Abstract
Skeletal and cardiac muscle have important roles in glucose uptake and utilization. However, changes in expression of protein coding genes and miRNAs that participate in glucose metabolism during development are not fully understood. In this study, we investigated the expression of genes related to glucose metabolism during muscle development. We found an age-dependent increase in gene expression in cardiac muscle, with enrichment in heart development- and energy-related metabolic processes. A subset of genes that were up-regulated until 30 or 180 days postnatally, and then down-regulated in psoas major muscle was significantly enriched in mitochondrial oxidative-related processes, while genes that up-regulated in longissimus doris muscle was significantly enriched in glycolysis-related processes. Meanwhile, expression of energy-related microRNAs decreased with increasing age. In addition, we investigated the correlation between microRNAs and mRNAs in three muscle types across different stages of development and found many potential microRNA–mRNA pairs involved in regulating glucose metabolism.
Graphical Abstract
Development-related Expression Profile of Genes and miRNAs Related to Glucose Metabolism in Pig Muscles
Skeletal muscle represents approximately 40% of body weight, and plays an important role in glucose disposal and plasma glucose utilization. Skeletal muscle is the primary site of glycogen storage, and therefore plays an important role in maintaining glucose homeostasis. In turn, glucose homeostasis is necessary to maintain skeletal muscle structure and function. Cardiac muscle readily consumes glucose aerobically without a ‘warm up’ period and always extracts the maximum ATP yield from each glucose molecule. Meanwhile, although glucose accounts for less than 35% of energy production in cardiac muscle under normal conditions, ATP can be obtained anaerobically through glycolysis even under ischemic conditions.Citation1) However, energy conservation and utilization of cardiac muscle may be abnormal in the event of cardiac hypertrophy and dysfunction.Citation2,3)
Muscle is the primary site of glucose uptake, which is mainly regulated by insulin signaling. Once transported into cells, glucose is phosphorylated to glucose-6-phosphate (G-6-P) and converted to ATP by glycolysis or mitochondrial oxidative phosphorylation, while excess glucose is stored as glycogen. Glucose metabolism relies on various enzymes, and studies have revealed important roles for several genes in regulating glucose metabolism in muscles.Citation4) In addition, microRNAs (miRNAs) are endogenous, non-coding RNAs that directly or indirectly regulate gene expression primarily at the translational level and play a major role in glucose homeostasis.Citation5,6) However, the expression pattern of protein coding genes and miRNAs that participate in glucose metabolism during development requires further investigation.
Recent studies indicate that pigs represent an attractive biomedical model for energy metabolism and obesity in humans because they have similar metabolic features, cardiovascular systems, and proportional organ sizes.Citation7) Here, we used pig as a model to investigate gene expression changes related to glucose uptake capacity and utilization in skeletal and cardiac muscles at different stages of development. We then performed functional enrichment analysis to identify the biological process of these genes. Furthermore, we performed correlation analysis between mRNA and miRNAs to uncover cooperative roles in energy metabolism of skeletal and cardiac muscles.
Materials and methods
Animals and tissue collection
Female Jinhua pigs, which are Chinese native pig breeds obtained from Zhejiang jiahua pig breeding company (Zhejiang, China), were used in the present study. Each stages contained three pigs, thus total of 15 healthy pigs were used to harvest tissues. The piglets were weaned at 28 ± 1 day of age and fed in the same conditions. A starter diet provided 3.40 Mcal kg−1 metabolizable energy (ME), 20.00% crude protein, and 1.15% lysine during 30–60th days after weaning. From 61st to 120th day, the diet contained 3.40 Mcal kg−1 ME, 17.90% crude protein, and 0.83% lysine. From 121st day to 7th year, the diet contained 3.40 Mcal kg−1 ME, 15.00% crude protein, and 1.15% lysine. All pigs were housed at the department (department of Zhejiang jiahua pig breeding) in pens with concrete floors and straw as bedding. The animals were fed twice daily ad libitum and had ad libitum access to water. The pigs were reared in compliance with national regulations for the humane care and use of animals in research. The pigs were sacrificed at the age of embryonic day 90, postnatal day zero, day 30, day 180, and 7 years. Then, the longissimus doris muscle (LDM), psoas major muscles (PMM), and cardiac muscles (CM) were immediately dissected from each pig. These samples were immediately frozen in liquid nitrogen, and then stored at −80 °C.
RNA isolation
Total RNA was isolated according to the manufacturer’s protocol of Trizol reagent (TaKaRa, Dalian, China). RNA integrity was verified by 1% agarose gel electrophoresis.
Quantitative polymerase chain reaction
mRNA was transcribed by SYBR® PrimeScriptTM Quantitative polymerase chain reaction (Q-PCR) Kit (TaKaRa) and Q-PCR was performed with SYBR Green Ⅰ(TaKaRa). MicroRNA transcription and Q-PCR were performed by SYBR® PrimeScript™ miRNA Q-PCR Kit (TaKaRa). Each experiment contained a negative control, and all the reactions were executed in triplicate.
All the primers used in Q-PCR for mRNA were designed using Primer 5.0 program (Supplemental Table ) and tested using a BLAST against the NCBI database (http://www.ncbi.nlm.nih.gov/tools/primer-blast). We firstly chose six internal control genes, then evaluated the normalized factors (M-value) using geNorm software (Supplemental Fig. )Citation8) and ranked them based on the M-values. The genes (YWHAZ, RPL4, and PPIA) with the lowest M value were chosen as the relative idea internal control genes in subsequent analysis. The primers of miRNA obtained from miRbase (http://www.mirbase.org/index.shtml) (Supplemental Table ), and the general used internal control genes, U6, 18S, and 5S were used to calculate miRNA relative expression.
Table 1. The biological process enrichment of genes in significant expression pattern.
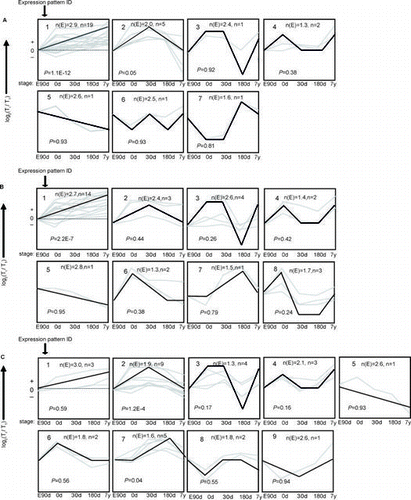
Fig. 1. The trends of 30 mRNA were divided into groups according to their dynamic expression patterns at the age of embryonic day 90 (E90d), postnatal day 0 (0d), day 30 (30d), day 180 (180d), and 7 years (7y) for (A) cardiac muscle, (B) longissimus dorsi Muscle, and (C) psoas major Muscle.
Notes: The dashed line indicates no change in expression at different age stages. The number in the top left corner of each square indicates the expression pattern ID. The blank, bold lines in the squares are trendlines of the expression patterns, and the gray lines represent gene expression from embryonic day 90 to 7 years postnatally. The p value is the corrected p value between the number of genes expected (n(E)) and the number of genes assigned (n), with p values < 0.05 considered to be statistically significant. Four other expressions were normalized to the highest one in four stages firstly, and then all expressions were log2-transformed.
The relative mRNA and miRNA expression level was quantified using the 2−△△Ct method and data are presented as mean ± SD. The differentially expressed genes were also identified by a two-way analysis of variance (ANOVA) using SigmaPlot 12.3.
Clustering and Gene class test analysis
Gene expression patterns during development were measured by short time-series expression miner (STEM) software.Citation9) The log2-transformed gene expression data were subjected to clustering. False discover rate (FDR)-corrected p value from multiple hypothesis test was obtained to determine the significance of gene enrichment for each pattern.
Genes in significant pattern (p < 0.05) were performed Gene class test analysis by ErmineJ using the following steps.Citation10) First, we performed the gene annotation by online software DAVID and imported into ErminJ. Then, the gene expression data and p values among the different age stages of each tissue calculated by two-way ANOVA are imported to analyze biological process enrichment. Genes with similar biological functions were clustered into a same gene group. The FDR adjusted permutation p values of above ANOVA were analyzed using gene score resampling algorithm to determine which gene group was significant with Benjamini–Hochberg FDR-corrected p value (pErmineJ) < 0.05 or 0.01.
Correlation analysis of mRNA and miRNA
To investigate the possible interactions between miRNA and its potential target mRNA, all the mRNAs and miRNAs expression data were imported into VANTED to calculated the Pearson’s correlation.Citation11) Only mRNA–miRNA pairs with a p value < 0.05 and a negative correlation efficiency were considered as possible interactions. Then we used TargetScan, PicTar, and miRanda databases to predict the target relationship.
Results
Expression patterns of protein-coding genes involved in glucose metabolism during development
To study gene expression pattern of protein-coding genes involved in glucose uptake and utilization during development, we measured expression of 30 genes at five stages in CM, LDM, and PMM, respectively. The predominant temporal expression patterns were identified using the STEM algorithm. Two expression patterns for PMM and one expression pattern for each of CM and LDM were found to be statistically significant between the number of genes expected (n(E)) and the number of genes assigned (n) (Fig. , Supplemental Table 2). These patterns potentially contain genes that were coordinately regulated.
Expression pattern 1 (p < 0.01) for CM contained 19 genes that were strongly up-regulated from embryonic day 90 to 7 years postnatally (Fig. (A)). To investigate the function of these genes, we used ErmineJ software to identify the enriched biological processes based on gene ontology (GO) annotation (Table ). We found that the process of heart development was enriched in CM (pErmineJ < 0.05), suggesting that heart development continues from embryonic day 90 to 7 years postnatally. Meanwhile, process of transmembrane transport containing genes of glucose transporter 4 (GLUT4) and monocarboxylate transporter 1 (MCT-1) was enriched in CM. The GLUT4 protein is mainly responsible for the transport of glucose into cells, thus up-regulated GLUT4 mRNA expression indicate increased glucose uptake in cardiac muscle with development.
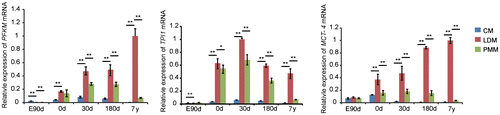
Using STEM analysis of gene expression in LDM, we identified eight temporal expression patterns, of which only one was significant (Fig. (B)). Similar to the results from CM, genes in expression pattern 1 (p < 0.01) were strongly up-regulated from embryonic day 90 to 7 years postnatally. We identified the enrichment of energy-related metabolic processes (Table ), such as phosphate metabolic process (pErmineJ < 0.05). Of these processes, transmembrane transport containing genes of GLUT4 and MCT-4 were enriched in LDM. The increased expression of GLUT4 also suggest increased glucose uptake in LDM with development. Although glycolysis was not an enriched process in LDM (p = 0.29), glycolytic enzymes were highly expressed in LDM compared with CM and PMM across the five age stages (Fig. ), indicating that LDM is a glycolytic skeletal muscle type that mainly consists of type IIb fibers.
Fig. 2. Gene expression related to glycolysis in cardiac and skeletal muscle during development.
Notes: Triose phosphate isomerase 1 (TPI1), muscle phosphofructokinase (PFKM), and monocarboxylate transporter 4 (MCT-4) are important glycolytic enzymes that are mainly expressed in white muscle, and significantly up-regulated in LDM compared with CM and PMM in the study. The data were normalized to the highest expression of the three muscles and presented as mean ± SD. n = 3 for each age stages of tissues. CM, cardiac muscle; LDM, longissimus dorsi muscle; PMM, psoas major muscles.
**p < 0.01, *p < 0.05.
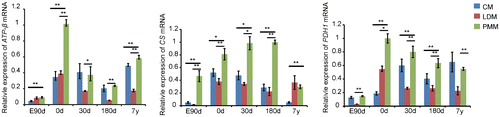
In expression pattern 2 (p < 0.01) of PMM, genes were first up-regulated from embryonic day 90 to 30 days postnatally and then down-regulated to 7 years postnatally (Fig. (C)). Expression pattern 7 (p < 0.05) represented genes that maintained steady expression levels from embryonic day 90 to birth, up-regulated soon after birth, and then down-regulated from 180 days to 7 years postnatally. We then annotated the 14 genes in patterns 2 and 7 and clustered them by biological process using ErminJ. Genes in pattern 7 had no significant enriched processes, perhaps because of the small number of genes in this pattern. Genes in pattern 2 were mainly related to oxidation and ATP synthesis (Table ). For example, citrate synthase (CS) and pyruvate dehydrogenase (PDH1) – genes related to mitochondrial oxidation, were enriched in the process of energy derivation by oxidation of organic compounds. Furthermore, we found that oxidation-related genes were strongly up-regulated in PMM compared with LDM and CM (Fig. ), which confirmed the oxidative capacity of PMM.Citation12)
Fig. 3. Gene expression related to oxidation in cardiac and skeletal muscle during development.
Notes: ATPase-ß, citrate synthase (CS), and pyruvate dehydrogenase (PDH1) are related to mitochondrial oxidation, which were up-regulated in PMM compared with CM and LDM in this study. The data were normalized to the highest expression of the three muscles and presented as mean ± SD. n = 3 for each age stages of tissues. CM: cardiac muscle, LDM, longissimus dorsi muscle, PMM: psoas major muscle.
**p < 0.01, *p < 0.05.
Expression pattern of miRNAs involved in glucose metabolism during development
The expression of miRNAs related to glucose metabolism was measured by Q-PCR, after which STEM analysis was used to profile miRNA expression patterns in CM, LDM, and PMM (Fig. , Supplemental Table 3).
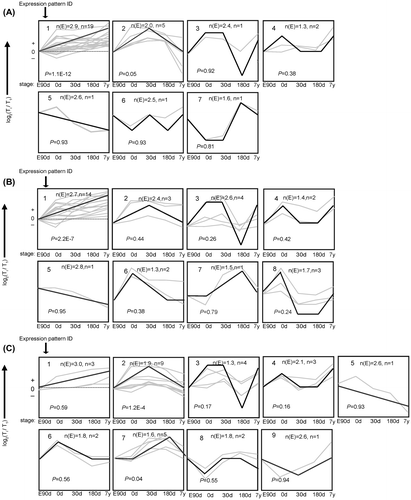
Fig. 4. The trends of 10 miRNA were divided into groups according to heir dynamic expression patterns for (A) Cardiac Muscle, (B) longissimus dorsi Muscle, and (C) psoas major muscles at the age of embryonic day 90 (E90d), postnatal day 0 (0d), day 30 (30d), day 180 (180d), and 7 years (7y).
Notes: The dashed line indicates no change in expression among different stages. The number in the top left corner of each square is the expression pattern ID. The blank, bold lines in the squares are trendlines of the expression patterns, and the gray lines represent gene expression from embryonic day 90 to 7 years postnatally. The p-value is the corrected P-value between the number of genes expected (n(E)) and the number of genes assigned (n), with p values < 0.05 considered to be statistically significant. Four other expressions were normalized to the highest one in four stages firstly, and then all expressions were log2-transformed.
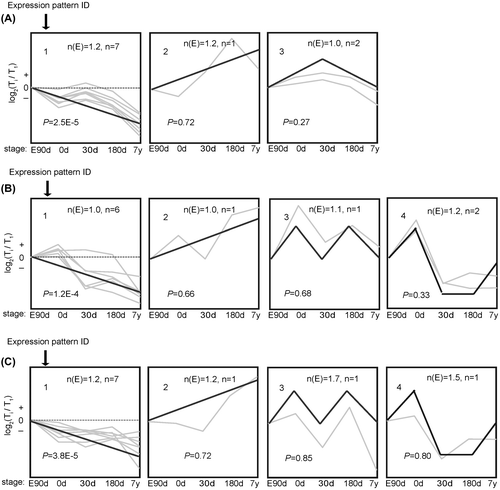
Expression pattern 1 showed differential gene numbers (p < 0.01) in the three muscles, where genes were strongly down-regulated from embryonic day 90 to 7 years postnatally. miR-126, let-7 g, miR-320, and miR-10b were co-expressed in all three muscle types in the same expression patterns, which were negatively correlated with age; while miR-133a was negatively correlated with age only in CM suggesting tissue-specific expression.
Relationship of miRNAs and potential target mRNAs related to glucose metabolism
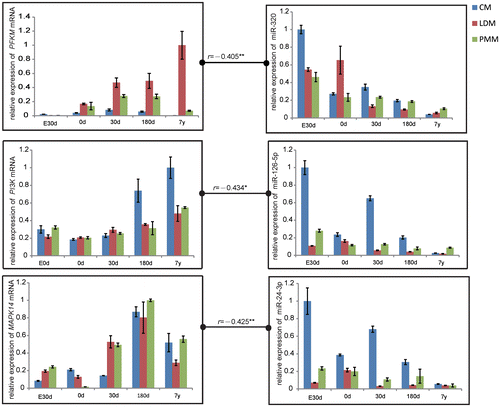
To investigate the possible interactions between miRNAs and mRNAs, we calculated the Pearson’s correlation between miRNAs and mRNAs using VANTED software. The significantly negative correlation between mRNA and miRNA (Supplemental Fig. ) suggests possible interaction of mRNA and miRNA. Of these potential miRNA–mRNA pairs, target prediction by TargetScan, PicTar, and miRanda databases found three miRNA–mRNA target relationship, muscle phosphofructokinase (PFKM) and miR-320, MAPK and miR-24, PI3 K and miR-126 (Fig. ), which were in agreement with previous studies, and each of the correlation of the three pairs was −0.405, −0.434, −0.425.
Fig. 5. Correlation in Gene Expression between PFKM and miR-320, PI3 K and miR-126, MAPK and miR-24 at the Stage of Embryonic day 90 (E90d), Postnatal day 0 (0 d), Day 30 (30d), Day 180 (180d), and 7 years (7y).
Notes: The data were normalized to the highest expression of the three muscles and presented as mean ± SD. n = 3 for each age stages of tissues. CM, cardiac muscle; LDM, longissimus dorsi muscle; PMM, psoas major muscles.
**p < 0.01, *p < 0.05.
Discussion
In the present study, we investigated expression of 30 mRNA and 10 miRNAs related to glucose uptake and utilization during development and profile the gene expression pattern of development in cardiac and skeletal muscle.
Glucose uptake is transported by GLUTs proteins. GLUT4 was the main transporter in pig, while other GLUTs showed weak signals.Citation13) Our results showed that GLUT4 was upregulated during cardiac and skeletal muscle development, which indicate enhanced glucose uptake with increasing age. Studies in rabbits hearts found that GLUT4 mRNA expression increased gradually after birth, which leads to improved post-ischemic contractile function with increasing age owing to an increase in glycolytic capacity,Citation14) suggesting that upregulated GLUT4 expression in CM may enhance glycolysis by increasing glucose uptake and contribute to provide energy for heart contractile. Meanwhile, studies in rat skeletal muscle demonstrated that GLUT4 protein levels decreased with age and led to age-related insulin resistance,Citation15) which might be caused by a shorter poly(A) tail in older rat skeletal muscle, leading to hamper GLUT4 translation efficiency.Citation16) On the other hand, pig at the age of 7 years is at the stage of young adult, which is similar to the age of 35–40 years old in humans. Though aging impairs glucose uptake and lead to insulin resistance, our results indicate that glucose uptake may increase during development and decreased in older age.
AKT, also known as protein kinase B (PKB), is an important signaling molecule to regulate glucose uptake by mediating insulin-induced translocation of the GLUT4 to the cell surface. AKT family consists of three different isoforms, AKT1, AKT2, and AKT3 encoded by separate genes. Although the three isoforms are expressed in muscle, researches showed that AKT3-deficient mice have a normal glucose metabolism,Citation17) which indicate AKT3 has no significant impact on glucose metabolism. And numerous studies analyzed glucose uptake by investigating AKT1 activity,Citation18−20) so we measured AKT1 mRNA expression to research its role of glucose metabolism. As we know, mRNA expression may influence protein expression, even protein activity. Previous studies demonstrated that decreased rat skeletal muscle AKT mRNA expression led to reducing protein level and inhibition of AKT phosphorylation, which inhibited AKT activity.Citation21) Thus, the present results of increased AKT1 mRNA level in LDM and CM may increase AKT1 activity, further increase GLUT4 translocation to cell surface, and enhance glucose uptake during development.
MCTs transport lactate in and out of muscle cells, mainly including MCT-1 and MCT-4. As we know, MCT-1 is primarily found in slow-twitch oxidative fibers and is highly correlated with the oxidative capacity of muscle, while MCT-4 is predominantly expressed in fast-twitch glycolytic fibers and its expression level is an index of glycolytic capacity.Citation22) Results showed that MCT-1 mRNA expression was increased during development and MCT-1 related process of membrane transport was enriched in CM suggesting enhanced oxidative capacities in CM with increasing age. While MCT-4 mRNA expression increased with age and MCT-4 related process of membrane transport was enriched in LDM, which suggests a concomitant increase in glycolytic capacity during development.
miRNA could regulate glucose metabolism by directly regulating target gene expression at the translational level. PFKM, the key regulator of glycolytic efficiency, has been identified as a target of miR-320.Citation23) Decreased miR-320 levels were accompanied by an increase in PFKM expression with increasing age in CM and LDM, suggesting enhanced glycolytic efficiency during development. Previous studies indicate that miR-24 and miR-126 negatively regulate glucose tolerance and uptake by MAPKCitation24) and PI3 K,Citation25) respectively. Increased levels of MAPK and PI3 K expression could increase the expression of GLUT4. We found that miR-24 and miR-126 were down-regulated during development, leading to activation of target genes and increased GLUT4 expression, which suggests a possible increase in glucose uptake during development. In addition, miRNA could also indirectly regulate glucose metabolism. Overexpression of let-7 in mice resulted in insulin resistance and impaired glucose tolerance by the insulin-PI3 K-mTOR pathway,Citation26) and miR-133a reduced insulin-induced glucose uptake in rat cardiomyocytes.Citation27) Our results show that let-7 g was negatively expressed with age in three muscle types and miR-133a expression decreased with age in CM, indicating increased muscle glucose uptake during development.
In conclusion, we investigated the expression pattern of genes related to glucose uptake and utilization during pig cardiac and skeletal muscle development. We identified genes related to heart development in CM, and genes related to glycolysis and oxidation that were differentially expressed in LDM and PMM during development. We also found that certain miRNAs that were down-regulated during development could regulate glucose metabolism by directly or indirectly regulating specific mRNAs. In addition, glucose uptake may be enhanced during pig cardiac and skeletal muscle development. However, gene transcription is only one factor to affect protein expression. Furthermore, the protein is generally activated by some protein modification, such as phosphorylation. Thus, to study the changes of glucose metabolism during development, these results will have to be confirmed by western blotting and enzyme activity assays.
Author contribution
In the present study, the authors Yanqin Guo, Long Jin, and Mingzhou Li designed the experiment. Then, Yanqin Guo, Fengjiao Wang, Mengnan He, and Rui Liu performed the Q-PCR. Yanqin Guo and Long Jin processed the data of Q-PCR and analyzed the results. Finally, the paper was writen by Yanqin Guo, and revised with the help of Long Jin and Mingzhou Li. Surong Shuai was as the Corresponding author.
Supplemental material
The supplemental material for this paper is available at http://dx.doi.org/10.1080/09168451.2014.915725.
Supplemental Tables 1-3 and Figs. 1 and 2
Download PDF (395.4 KB)Funding
This work was supported by grants from the National High Technology Research and Development Program of China (863 Program) [grant number 2013AA102502]; the Fund for Distinguished Young Scientists of Sichuan Province [grant number 2013JQ0013]; the National Special Foundation for Transgenic Species of China [grant numbers 2014ZX0800950B and 2011ZX08006-003]; the Project of Provincial Twelfth Five Years’ Animal Breeding of Sichuan Province [grant number 2011YZGG15].
Notes
Abbreviations: CM, cardiac muscles; CS, citrate synthase; FDR, False discover rate; GLUT 4, glucose transporter 4; G-6-P, Glucose-6-phosphate; GCT, Gene class test; GSR, gene score resampling; GO, gene ontology; LDM, longissimus doris muscle; miRNA, microRNA; MHT, multiple hypothesis test; MCT, monocarboxylate transporter; PMM, psoas major muscles; PDH1, pyruvate dehydrogenase 1; Q-PCR, Quantitative polymerase chain reaction; STEM, Short Time-series Expression Miner; PFKM, Phosphofructokinase (muscle).
References
- Cross HR, Opie LH, Radda GK, Clarke K. Is a high glycogen content beneficial or detrimental to the ischemic rat heart? A controversy resolved. Circ. Res. 1996;78:482–491.10.1161/01.RES.78.3.482
- Perciaccante A, Fiorentini A. Insulin resistance may be involved in relationship between cardiac autonomic dysfunction and polycystic ovary syndrome. Ann. Noninvas. Electro. 2007;12:388.10.1111/anec.2007.12.issue-4
- Xu X, Ren J. Unmasking the janus faces of autophagy in obesity-associated insulin resistance and cardiac dysfunction. Clin. Exp. Pharmacol. Physiol. 2012;39:200–208.10.1111/j.1440-1681.2011.05638.x
- Xu Y, Jin M, Wang L, Zhang A, Zuo B, Xu DQ, Ren ZQ, Lei MG, Mo XY, Li FE, Zheng R, Deng CY, Xiong YZ. Differential proteome analysis of porcine skeletal muscles between Meishan and Large White. J Anim. Sci. 2009;87:2519–2527.10.2527/jas.2008-1708
- Gallagher IJ, Scheele C, Keller P, Nielsen AR, Remenyi J, Fischer CP, Remenyi J, Fischer, CP, Roder K, Babraj J, Wahlestedt C, Hutvagner G, Pedersen BK, Timmons JA. Integration of microRNA changes in vivo identifies novel molecular features of muscle insulin resistance in type 2 diabetes. Genome Med. 2010;2:9.10.1186/gm130
- Ferland-McCollough D, Ozanne SE, Siddle K, Willis AE, Bushell M. The involvement of microRNAs in Type 2 diabetes. Biochem. Soc. Trans. 2010;38:1565–1570.10.1042/BST0381565
- Spurlock ME, Gabler NK. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. J. Nutr. 2008;138:397–402.
- Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, and Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3, RESEARCH0034.
- Ernst J, Bar-Joseph Z. ErmineJ: tool for functional analysis of gene expression data sets. BMC Bioinf. 2006;7:191.10.1186/1471-2105-7-191
- Lee HK, Braynen W, Keshav K, Pavlidis P. ErmineJ: tool for functional analysis of gene expression data sets. BMC Bioinf. 2005;6:269.10.1186/1471-2105-6-269
- Junker BH, Klukas C, Schreiber F. Identification of differences in microRNA transcriptomes between porcine oxidative and glycolytic skeletal muscles. BMC Bioinf. 2006;7:109.10.1186/1471-2105-7-109
- Liu Y, Li M, Ma J, Zhang J, Zhou C, Wang T, Gao X, Li X. Identification of differences in microRNA transcriptomes between porcine oxidative and glycolytic skeletal muscles. BMC Mol. Biol. 2013;14:7.10.1186/1471-2199-14-7
- Aschenbach JR, Steglich K, Gäbel G, Honscha KU. Expression of mRNA for glucose transport proteins in jejunum, liver, kidney and skeletal muscle of pigs. J. Physiol. Biochem. 2009;65:251–266.10.1007/BF03180578
- Friehs I, Cao-Danh H, Stamm C, Cowan DB, McGowan FX, del Nido PJ. Postnatal increase in insulin-sensitive glucose transporter expression is associated with improved recovery of postischemic myocardial function. J. Thorac. Cardiov. Sur. 2003;126:263–271.10.1016/S0022-5223(03)00034-5
- Santos JM, Benite-Ribeiro SA, Queiroz G, Duarte JA. The effect of age on glucose uptake and GLUT1 and GLUT4 expression in rat skeletal muscle. Cell Biochem. Funct. 2012;30:191–197.10.1002/cbf.v30.3
- Seraphim PM, Nunes MT, Giannocco G, Machado UF. Age related obesity-induced shortening of GLUT4 mRNA poly(A) tail length in rat gastrocnemius skeletal muscle. Mol. Cell Endocrinol. 2007;276:80–87.10.1016/j.mce.2007.07.004
- Easton RM, Cho H, Roovers K, Shineman DW, Mizrahi M, Forman MS, Lee VM, Szabolcs M, de Jong R, Oltersdorf T, Ludwig T, Efstratiadis A, Birnbaum MJ. Role for Akt3/protein kinase Bgamma in attainment of normal brain size. Mol. Cell. Biol. 2005;25:1869–1878.10.1128/MCB.25.5.1869-1878.2005
- Pu J, Peng G, Li L, Na H, Liu Y, Liu P. Skeletal muscle insulin resistance induced by adipocyte-conditioned medium: underlying mechanisms and reversibility. J. Lipid Res. 2011;52:1319–1327.10.1194/jlr.M011254
- Sell H, Eckardt K, Taube A, Tews D, Gurgui M, Van Echten-Deckert G, Eckel J. Skeletal muscle insulin resistance induced by adipocyte-conditioned medium: underlying mechanisms and reversibility. Am. J. Physiol-endoc. M. 2008;294:E1070–E1077.
- Yu J, Shi L, Wang H, Bilan PJ, Yao Z, Samaan MC, He Q, Klip A, Niu W. Conditioned medium from hypoxia-treated adipocytes renders muscle cells insulin resistant. Eur. J. Cell Biol. 2011;90:1000–1015.10.1016/j.ejcb.2011.06.004
- Liu Y, Huo X, Pang XF, Zong ZH, Meng X, Liu GL. Musclin inhibits insulin activation of Akt/protein kinase B in rat skeletal muscle. J. Int. Med. Res. 2008;36:496–504.10.1177/147323000803600314
- Enoki T, Yoshida Y, Lally J, Hatta H, Bonen A. Testosterone increases lactate transport, monocarboxylate transporter (MCT) 1 and MCT4 in rat skeletal muscle. J. Phys. 2006;577:433–443.
- Tang H, Lee M, Sharpe O, Salamone L, Noonan EJ, Hoang CD, Levine S, Robinson WH, Shrager JB. Oxidative stress-responsive microRNA-320 regulates glycolysis in diverse biological systems. FASEB J. 2012;26:4710–4721.10.1096/fj.11-197467
- Huang B, Qin W, Zhao B, Shi Y, Yao C, Li J, Xiao H, Jin Y. MicroRNA expression profiling in diabetic GK rat model. Acta Biochim. Biophys. Sin. 2009;41:472–477.10.1093/abbs/gmp035
- Guo C, Sah JF, Beard L, Willson JK, Markowitz SD, Guda K. The noncoding RNA, miR-126, suppresses the growth of neoplastic cells by targeting phosphatidylinositol 3-kinase signaling and is frequently lost in colon cancers. Genes Chromosomes Canc. 2008;47:939–946.10.1002/gcc.v47:11
- Zhu H, Shyh-Chang N, Segrè AV, Shinoda G, Shah SP, Einhorn WS, Takeuchi A, Engreitz JM, Hagan JP, Kharas MG, Urbach A, Thornton JE, Triboulet R, Gregory RI, Diagram Consortium, Magic Investigators, Altshuler D, Daley GQ. The Lin28/let-7 axis regulates glucose metabolism. Cell. 2011;147:81–94.10.1016/j.cell.2011.08.033
- Horie T, Ono K, Nishi H, Iwanaga Y, Nagao K, Kinoshita M, Kuwabara Y, Takanabe R, Hasegawa K, Kita T, Kimura T. MicroRNA-133 regulates the expression of GLUT4 by targeting KLF15 and is involved in metabolic control in cardiac myocytes. Biochem. Biophys. Res. Commun. 2009;389:315–320.10.1016/j.bbrc.2009.08.136
