Abstract
Production and utilization of cellulosic ethanol has been limited, partly due to the difficulty in degradation of cellulosic feedstock. β-Glucosidases convert cellobiose to glucose in the final step of cellulose degradation, but they are inhibited by high concentrations of glucose. Thus, in this study, we have screened, isolated, and characterized three β-glycosidases exhibiting highly glucose-tolerant property from Aspergillus niger ASKU28, namely β-xylosidase (P1.1), β-glucosidase (P1.2), and glucan 1,3-β-glucosidase (P2). Results from kinetic analysis, inhibition study, and hydrolysis of oligosaccharide substrates supported the identification of these enzymes by both LC/MS/MS analysis and nucleotide sequences. Moreover, the highly efficient P1.2 performed better than the commercial β-glucosidase preparation in cellulose saccharification, suggesting its potential applications in the cellulosic ethanol industry. These results shed light on the nature of highly glucose-tolerant β-glucosidase activities in A. niger, whose kinetic properties and identities have not been completely determined in any prior investigations.
Graphical Abstract
This study presents the identities and kinetic properties of three glucose-tolerant β-glycosidases from A. niger, and their applications in cellulose hydrolysis.
Energy shortage is likely to develop into a major problem in most countries of the world due to increasing energy consumption and depletion of fossil fuels. It is therefore necessary to search for alternative fuels to replace fossil fuels. Cellulosic biomass represents one of the most attractive alternative raw materials for bioethanol production since it is the most abundant glycan in nature. Cellulose, a linear polymer of β-1,4 glucose, is hydrolyzed by either acid or enzymes to its glucose monomers, which are then fermented into ethanol.Citation1−3) Acid hydrolysis is performed by using either dilute acid at high temperature and pressure, or concentrated acid at relatively low temperature and atmospheric pressure.Citation1) The disadvantages of this method are the extreme conditions, the resulting by-products, and the release of hazardous acidic wastes.Citation1,2) On the other hand, enzymatic hydrolysis of cellulose proceeds under ambient conditions, and produces higher yield of glucose with fewer by-products than the acid hydrolysis.Citation2) However, problems in enzymatic degradation of cellulose, partly due to the product inhibition, have prevented widespread production and utilization of cellulosic ethanol.
Enzymatic hydrolysis of cellulose can be accomplished by synergistic action of endoglucanases, exoglucanases, and β-glucosidases. Endoglucanases (E.C. 3.2.1.4) randomly hydrolyze amorphous regions of cellulose to produce cello-oligosaccharides. Exoglucanases, including cellodextrinases (E.C. 3.2.1.74) and cellobiohydrolases (E.C. 3.2.1.91), remove glucose and cellobiose units, respectively, from cellulose polysaccharide chains. β-Glucosidases (E.C. 3.2.1.21) then liberate the non-reducing end glucose units from cello-oligosaccharides and cellobiose.Citation3) However, the activities of endo- and exo-glucanases, and β-glucosidases can be inhibited by high concentrations of their products, which are cellobiose and glucose, respectively.Citation4,5) Therefore, a highly glucose-tolerant β-glucosidase (HGT-BG) is needed to overcome the product inhibition problem. Since HGT-BG will work efficiently even at a high concentration of glucose, it will help to remove the cellobiose intermediate that inhibits the activity of cellulases. Indeed, HGT-BGs have been reported from many fungal sources (Supplemental Table 1; see http://dx.doi.org/10.1080/09168451.2014.915727)Citation5−10) and termite Nasutitermes takasagoensis.Citation11) However, complete kinetic parameters were mostly lacking. Only HGT-BG from Aspergillus niger CCRC 31494 was reported with the kcat and kcat/Km values toward para-nitrophenyl-β-d-glucopyranoside (pNP-Glc), but not toward cellobiose, which was the target substrate in cellulolysis.Citation6) Furthermore, none of the reported HGT-BGs had been identified by LC/MS/MS analysis or by nucleotide sequences as being a true 1,4-β-glucosidase. Only HGT-BG F2 from A. fortidus was identified by N-terminal sequencing, but had sequence homology to glucan 1,3-β-glucosidase.Citation10)
Aspergillus species are important sources for β-glucosidases, and among them A. niger is the most common species for commercial β-glucosidase production.Citation12−14) We were thus interested in searching for HGT-BG activities in A. niger and determining their kinetic properties and identifying their sequences. We found that the isolate of A. niger ASKU28 showed the highest HGT-BG activity when grown in minimal medium supplemented with cellobiose. Its HGT-BG activity increased further when grown with beechwood xylan supplement. Three HGT-BGs were purified to homogeneity from culture medium, and their identities determined by LC/MS/MS as β-xylosidase, β-glucosidase, and glucan 1,3-β-glucosidase A from A. niger. Results from enzymatic characterization, including optimal reaction conditions and stability, kinetic properties, glucose inhibition, and hydrolysis of oligosaccharides and cellulose, supported their identification. The present data help to shed light on the identities and properties of HGT-BG enzymes from Aspergillus sp. and their applications in cellulose saccharification.
Materials and methods
Materials
Cello-oligosaccharides (degree of polymerization from 2 to 6) and laminarioligosaccharides (degree of polymerization from 2 to 5) were purchased from Seikagaku Kogyo Co. (Tokyo, Japan). Gel filtration calibration kit (high molecular weight) and blue dextran 2000 were purchased from GE Healthcare (Buckinghamshire, UK). Fungal media components, all aryl-glycosides, 4-methylumbelliferyl-β-d-glucopyranoside (4MU-Glc), amygdalin, gentiobiose, laminarin, glucose oxidase/peroxidase reagent, Sigmacell® cellulose (type 50), A. niger cellobiase, and T. reesei ATCC 26921 cellulase were purchased from Sigma Chemical (St. Louis, MO, USA). All other chemicals were of analytical grade.
Micro-organisms and culture conditions
One hundred and twenty-six isolates of Aspergillus spp. were obtained from an existing stock collection from the laboratory of Dr. Kitpreechavanich at the Department of Microbiology, Kasetsart University, Thailand. Aspergillus spp. isolates were maintained for 4 days on potato dextrose agar slant (20% potato, 2% dextrose, and 1.5% agar) at room temperature, and used to prepare a spore suspension in 0.15% (v/v) Tween 80. For enzyme production, the spore suspension was grown at a concentration of 1 × 105 viable spores mL−1 in minimal medium (0.2% NaNO3, 0.2% KCl, 0.1% KH2PO4, 0.1% NH4NO3, 0.1% (NH4)H2PO4, 0.05% MgSO4.7H2O, and 0.05% yeast extract, pH 6.0) containing 0.5% (w/v) various carbon sources (cellulose powder, cellobiose, quercetin, lactose, or beechwood xylan), at 30 °C and 150 rpm.Citation7) Then culture medium was collected on day 4, 7, and 14 of cultivation and centrifuged at 4 °C, 5000 rpm for 10 min to remove fungal biomass. The supernatant was used as crude β-glucosidase preparation.
Enzyme assays for screening and purification of HGT-BGs from Aspergillus spp
The HGT-BG activities were measured by incubating 20 μL of the crude β-glucosidase preparation with 1 mM pNP-Glc in 0.1 M sodium acetate, pH 5.0, containing 20% (w/v) glucose in a total volume of 50 μL. After incubation at 50 °C for 30 min, the reactions were stopped by adding 100 μL of 2 M sodium carbonate. The absorbance of para-nitrophenol (pNP) released from the reaction was measured at 405 nm, and compared to a standard curve of pNP. The isolate of Aspergillus sp. which showed the highest HGT-BG activity was chosen for further study.
Determination of factors affecting HGT-BG production
Nine carbon sources, including arabinose, beechwood xylan, cellulose powder, cellobiose, glucose, lactose, quercetin, methyl xylose, and xylose, were added to the minimal medium at the concentration of 0.5% (w/v) to study the effect of carbon source on the enzyme production. Then, the carbon source that produced the highest HGT-BG activity was selected for determination of its optimum concentration for HGT-BG production. Finally, the appropriate concentration of the selected carbon source was used for determination of the optimum rotational speed and culturing temperature for HGT-BG production.
Purification of HGT-BGs from the selected Aspergillus sp
The selected isolate of Aspergillus sp. was grown in the culture condition that produced the highest HGT-BG activity as determined earlier. Culture medium was collected for measurement of HGT-BG activity every 2 days until the HGT-BG activity did not increase further. Culture medium was harvested by filtering through filter paper (Whatman no. 1). The volume of culture media was reduced by ultrafiltration (10,000 MWCO, Stirred Ultrafiltration cell, Millipore, Billerica, MA, USA). Then, the crude HGT-BG was further concentrated to 2 mL by centrifugal ultrafiltration (10,000 MWCO, Millipore, Billerica, MA, USA) at 4000 rpm and 4 °C. The crude HGT-BG preparation was applied to a Sephacryl S-300 column (GE Healthcare, Buckinghamshire, UK) that was previously equilibrated with 10 mM sodium acetate, pH 5.0, containing 50 mM NaCl and 8 mM EDTA, at a flow rate of 1 mL min−1. HGT-BG was eluted by using the same buffer as in the equilibration step, and was separated into two fractions of about 110 kDa (P1) and 33–66 kDa (P2).
P1 was further purified to homogeneity by using Hitrap Q anion exchange chromatography (GE Healthcare) followed by Mono Q HR 5/5 anion exchange chromatography (GE Healthcare). Both steps were performed by using 10 mM sodium acetate, pH 5.0, as a start buffer, and a linear gradient of 0–1 M NaCl in 10 mM sodium acetate, pH 5.0, as an elution buffer. After Mono Q chromatography, P1 was separated into two fractions namely P1.1 and P1.2.
P2 was further purified by chromatofocusing on a Mono PTM HR 5/5 column (GE Healthcare). P2 was dissolved in 10 mM sodium phosphate, pH 6.0, and then applied to the column, which was previously equilibrated with 10 mM sodium phosphate, pH 6.0. The protein was eluted with 1/10 dilution of polybuffer 74, pH 4.0 (GE Healthcare), at a flow rate of 0.5 mL min−1.
At each step of purification, fractions containing HGT-BG activities were pooled, and concentrated by ultrafiltration. Protein concentration was determined with the Bio-Rad protein assay reagent kit (Bio-Rad, Hercules, CA, USA), and compared to a standard curve of bovine serum albumin. SDS-PAGE was performed to check the protein purity on 7.5 and 10% resolving gels.Citation15) Eight percent non-denaturing PAGE was performed to check the in-gel activity of the purified HGT-BGs using 1 mM 4MU-Glc as substrate.Citation16)
Determination of glycosylation modifications
The glycosylation modifications of the purified HGT-BGs were assayed by treatment with endoglycosidase Hf (New England Biolabs, Ipswich, MA, USA), which cleaves the β-glycosidic bond between the two N-acetylglucosamine subunits next to asparagine residue of N-glycosylated proteins. The purified HGT-BGs were denatured in glycoprotein denaturing buffer (0.5% SDS, 0.04 M DTT) at 100 °C for 10 min, and cooled to room temperature. Then, the denatured proteins were incubated with 1000 U of endoglycosidase Hf in 50 mM sodium citrate, pH 5.5, at 37 °C for 1 h. The cleavage products were analyzed by 10% SDS-PAGE.
Determination of native molecular weights
The purified HGT-BGs and the gel filtration calibration kit (high molecular weight), which comprises ferritin (440 kDa), aldolase (158 kDa), conalbumin (75 kDa), and ovalbumin (43 kDa) were applied onto a Sephacryl S-300 HR column that was previously equilibrated with 50 mM sodium phosphate, pH 6.0, containing 150 mM NaCl, at a flow rate of 1 mL min−1. All proteins were eluted by the same buffer as in the equilibration step. A calibration curve was constructed by plotting the values of a partition coefficient of the protein markers against the log values of their molecular weights, and used to calculate the native molecular weights of the purified HGT-BGs. Void volume was determined by using blue dextran 2000 (GE Healthcare).
Determination of amino acid sequence
N-terminal sequence analysis via Edman degradation reaction was performed by AltaBioscience, University of Birmingham, UK. For the LC/MS/MS analysis, the protein bands of the purified HGT-BGs were cut from the SDS-PAGE gel and subjected to in-gel digestion with trypsin.Citation17) Then, the tryptic peptides were identified by nanoflow liquid chromatography coupled with electrospray ionization (nano ESI MS/MS) quadrupole-time of flight tandem mass spectrometry (Q-ToF micro; Micromass, UK). The obtained MS/MS spectra were analyzed by the Mascot search program.Citation18)
Determination of hydrolytic properties
The hydrolytic activity of the purified HGT-BGs was assayed by incubating the enzymes with 10 mM pNP-Glc in 0.1 M sodium acetate, pH 5.0, containing 20% (w/v) glucose for 5 min. The reaction mixtures were then stopped by adding 2 M sodium carbonate, and the amount of pNP released from the reactions was determined by reading the absorbance of 405 nm and comparing it to a standard curve of pNP.
The optimal temperature for enzyme activity was determined by incubating the reaction mixtures at the temperature ranging from 30 to 100 °C. The optimal pH for enzyme activity was determined at their optimal temperature in 50 mM sodium citrate, pH 2.1–6.4; 50 mM sodium formate, pH 3.0–4.5; 50 mM sodium acetate, pH 4.0–5.5; and 0.1 M sodium phosphate, pH 2–9.
The temperature stability for enzyme activity was determined by incubating the enzymes at 30–80 °C for 30 min before measuring the residual activities at their optimal temperature and pH. The pH stability for enzyme activity was determined by incubating the enzymes in 0.1 M sodium phosphate, pH 2–9, at 4 °C for 4 h before measuring the residual activities at their optimal temperature and pH.
Kinetic analysis
The kinetic parameters of the purified HGT-BGs were determined toward various substrates, namely pNP-Glc, para-nitrophenyl-β-d-xylopyranoside (pNP-Xyl), para-nitrophenyl-α-l-arabinopyranoside (pNP-Ara), para-nitrophenyl-β-d-fucopyranoside (pNP-Fuc), para-nitrophenyl-β-d-galactopyranoside (pNP-Gal), para-nitrophenyl-β-d-mannopyranoside (pNP-Man), cellobiose, laminaribiose, and xylobiose. The reactions were performed in 0.1 M sodium citrate for 10 min at 40 °C and at their optimal pH. The reactions with pNP-glycosides were stopped by adding 2 M sodium carbonate, and the amount of pNP released was determined by reading the absorbance of 405 nm and comparing it to a standard curve of pNP. The reactions with cellobiose and laminaribiose were stopped by heating to 95 °C for 5 min, and glucose released from the reaction was reacted with a glucose oxidase/peroxidase reagent for 15 min at 37 °C. The amount of glucose was determined by reading the absorbance at 405 nm, and comparing it to a standard curve of glucose. The reactions with xylobiose were stopped by reacting with 1% dinitrosalicylic acid, then heating to 95 °C for 5 min, and cooling on ice. The amount of xylose was determined by reading the absorbance at 540 nm, and comparing it to a standard curve of xylose.Citation19) Kinetic parameters were calculated from the Michaelis–Menten equation (or Lineweaver–Burk equation when the concentration of substrate is low compared with the Km value) using KaleidaGraph program (Synergy Software).
Determination of inhibition constant (Ki)
The inhibitory effect of glucose and xylose on HGT-BG activity was measured by adding a range of concentrations of glucose or xylose into the reaction mixture of the purified enzymes and various concentrations of substrate (pNP-Glc or pNP-Xyl) in 0.1 M sodium citrate for 5 min at their optimal temperature and pH. The amount of pNP released from the reactions was determined as described above. The Ki of competitive inhibitor was obtained by using Dixon plot.
Hydrolytic activity toward oligosaccharides
The hydrolytic activities of purified HGT-BGs were determined toward 1 mM of various oligosaccharide substrates, namely cello-oligosaccharides (cellobiose, cellotriose, cellotetraose, cellopentaose, and cellohexaose), laminarioligosaccharides (laminariobiose, laminaritriose, laminaritetraose, and laminaripentaose), amygdalin, gentiobiose, and laminarin, in 0.1 M sodium citrate for 5 min at 40 °C and at their optimal pH. The amount of glucose released from the reactions was determined as described above, and expressed as the percent of relative activity to the amount of glucose released from 1 mM pNP-Glc under the same reaction condition.
Hydrolytic activity toward cellulose
Suspensions of 2.5% (w/v) Sigmacell® cellulose, type 50, were hydrolyzed by 5 μg of each enzyme, namely the purified P1.1, P1.2, and P2, a commercial cellobiase from A. niger (equivalent to Celluclast 1.5 L from Novozyme) and a commercial cellulase from T. reesei ATCC 26921 (equivalent to Novozym 188 from Novozyme) in 0.1 M sodium citrate at their optimal pH, for 24 h at 40 °C, 220 rpm. The amount of glucose released from the reaction at various times was determined as described above.
Results and discussion
Screening and optimization of HGT-BG production
HGT-BG activities were screened from 126 isolates of Aspergillus spp. grown in minimal mediumCitation7) containing 0.5% (w/v) of five different carbon sources (cellulose, cellobiose, lactose, quercetin, or beechwood xylan). Enzyme activities were generally 3- to 5-fold higher at 50 °C than at 30 °C, and at least 20-fold higher in the absence of glucose (results not shown). However, since we were interested in HGT-BG production, the activity screening was performed in the presence of 20% (w/v) glucose at 50 °C. The isolate of A. niger ASKU28 showed the highest HGT-BG activity when grown in minimal medium supplemented with cellobiose (data not shown), and was thus selected for further study. In addition, beechwood xylan and quercetin could induce even higher expression of HGT-BG activity by A. niger ASKU28 than cellobiose. Previously, beechwod xylan was reported to induce the expression of a β-glucosidase gene from A. fumigatus and Talaromyces emersonii,Citation20,21) while quercetin, a plant-derived flavonoid, was the best inducer for HGT-BG production in A. oryzae and A. niger.Citation7,8) A. niger has been reported to produce many isoforms of β-glucosidase with different molecular weights and enzymatic properties when grown in different culture conditions and carbon sources.Citation22) So, in this study, beechwood xylan was used as an inducer for HGT-BG production by A. niger ASKU28, in order to elicit enzyme profiles that might differ from previous studies due to different culture conditions.
The optimal growth condition for HGT-BG production by A. niger ASKU28 was found to be minimal medium supplemented with 2% (w/v) beechwood xylan, at 30 °C, 100 rpm. The highest HGT-BG activity by A. niger ASKU28 was obtained after 12–14 days of cultivation, as β-glucosidases are involved in recycling of cell wall components (which include β-1,3-glucan) when nutrients are depleted. This result agreed well with the previous study of β-glucosidases from A. oryzae.Citation7,8)
Purification and characterization of HGT-BGs from A. niger ASKU28
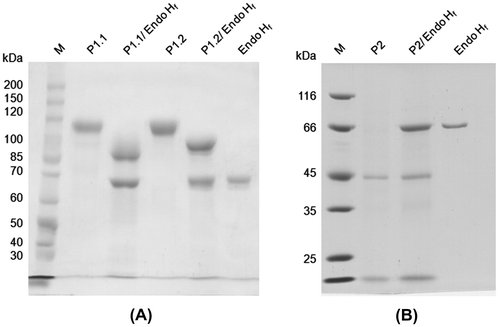
A. niger ASKU28 was grown in minimal medium supplemented with 2.0% (w/v) beechwood xylan for 12–14 days under its optimal growth condition. The culture medium was used as the source of crude HGT-BGs and concentrated by ultrafiltration. Following gel filtration chromatography, HGT-BGs were separated into two fractions of about 110 kDa (P1) and 33–66 kDa (P2). P1 was further purified to homogeneity by two steps of anion exchange chromatography (Hitrap Q followed by Mono Q), yielding P1.1 and P1.2 (Fig. (A)). P2 was further purified to homogeneity by chromatofocusing chromatography (Fig. (B)). The summary of their purification is presented in Table . The activities reported in Table appeared rather low as they were measured in the presence of 20% glucose. However, when glucose was omitted from the reactions, the total β-glucosidase activities of the purified P1.1, P1.2, and P2 were 18, 54, and 3.7 μmol min−1, respectively. The fact that these values do not correlate with the total activities in Table of 0.6, 0.6, and 1.1 μmol min−1, respectively, may be due to differences in glucose tolerance of the three enzymes. Gel filtration chromatography was selected as the first purification step since the other chromatography techniques resulted in the co-elution of P1 and P2. For the purification of P1.1 and P1.2, Hitrap Q was used to remove bulk impurities in the intermediate step of purification, while Mono Q was used to remove trace impurities in the polishing step of purification and to separate P1.1 and P1.2. However, their purification fold and percent recovery were very low (Table ), since fungi secrete a lot of proteins into culture medium and most of these proteins had similar properties as the purified glycosidases, making it impossible to pool all fractions containing HGT-BG activities. For the purification of P2, Mono-P was a suitable technique to remove impurities as the specific activity obtained from this step increased 13-fold (Table ).
Fig. 1. Ten percent SDS-PAGE of protein samples at each purification step.
Notes: (A) P1.1 and P1.2. (B) P2. The purification steps are indicated above the lanes. Lane M is protein size markers.
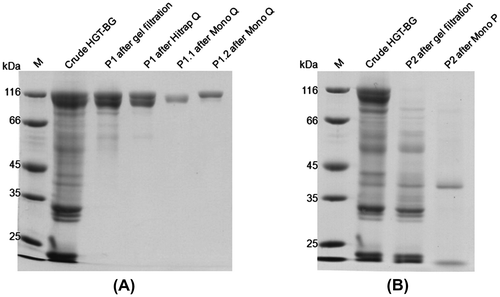
Table 1. Summary of purification steps of P1.1, P1.2, and P2 produced by 1 L of A. niger ASKU28 culture.
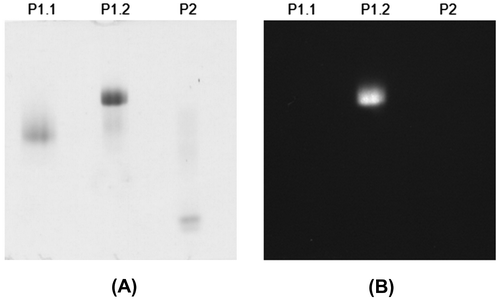
The apparent subunit molecular weights of P1.1, P1.2, and P2 were found to be 112, 117, and 40 kDa, respectively (Supplemental Fig. 1 and Fig. 1(B)). Only that of P2 is in the same range as the previously reported HGT-BGs (43–54 kDa),Citation5−10) suggesting similar properties, while P1.1 and P1.2 were probably different enzymes. P1.1 and P1.2 showed the same native molecular weight of 390 kDa, while that of P2 was 45 kDa. Thus, P1.1 and P1.2 may contain 3–4 subunits, while P2 appeared to be monomeric. The native and subunit molecular weights of P1.1 and P1.2 are similar to β-xylosidase and β-glucosidase from A. fumigatus,Citation20) and β-glucosidase II from A. niger CCRC 31494.Citation23) P1.1 and P1.2 were found to contain high mannose glycosylation, and showed the apparent subunit molecular weights of 89 and 96 kDa, respectively, after deglycosylation by endoglycosidase Hf (Fig. (A)), whereas P2 did not appear to contain N-linked glycosylation (Fig. (B)). Activity staining of these purified enzymes with 1 mM 4MU-Glc on a non-denaturing PAGE revealed a fluorogenic signal of the 4-methylumbelliferone product under UV light from only P1.2 (Fig. ), suggesting different substrate specificities among the three enzymes.
Identification of HGT-BGs by amino sequence analysis
The internal amino acid sequences of the purified P1.1, P1.2, and P2 were determined by tryptic digestion and LC/MS/MS analysis. Their peptide sequences were similar to those of glycoside hydrolase family 3 β-xylosidase (GenBank: AAD13106), glycoside hydrolase family 3 β-glucosidase (GenBank: CAB75696), and glycoside hydrolase family 5 glucan 1,3-β-glucosidase A (GenBank: XP_001398868) from A. niger, respectively, with 10, 19, and 10% sequence coverage, respectively (Supplemental Figs. 2–4). N-terminal sequencing by Edman degradation found that P1.1 contained a blocked N-terminus, but the amino acid sequence of A. niger β-xylosidase was predicted to have a 26-amino acid signal sequence by SignalP 3.0 prediction program.Citation24) Thus, the mature N-terminal sequence of P1.1 is likely to begin at glutamine 27, that might cyclize to pyroglutamate, blocking the Edman degradation reaction.Citation25) On the other hand, the mature N-terminal amino acid sequence obtained for P1.2 was found to be DELAYS, which starts at position 20 of A. niger β-glucosidase. The N-terminal amino acid sequence of P2 was not analyzed by Edman degradation. However, the amino acid sequence of A. niger glucan 1,3-β-glucosidase A was predicted to have a 22 amino acid signal sequence, and thus the mature N-terminus of P2 is likely to begin at valine 23.
Since the carbon source used in this study was beechwood xylan, the expression of β-xylosidase (P1.1) was most likely induced for utilization of xylan (a complex polysaccharide with β-1,4-linked xylose backbone). In addition, since the HGT-BG enzymes were harvested from the culture medium on day 12–14 of cultivation, glucan 1,3-β-glucosidase (P2) was probably expressed for turnover of cell wall β-glucan (β-1,3-linked glucose polymer). Similarly, HGT-BG F2 from A. fortidus was also identified by N-terminal amino acid sequencing as glucan 1,3-β-glucosidase, in agreement with their similar subunit molecular weights.Citation10) The identities of other reported fungal HGT-BGs were not determined,Citation5−9) so they could well be any carbohydrate-active enzymes having β-glucosidase activity. Thus, we are the first to identify HGT-BG activities in A. niger as β-xylosidase, β-glucosidase, and glucan 1,3-β-glucosidase.
Determination of optimal reaction conditions and stability of HGT-BGs
The optimal temperature of the purified P1.1, P1.2, and P2 was found to be 80, 70, and 70 °C, respectively (Supplemental Fig. 5). The optimal pH of the purified P1.1, P1.2, and P2 was found to be at pH 3–4, 3.5–4, and 4.5–5.5, respectively (Supplemental Fig. 6). These enzymes were stable up to 40, 50, and 50 °C, respectively, with at least 80% activity remaining after incubation at these temperatures for 30 min, but lost all activities at 60, 70, and 70 °C, respectively (Supplemental Fig. 7). P1.1 and P2 were stable at pH 3–5, and pH 3–7, respectively, with at least 80% activity remaining after incubation at the above pHs for 4 h (Supplemental Fig. 8). P1.1 was more stable at acidic pH values, while P2 was more stable at basic pH values. P1.2 was less stable than the other two enzymes, with only 60–70% remaining activities after incubation at pH 3–6, but retained more activities at extreme pHs.
In comparison, P1.1 (β-xylosidase) and P2 (glucan 1,3-β-glucosidase) showed 10 °C higher optimal temperature than the previously reported A. niger β-xylosidase and A. fortidus glucan 1,3-β-glucosidase, respectively, while their optimal pHs were in the same range.Citation10,26) P1.2 (β-glucosidase) showed similar optimal temperature and pH to most reported fungal β-glucosidases.Citation14,20,22,23,27,28)
Kinetic analysis of HGT-BGs
Kinetic parameters of the purified HGT-BGs were determined toward selected para-nitrophenyl-glycosides, cellobiose, laminaribiose, and xylobiose, at their optimal pH and 40 °C (Table ). The reactions were not carried out at optimal temperature due to possible thermal denaturation. The reported kcat values were calculated per subunit rather than per native molecule. None of these three enzymes could appreciably hydrolyze pNP-Gal and pNP-Man (not shown). P1.1 was most efficient toward pNP-Xyl, supporting its identification by LC/MS/MS as a β-xylosidase, and also showed good catalytic efficiency toward pNP-Ara. Similarly, numerous enzymes in glycoside hydrolase family 3, such as α-l-arabinofuranosidase from barley (Hordeum vulgare L.) and β-xylosidase from A. japonicas, have been characterized as bifunctional enzymes having both α-l-arabinofuranosidase and β-d-xylopyranosidase activities.Citation24,29)
Table 2. Apparent kinetic parameters for the hydrolysis of pNP-glycosides and natural diglycosides.
P1.2, which was identified as a β-glucosidase, showed slightly higher efficiency toward laminaribiose than pNP-Glc and about 24-fold higher than cellobiose, suggesting its preference for the β-1,3 over the β-1,4 glucosidic bond. Nonetheless, among the three enzymes obtained in this study, P1.2 appeared the most efficient in hydrolysis of tested natural disaccharides, cellobiose, and laminaribiose. Comparison of our kinetic parameters to those reported in the literature is quite difficult since the values of kcat and kcat/Km are often lacking. For hydrolysis of pNP-Glc, the Km value of P1.2 is similar to or lower than those of other A. niger β-glucosidases (Supplemental Table 1). Among the reported kcat and kcat/Km values of A. niger enzymes, only β-glucosidase II from A. niger CCRC 31494 had a higher kcat value (920 s−1) than our P1.2, but since its Km (2.2 mM) was also higher, its kcat/Km value was similar (420 s−1 mM−1) to our enzyme.Citation23) Interestingly, characterization of A. niger β-glucosidase from a commercial preparation (Novozymes SP188) showed a similar Km value (0.57 mM) to our P1.2, but its kcat value (26 s−1) was 10-fold lower, resulting in a 10-fold lower kcat/Km value (46 s−1 mM−1).Citation14) For hydrolysis of cellobiose, our P1.2 showed the lowest values of Km and kcat among fungal β-glucosidases, but its kcat/Km value was in the same order of magnitude as those of Novozymes SP188 (36 s−1 mM−1).Citation14) So, our data suggested that the hydrolytic efficiency of P1.2 is comparable to that of the commercial Novozymes SP188.
P2 exhibited the highest catalytic efficiency toward laminaribiose, supporting its identification by LC/MS/MS as a glucan 1,3-β-glucosidase. Unlike the other two enzymes obtained in this study, P2 showed no activity toward pNP-Ara and cellobiose. However, the enzyme exhibited 8-fold higher Km and 4-fold higher kcat for laminaribiose compared with glucan 1,3-β-glucosidase from Phanerochaete chrysosporium.Citation30) The Km value of P2 toward pNP-Glc was also higher than that of HGT-BG F2 from A. fortidus (also identified as glucan 1,3-β-glucosidase, Supplemental Table 1), but its kcat and kcat/Km values were not available for comparison.Citation10)
Inhibition constants (Ki)
The inhibitory effect of glucose and xylose on P1.1, P1.2, and P2 was measured in the reactions containing pNP-Glc and pNP-Xyl as substrates (Table ). For all cases, competitive inhibition was confirmed by Lineweaver–Burk plots (results not shown). P1.1 (β-xylosidase) could tolerate high concentrations of glucose with both substrates, but were moderately inhibited by xylose. Others have reported Ki values for xylose between 2.9 and 8.3 mM against A. niger β-xylosidasesCitation26,31,32) and 4.5 mM against A. fumigatus β-xylosidase,Citation20) which are about 20- to 50-folds lower than the values reported in this study. On the other hand, P1.2 (β-glucosidase) was strongly inhibited by glucose with pNP-Glc as substrate and by xylose with pNP-Xyl as substrate, but only moderately inhibited when the substrates were reversed. For P2 (glucan 1,3-β-glucosidase), only glucose showed inhibitory effect on its activity toward pNP-Glc, while xylose did not show the inhibitory effect for either substrate. The inhibitory effects of xylose on P1.1, and glucose on P1.2 and P2 agree well with the LC/MS/MS analysis and kinetic studies of these enzymes.
Table 3. Inhibition constants for glucose and xylose on the activities of P1.1, P1.2, and P2.
In comparison, previous studies have found the Ki values for glucose to be between 1.3 and 9.2 mM against fungal β-glucosidases, and 0.5 and 1.4 M against those reported to be glucose tolerant (Supplemental Table 1). However, since the identities of most reported HGT-BGs were not known (except for F2 from A. fortidus) and their subunit molecular weights were much less than our P1.2, it is likely that those HGT-BGs were not truly 1,4-β-glucosidases and their high Ki values could be toward activities of other types of glycoside hydrolases, such as our P2 (glucan 1,3-β-glucosidase) whose Ki value for glucose was 0.29 M. In this study, the Ki values for glucose against P1.2 (β-glucosidase) are higher than the “regular” fungal β-glucosidases, suggesting that our P1.2 is superior to these enzymes in terms of glucose tolerance.
Hydrolysis of oligosaccharides
The hydrolytic activities of the purified P1.1, P1.2, and P2 were determined toward 1 mM of various oligosaccharide substrates at their optimal pH and 40 °C for 10 min (Table ). P1.1 and P1.2 exhibited activities toward all oligosaccharides used. Both enzymes exhibited slightly higher activities toward cello-oligosaccharides (β-1,4-linked glucose polymer) than laminarioligosaccharides (β-1,3-linked glucose polymer) of comparable chain length, and their activities decreased as the chain length increased from 3 to 6. They also had higher activities toward gentiobiose (β-1,6-linked diglucoside) than amygdalin ([(6-O-β-d-glucopyranosyl-β-d-glucopyranosyl)oxy](phenyl)acetonitrile), but the activities toward laminarin (β-1,3 glucan with β-1,6 linkages) were relatively poor. On the other hand, P2 could not hydrolyze cello-oligosaccharide substrates. However, P2 exhibited high activity toward laminaribiose, and extremely high activities towards longer laminarioligosaccharides and laminarin. Amygdalin and gentiobiose are poor substrates for P2. These results are in agreement with its identification as glucan 1,3-β-glucosidase.
Table 4. Hydrolytic activity of P1.1, P1.2, and P2 toward 1 mM oligosaccharides and glycosides.
Hydrolysis of cellulose
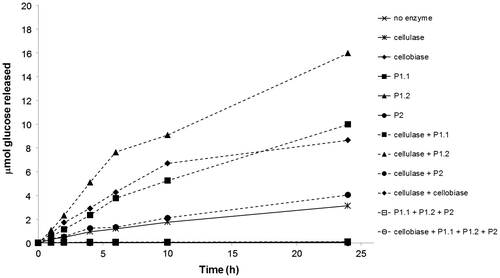
The hydrolysis of 2.5% (w/v) Sigmacell® cellulose, type 50, by 5 μg of various cellulolytic enzymes, namely P1.1, P1.2, and P2, commercial cellobiase from A. niger and commercial cellulase from Trichoderma reesei ATCC 26921, was tested in 0.1 M sodium citrate at their optimal pH, 40 °C, for 24 h with shaking at 220 rpm (Fig. ). The purified P1.1, P1.2, P2, or cellobiase alone could not significantly hydrolyze Sigmacell® cellulose since only small amounts of glucose were released. The combination of P1.1, P1.2, and P2, or the combination of P1.1, P1.2, P2, and cellobiase still could not hydrolyze Sigmacell® cellulose, suggesting that cellulase activity is required for cellulolysis. Treatment of the commercial cellulase alone resulted in the release of approximately 3 μmol glucose after 24 h incubation. While the combination of the commercial cellulase with P2 only slightly improved the glucose yield, the combination of the commercial cellulase with either P1.1 or the commercial cellobiase further increased the glucose yield to 8–10 μmol. Significantly, the combination of the commercial cellulase and P1.2 produced the highest yield of glucose from Sigmacell® cellulose, which was approximately 16 μmol (or about 12% (w/w) conversion). These results suggested that our P1.2 is superior to the commercial A. niger cellobiase in cellulose saccharification.
Sequence comparison of P1.1 and P1.2
Subsequent to the enzymatic characterization of the 3 glucose-tolerant β-glycosidases from A. niger ASKU28, the coding sequences of mature P1.1 and P1.2 were cloned (but cloning of P2 is still unsuccessful). The 2334-bp coding sequence of mature P1.1 (GenBank: KF793932) encoded 777 amino acid residues (Choengpanya K, et al., unpublished results). The deduced amino acid sequence of P1.1 corresponds to residue 28 to the stop codon of glycoside hydrolase family 3 A. niger β-xylosidase (GenBank: AAD13106) that matched with the LC/MS/MS analysis of P1.1, with 98% sequence identity.Citation31) The fact that P1.1 is identified at a nucleotide level as a β-xylosidase explains its high catalytic efficiency toward pNP-Xyl and its tolerant toward glucose inhibition.
The 2526-bp coding sequence of mature P1.2 (GenBank: JX127252) encoded 841 amino acid residues.Citation33) After translation to protein, the sequence of P1.2 corresponds to residue 20 of the stop codon of glycoside hydrolase family 3 A. niger β-glucosidase (GenBank: CAB75696) that matched with the LC/MS/MS analysis of P1.2, with only one amino acid difference.Citation34) The amino acid sequence of P1.2 is thus very similar to that of A. niger β-glucosidases BG3 and BGs, which were reported to be identical.Citation35) While normal hydrolysis takes place at low substrate concentrations, the same enzyme was reported to carry out a transglucosidic reaction at high substrate concentrations, where the second substrate molecule (such as pNP-Glc) or the product (glucose) competes with water for the glycosyl moiety covalently bound to the enzyme.Citation36) Thus, the apparent HGT-BG activities of our P1.2 in the presence of 20% glucose could also include transglucosidic reaction between the high concentration of glucose and the covalently bound glucose that accelerated the breakdown of pNP-Glc. Nonetheless, the kinetic parameters for hydrolytic reaction of our P1.2 (derived from reactions at low substrate concentrations) are in the same range as the corresponding parameters reported for the A. niger β-glucosidase with transglucosidic activity.Citation35,37)
Although there have been many reports on β-glucosidases and some HGT-BGs from Aspergillus spp. and other fungi, only a small number of them were fully characterized in terms of kinetic studies (Supplemental Table 1). Among the reported HGT-BGs, only F2 from A. fortidus has been identified as glucan 1,3-β-glucosidase.Citation10) The identities of other fungal HGT-BGs reported were not known, but were likely to be different from our P1.1 (β-xylosidase) or P1.2 (β-glucosidase), as judged by the subunit molecular weights. Our study presented here provides comprehensive data on characterization and identification of HGT-BG activities from A. niger ASKU28. The synergistic action of P1.2 and cellulase shown in this study suggests that our enzyme may be useful in enzymatic degradation of cellulose to glucose. Since the coding sequence of mature P1.2 has been cloned, the problems of its low production and purification yields were overcome by expression of recombinant enzyme in Pichia pastoris.Citation33) The substitution of Trp-49 in A. niger β-glucosidase has been shown to reduce the efficiencies of the transglucosidic reactions.Citation38) Further improvement of enzymatic properties, such as thermostability, may be achieved by protein engineering strategies. As such, P1.2 showed a high potential in industrial application for production of cellulosic ethanol.
Supplemental material
The supplemental material for this paper is available at http://dx.doi.org/10.1080/09168451.2014.915727.
Supplemental Figs. 1-8
Download PDF (337 KB)Supplemental Fig. 9
Download MS Power Point (158 KB)Supplemental Table 1
Download PDF (97.9 KB)Supplemental Fig. 9 caption
Download MS Word (12.8 KB)Acknowledgments
The authors especially thank Professor James R. Ketudat-Cairns, Suranaree University of Technology, Thailand, for kindly providing glycosides and oligosaccharides, Dr Nonlawat Boonyalai, Kasetsart University, Thailand, for kindly providing Gel filtration calibration kit and blue dextran 2000, and Professor Harry Brumer, University of British Columbia, Canada, for helpful discussion. The project was supported by grants from the National Research Council of Thailand, the Office of the Higher Education Commission, Thailand, and the Higher Education Research Promotion and National Research University Project of Thailand. P.T. is a recipient of the Ph.D. scholarship from the Office of the Higher Education Commission, Thailand.
Notes
Abbreviations: HGT-BG, highly glucose-tolerant β-glucosidase; 4MU-Glc, 4-methylumbelliferyl-β-d-glucopyranoside; pNP, para-nitrophenol; pNP-Ara, para-nitrophenyl-α-l-arabinopyranoside; pNP-Fuc, para-nitrophenyl-β-d-fucopyranoside; pNP-Gal, para-nitrophenyl-β-d-galactopyranoside; pNP-Glc, para-nitrophenyl-β-d-glucopyranoside; pNP-Man, para-nitrophenyl-β-d-mannopyranoside; pNP-Xyl, para-nitrophenyl-β-d-xylopyranoside.
References
- Iranmahboob J, Nadim F, Monemi S. Optimizing acid-hydrolysis: a critical step for production of ethanol from mixed wood chips. Biomass Bioenergy. 2002;22:401–404.10.1016/S0961-9534(02)00016-8
- Sukumaran RK, Singhania RR, Mathew GM, Pandey A. Cellulase production using biomass feed stock and its application in lignocellulose saccharification for bio-ethanol production. Renewable Energy. 2009;34:421–424.10.1016/j.renene.2008.05.008
- Lynd LR, Weimer PJ, van Zyl WH, Pretorius IS. Microbial cellulose utilization: fundamentals and biotechnology. Microbiol. Mol. Biol. Rev. 2002;66:506–577.10.1128/MMBR.66.3.506-577.2002
- Sonia K, Chadha B, Badhan A, Saini H, Bhat M. Identification of glucose tolerant acid active β-glucosidases from thermophilic and thermotolerant fungi. World J. Microbiol. Biotechnol. 2008;24:599–604.10.1007/s11274-007-9512-6
- Saha BC, Bothast RJ. Production, purification, and characterization of a highly glucose-tolerant novel β-glucosidase from Candida peltata. App. Environ. Microbiol. 1996;62:3165–3170.
- Yan TR, Lin CL. Purification and characterization of a glucose-tolerant β-glucosidase from Aspergillus niger CCRC 31494. Biosci. Biotechnol. Biochem. 1997;61:965–970.10.1271/bbb.61.965
- Riou C, Salmon J-M, Vallier M-J, Günata Z, Barre P. Purification, characterization, and substrate specificity of a novel highly glucose-tolerant β-glucosidase from Aspergillus oryzae. Appl. Environ. Microbiol. 1998;64:3607–3614.
- Günata Z, Vallier M-J. Production of a highly glucose-tolerant extracellular β-glucosidase by three Aspergillus strains. Biotechnol. Lett. 1999;21:219–223.10.1023/A:1005407710806
- Decker CH, Visser J, Schreier P. β-Glucosidase multiplicity from Aspergillus tubingensis CBS 643.92: purification and characterization of four β-glucosidases and their differentiation with respect to substrate specificity, glucose inhibition and acid tolerance. Appl. Microbiol. Biotechnol. 2001;55:157–163.10.1007/s002530000462
- Decker CH, Visser J, Schreier P. β-Glucosidases from five black Aspergillus species: study of their physico-chemical and biocatalytic properties. J. Agric. Food Chem. 2000;48:4929–4936.10.1021/jf000434d
- Uchima CA, Tokuda G, Watanabe H, Kitamoto K, Arioka M. Heterologous expression in Pichia pastoris and characterization of an endogenous thermostable and high-glucose-tolerant β-glucosidase from the termite Nasutitermes takasagoensis. Appl. Environ. Microbiol. 2012;78:4288–4293.10.1128/AEM.07718-11
- van den Brink J, de Vries RP. Fungal enzyme sets for plant polysaccharide degradation. Appl. Microbiol. Biotechnol. 2011;91:1477–1492.10.1007/s00253-011-3473-2
- Pel HJ, de Winde JH, Archer DB, Dyer PS, Hofmann G, Schaap PJ, Turner G, de Vries RP, Albang R, Albermann K, Andersen MR, Bendtsen JD, Benen JAE, van den Berg M, Breestraat S, Caddick MX, Contreras R, Cornell M, Coutinho PM, Danchin EGJ, Debets AJM, Dekker P, van Dijck PWM, van Dijk A, Dijkhuizen L, Driessen AJM, d'Enfert C, Geysens S, Goosen C, Groot GSP, de Groot PWJ, Guillemette T, Henrissat B, Herweijer M, van den Hombergh JPTW, van den Hondel CAMJJ, van der Heijden RTJM, van der Kaaij RM, Klis FM, Kools HJ, Kubicek CP, van Kuyk PA, Lauber J, Lu X, van der Maarel MJEC, Meulenberg R, Menke H, Mortimer MA, Nielsen J, Oliver SG, Olsthoorn M, Pal K, van Peij NNME, Ram AFJ, Rinas U, Roubos JA, Sagt CMJ, Schmoll M, Sun J, Ussery D, Varga J, Vervecken W, van de Vondervoort PJJ, Wedler H, Wösten HAB, Zeng A-P, van Ooyen AJJ, Visser J, Stam H. Genome sequencing and analysis of the versatile cell factory Aspergillus niger CBS 513.88. Nat. Biotechnol. 2007;25:221–231.10.1038/nbt1282
- Chauve M, Mathis H, Huc D, Casanave D, Monot F, Ferreira NL. Comparative kinetic analysis of two fungal β-glucosidases. Biotechnol. Biofuels. 2010;3.10.1186/1754-6834-3-3
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685.10.1038/227680a0
- Srisomsap C, Svasti J, Surarit R, Champattanachai V, Sawangareetrakul P, Boonpuan K, Subhasitanont P, Chokchaichamnankit D. Isolation and characterization of an enzyme with β-glucosidase and β-fucosidase activities from Dalbergia cochinchinensis Pierre. J. Biochem. 1996;119:585–590.10.1093/oxfordjournals.jbchem.a021282
- Shevchenko A, Tomas H, Havlis J, Olsen JV, Mann M. In-gel digestion for mass spectrometric characterization of proteins and proteomes. Nat. Protoc. 2007;1:2856–2860.10.1038/nprot.2006.468
- Perkins DN, Pappin DJC, Creasy DM, Cottrell JS. Probability-based protein identification by searching sequence databases using mass spectrometry data. Electrophoresis. 1999;20:3551–3567.10.1002/(ISSN)1522-2683
- Miller GL. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959;31:426–428.10.1021/ac60147a030
- Kitpreechavanich V, Hayashi M, Nagai S. Purification and characterization of extracellular β-xylosidase and β-glucosidase from Aspergillus fumigatus. Agric. Biol. Chem. 1986;50:1703–1711.10.1271/bbb1961.50.1703
- Collins CM, Murray PG, Denman S, Morrissey JP, Byrnes L, Teeri TT, Tuohy MG. Molecular cloning and expression analysis of two distinct β-glucosidase genes, bg1 and aven1, with very different biological roles from the thermophilic, saprophytic fungus Talaromyces emersonii. Mycol. Res. 2007;111:840–849.10.1016/j.mycres.2007.05.007
- Rashid M, Siddiqui K. Purification and characterization of a β-glucosidase from Aspergillus niger. Folia Microbiol. 1997;42:544–550.10.1007/BF02815462
- Yan T-R, Lin Y-H, Lin C-L. Purification and characterization of an extracellular β-glucosidase II with high hydrolysis and transglucosylation activities from Aspergillus niger. J. Agric. Food Chem. 1998;46:431–437.10.1021/jf9702499
- Bendtsen JD, Nielsen H, von Heijne G, Brunak S. Improved prediction of signal peptides: signalp 3.0. J. Mol. Biol. 2004;340:783–795.10.1016/j.jmb.2004.05.028
- Wakiyama M, Yoshihara K, Hayashi S, Ohta K. Purification and properties of an extracellular β-xylosidase from Aspergillus japonicus and sequence analysis of the encoding gene. J. Biosci. Bioeng. 2008;106:398–404.10.1263/jbb.106.398
- Rodionova NA, Tavobilov IM, Bezborodov AM. β-Xylosidase from Aspergillus niger 15: purification and properties. J. Appl. Biochem. 1983;5:300–312.
- Eyzaguirre J, Hidalgo M, Leschot A. β-Glucosidases from filamentous fungi: properties, structure, and applications. In Yarema KJ, editor. Handbook of carbohydrate engineering. Boca Raton: CRC Press; 2005. p. 645–685.
- Jäger S, Brumbauer A, Fehér E, Réczey K, Kiss L. Production and characterization of β-glucosidases from different Aspergillus strains. World J. Microbiol. Biotechnol. 2001; 17: 455–461.10.1023/A:1011948405581
- Lee RC, Hrmova M, Burton RA, Lahnstein J, Fincher GB. Bifunctional family 3 glycoside hydrolases from barley with α-l-arabinofuranosidase and β-d-xylosidase activity. J. Biol. Chem. 2003;278:5377–5387.10.1074/jbc.M210627200
- Kawai R, Igarashi K, Kitaoka M, Ishii T, Samejima M. Kinetics of substrate transglycosylation by glycoside hydrolase family 3 glucan (1→3)-β-glucosidase from the white-rot fungus Phanerochaete chrysosporium. Carbohydr. Res. 2004; 339: 2851–2857.10.1016/j.carres.2004.09.019
- La Grange DC, Pretorius IS, Claeyssens M, van Zyl WH. Degradation of xylan to d-xylose by recombinant Saccharomyces cerevisiae coexpressing the Aspergillus niger β-xylosidase (xlnD) and the Trichoderma reesei xylanase II (xyn2) genes. Appl. Environ. Microbiol. 2001;67:5512–5519.10.1128/AEM.67.12.5512-5519.2001
- Selig M, Knoshaug E, Decker S, Baker J, Himmel M, Adney W. Heterologous expression of Aspergillus niger β-d-xylosidase (XlnD): characterization on lignocellulosic substrates. Appl. Biochem. Biotechnol. 2008;146:57–68.10.1007/s12010-007-8069-z
- Thongpoo P, McKee LS, Araújo AC, Kongsaeree PT, Brumer H. Identification of the acid/base catalyst of a glycoside hydrolase family 3 (GH3) β-glucosidase from Aspergillus niger ASKU28. Biochim. Biophys. Acta - General Subjects. 2013;1830:2739–2749.10.1016/j.bbagen.2012.11.014
- Dan S, Marton I, Dekel M, Bravdo B-A, He S, Withers SG, Shoseyov O. Cloning, expression, characterization, and nucleophile identification of family 3, Aspergillus niger β-glucosidase. J. Biol. Chem. 2000;275:4973–4980.10.1074/jbc.275.7.4973
- Seidle HF, Marten I, Shoseyov O, Huber RE. Physical and kinetic properties of the family 3 β-glucosidase from Aspergillus niger which is important for cellulose breakdown. Protein J. 2004;23:11–23.10.1023/B:JOPC.0000016254.58189.2a
- Seidle HF, Huber RE. Transglucosidic reactions of the Aspergillus niger family 3 β-glucosidase: qualitative and quantitative analyses and evidence that the transglucosidic rate is independent of pH. Arch. Biochem. Biophys. 2005;436:254–264.10.1016/j.abb.2005.02.017
- Seidle HF, McKenzie K, Marten I, Shoseyov O, Huber RE. Trp-262 is a key residue for the hydrolytic and transglucosidic reactivity of the Aspergillus niger family 3 β-glucosidase: substitution results in enzymes with mainly transglucosidic activity. Arch. Biochem. Biophys. 2005;444:66–75.10.1016/j.abb.2005.09.013
- Seidle HF, Allison SJ, George E, Huber RE. Trp-49 of the family 3 β-glucosidase from Aspergillus niger is important for its transglucosidic activity: creation of novel β-glucosidases with low transglucosidic efficiencies. Arch. Biochem. Biophys. 2006;455:110–118.10.1016/j.abb.2006.09.016