.
- Roe JH, Oesterling MJ. The Determination of dehydroascorbic acid and ascorbic acid in plant tissues by the 2, 4-dinitrophenylhyrazine method. J. Biol. Chem. 1944;152:511–517.
- Teng HM, Fang MF, Cai X, Hu ZH. Localization and dynamic change of saponin in vegetative organs of Polygala tenuifolia. J. Integ. Plant Biol. 2009;51:529–536.10.1111/jipb.2009.51.issue-6
- Brand-Williams W, Cuvelier ME, Berset C. Use of a free radical method to evaluate antioxidant activity. LWT—Food Sci. Technol. 1995;28:25–30.10.1016/S0023-6438(95)80008-5
- Hovius MHY, Goldman ILG. Flavor precursor (S-alk(en)yl-L-cysteince sulfoxides) concentration and composition in onion plant organs and predictability of field white rot reaction of onions. J. Amer. Soc. Hort. Sci. 2005;130:196–202.
- Yoo KS, Pike LM. Determination of flavor precursor compound S-alk(en)yl-L-cysteine sulfoxides by an HPLC method and their distribution in Allium species. Sci. Hort. 1998;75:1–10.
- Fritsch RM, Keusgen M. Occurrence and taxonomic significance of cysteine sulphoxides in the genus Allium L. (Alliaceae). Phytochemistry. 2006;67:1127–1135.10.1016/j.phytochem.2006.03.006
- Lawson LD, Wang ZY, Hughes BG. ɣ-Glutamyl-S-alkylcysteines in garlic and other Allium species: precursors of age-dependent trans-1-propenyl thiosulfinates. J. Nat. Prod. 1991a;54:436–444.10.1021/np50074a014
- Krest I, Glodek J, Keusgen M. Cysteine sulfoxides and alliinase activity of some Allium species. J. Agric. Food Chem. 2000;48:3753–3760.10.1021/jf990521+
- Kusterer J, Keusgen M. Cysteine sulfoxides and volatile sulfur compounds from Allium tripedale. J. Agric. Food Chem. 2010;58:1129–1137.10.1021/jf903581f
- Kohli B, Gohil RN. Need to conserve Allium roylei Stearn: a potential gene reservoir. Genet. Resour. Crop Evol. 2009;56:891–893.10.1007/s10722-009-9482-7
- Hirota S, Shimoda T, Takahama U. Distribution of flavonols and enzymes participating in the metabolism in onion bulbs: Mechanism of accumulation of quercetin and its glycosides in the abaxial epidermis. Food Sci. Technol. Res. 1999;5:384–387.10.3136/fstr.5.384
- Rodrigues AS, Pérez-Gregorio M.R., García-Falcón MS, Simal-Gándara J, Almeida DPF. Effect of meteorological conditions on antioxidant flavonoids in cultivars of white and red onions. Food Chem. 2011;124:303–308.10.1016/j.foodchem.2010.06.037
- Rhodes MJC, Price KR. Analytical problems in the study of flavonoid compounds in onions. J. Agric. Food Chem. 1996;57:113–117.
- Lee SU, Lee JH, Choi SH, Lee JS, Ohnisi-Kameyama M, Kozukue N, Levin CE, Friedman M. Flavonoid content in fresh, home-processed, and light-exposed onions and in dehydrated commercial onion products. J. Agric. Food Chem. 2008;56:8541–8548.10.1021/jf801009p
- Sellappan S, Akoh CC. Flavonoids and antioxidant capacity of Georgia-grown Vidalia onions. J. Agric. Food Chem. 2002;50:5338–5342.10.1021/jf020333a
- Bonaccorsi P, Caristi C, Gargiulli C, Leuzzi U. Flavonol glucosides in Allium species: A comparative study by means of HPLC-DAD-ESI-MS-MS. Food Chem. 2008;107:1668–1673.10.1016/j.foodchem.2007.09.053
- Miean KH, Mohamed S. Flavonoid (Myricetin, Querceting, kaempferol, Luteolin, and Apigenin) content of edible tropical plants. J. Agric. Food Chem. 2001;49:3106–3112.10.1021/jf000892m
- Pérez-Gregorio RM, García-Falcón MS, Simal-Gándara J, Rodrigues AS, Almeida DPF. Identification and quantification of flavonoids in traditional cultivars of red and white onions at harvest. J. Food Compos. Anal. 2010;23:592–598.10.1016/j.jfca.2009.08.013
- Patil BS, Pike LM, Yoo KS. Variation in the quercetin content in different colored onions (Allim cepa L.). J. Amer. Soc. Hort. Sci. 1995;120:909–913.
- Lake JA, Field kJ, Davey MP, Beerling DJ, Lomax BH. Metabolomic and physiological responses reveal multi-phasic acclimation of Arabidopsis thaliana to chronic UV radiation. Plant Cell Environ. 2009;32:1377–1389.
- Popa VI, Dumitru M, Volf I, Anghel N. Lignin and polyphenols as allelochemicals. Ind. Crops Prod. 2008;27:144–149.10.1016/j.indcrop.2007.07.019
- Nencini C, Menchiari A, Franchi GC, Micheli L. In vitro antioxidant activity of aged extracts of some Italian Allium species. Plant Foods Hum. Nutr. 2011;66:11–16.10.1007/s11130-010-0204-2
- Ignat I, Volf I, Popa VI. A critical review of methods for characterization of polyphenolic compounds in fruits and vegetables. Food Chem. 2011;126:1821–1835.10.1016/j.foodchem.2010.12.026
- Dziri S, Hassen I, Fatnassi S, Mrabet Y, Casabianca H, Hanchi B, Hosni K. Phenolic constituents, antioxidant and antimicrobial activities of rosy garlic (Allium roseum var. odoratissimum). J. Funct. Food. 2012; 4: 423–432.10.1016/j.jff.2012.01.010
- Lombard KA. Investigation of the flavonol quercetin in onion (Allium cepa L.) by high-performance liquid (HPLC) and spectrophotometric methodology [MSc thesis]. Lubbock (TX): Texas Tech. Univ; 2000.
- Rodríguez Galdón B, Rodríguez Rodríguez EM, Díaz Romero C. Flavonoids in onion cultivars (Allium cepa L.). J. Food Sci. 2008;73:C599–C605.10.1111/jfds.2008.73.issue-8
- Bilyk A, Cooper P, Sapers G. Varietal difference in distribution of quercetin and kaempferol in onion tissue. J. Agric. Food Chem. 1984;32:274–276.10.1021/jf00122a024
- Jimenez L, Alarcón E, Trevithick-Sutton C, Gandhi N, Scaiano JC. Effect of ɣ-radiation on green onion DNA integrity: Role of ascorbic acid and polyphenols against nucleic acid damage. Food Chem. 2011;128:735–741.10.1016/j.foodchem.2011.03.098
- Bernaert N, De Paepe D, Bouten C, De Clercq H, Stewart D, Van Bockstaele E, De Loose M, Van Droogenbroeck B. Antioxidant capacity, total phenolic and ascorbate content as a function of the genetic diversity of leek (Allium ampeloprasum var. porrum). Food Chem. 2012;134:669–677.10.1016/j.foodchem.2012.02.159
- Andarwulan N, Kurniasih D, Apriady RA, Rahmat H, Roto AV, Bolling BW. Polyphenols, carotenoids, and ascorbic acid in underutilized medicinal vegetables. J. Funct. Food. 2012;4:339–347.10.1016/j.jff.2012.01.003
- Barile E, Bonanomi G, Antignani V, Zolfaghari B, Sajjadi SE, Scala F, Lanzotti V. Saponin from Allium minutiflorum with antifungal activity. Phytochemistry. 2007;68:596–603.10.1016/j.phytochem.2006.10.009
- Dong TT, Cui XM, Song ZH, Zhao KJ, Ji ZN, Lo CK, Tsim KW. Chemical assessment of roots of Panax notoginseng in China: regional and seasonal variations in its active constituents. J. Agric. Food Chem. 2003;51:4617–4623.10.1021/jf034229k
- Lim W, Mudge KW, Vermeylen F. Effects of population, age and cultivation methods on ginsenoside content of wild American ginseng (Panax quinquefolium). J. Agric. Food Chem. 2005;53:8498–8505.10.1021/jf051070y
- Huhman DV, Berhow MA, Sumner LW. Quantification of saponins in aerial and subterranean tissues of Medicago truncatula. J. Agric. Food Chem. 2005;53:1914–1920.10.1021/jf0482663
- Szakiel A, Pączkowski C, Henry M. Influence of environmental abiotic factors on the content of saponins in plants. Phytochem. Rev. 2011;10:471–491.10.1007/s11101-010-9177-x
- Tepe B, Sokmen M, Akpulat HA, Sokmen A. In vitro antioxidant activities of the methanol extracts of five Allium species from Turkey. Food Chem. 2005;92:89–92.10.1016/j.foodchem.2004.07.016
- Diplock AT, Charuleux JL, Crozier-Willi G, Kok FJ, Rice-Evans C, Roberfroid M, Stahl W, Viña-Ribes J. Functional food science and defence against reactive oxidative species. British J. Nutr. 1998;80:S77–112.10.1079/BJN19980106
- Kim SM, Kubota K, Kobayashi A. Antioxidative activity of sulfur-containing flavor compounds in garlic. Biosci Biotechnol Biochem. 1997;61:1482–1485.10.1271/bbb.61.1482
- Griffiths G, Trueman L, Crowther T, Thomas B, Smith B. Onions-A global benefits to health. Phytother. Res. 2002;16:603–615.10.1002/(ISSN)1099-1573
- Morrissey JP, Osbourn AE. Fungal resistance to plant antibiotics as a mechanism of pathogenesis. Microbiol. Mol. Biol. 1999;63:708–724.
- Avato P, Bucci R, Tava A, Vitali C, Rosato A, Bialy Z, Jurzysta M. Antimicrobial activity of saponin from Medicago sp.: structure-activity relationship. Phytother. Res. 2006;20:454–457.10.1002/(ISSN)1099-1573
- Rauha JP, Remes S, Heinonen M, Hopia A, Kähkönen M, Kujala T, Pihlaja K, Vuorela H, Vuorela P. Antimicrobial effect of Finnish plant extracts containing flavonoids and other phenolic compounds. Int. J. Food Microbiol. 2000;56:3–12.10.1016/S0168-1605(00)00218-X
- Skerget M, Majhenic L, Bezjak M, Knez Z. Antioxidant, Radical Scavenging and Antimicrobial Activities of Red Onion (Allium cepa L.) Skin and Edible Part Extracts. Chem. Biochem. Eng. 2009;23:435–444.
- Santas J, Almajano MP, Carbó R. Antimicrobial and antioxidant activity of crude onion (Allium cepa L.) extracts. Int. J. Food Sci. Technol. 2010;45:403–409.10.1111/ifs.2010.45.issue-2