Abstract
As a component of the renin-angiotensin system, the (pro)renin receptor [(P)RR] activates prorenin along with intracellular signaling pathways. In this study, the glutathione S-transferase-fused extracellular domain of (P)RR expressed in mammalian cells was recovered in the detergent phase in detergent-based two-phase separation experiments, and intracellular localization was observed by immunocytochemistry, suggesting retention inside the cell through stable membrane association.
The (pro)renin receptor [(P)RR] is a newly identified regulator of the renin-angiotensin system that plays an essential role in blood pressure control and electrolyte balance.Citation1) (P)RR has two distinct components: the evolutionarily ancient transmembrane and cytoplasmic domains, as well as a vertebrate-specific extracellular domain (ECD).Citation2) The binding of renin (EC 3.4.23.15) and its inactive precursor prorenin to the N-terminal ECD of (P)RR induces the activation of the ERK1/2 MAP kinase signaling pathwayCitation1) as well as the non-proteolytic activation of prorenin.Citation1,3) We previously purified the (P)RR ECD for binding studies using a cell-free system;Citation3) however, (P)RR tended to aggregate, and a mild detergent (0.1% Brij35) was required for optimum purification. As an alternative method, in the present study, the (P)RR ECD was expressed and characterized in cultured mammalian cells. In addition, phase separation and immunocytochemistry experiments were carried out to determine the cellular localization of the (P)RR ECD.
Expression constructs encoding the full-length (FL), 350-amino acid rat (P)RR protein (DNA Data Bank of Japan, No. AB188298), and ECD (residues 1–304) of (P)RR were generated in the backbone vector pcDNA3 (Invitrogen, Carlsbad, CA, USA). In the FL construct (FLAG-FL), a FLAG epitope tag was inserted between the signal peptide and ECD (Fig. (A)), while the ECD construct (FLAG-ECD) contained an additional decahistidine tag fused to the C-terminus. COS-7 cells (RCB0539; RIKEN Bioresource Center Cell Bank, Tsukuba, Japan) maintained in Dulbecco’s modified Eagle medium supplemented with 10% fetal bovine serum (FBS), 2 mmol/L l-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin at 37 °C and 5% CO2 were transiently transfected for 24 h with the FLAG constructs or empty vector (which served as a control) by using the calcium phosphate method. The medium was replaced with serum-free medium, and 48 h later, cell lysates and culture supernatants were analyzed by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) followed by immunoblotting using anti-FLAG M1 antibody (Covance, San Diego, CA, USA). FLAG-ECD as well as FLAG-FL was detected in the cell lysate, but not in the culture supernatant (Fig. (A)), indicating the intracellular retention of the (P)RR ECD.
Fig. 1. Schematic representation of FLAG-tagged and GST-fused (P)RR constructs used in this study.
Notes: (A) The full-length (FL) (P)RR construct, which includes the transmembrane (TM) and cytoplasmic (Cyto) domains, and the (P)RR extracellular domain (ECD) were tagged with an N-terminal FLAG epitope (FLAG). FLAG-ECD also contained a C-terminal decahistidine (His) tag. (B) Each GST fusion construct contained a foreign signal peptide (SP) at the N-terminus of GST that targets the protein for secretion. GST-KDEL contained a KDEL C-terminal ER retention sequence. Numbers and those in parentheses indicate amino acid positions in rat or human (P)RR sequences and those in GST with SP, respectively.
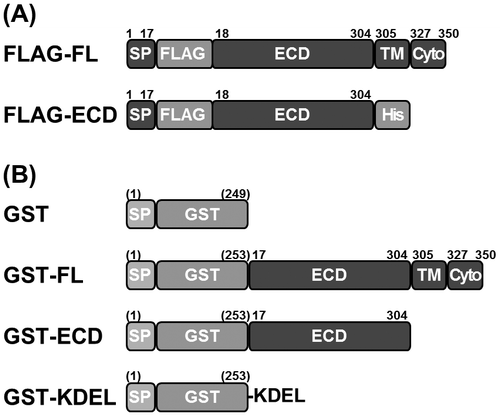
Fig. 2. Localization of the (P)RR ECD in COS-7 cells.
Notes: (A) Cells were transfected with the empty plasmid pcDNA3 (Vector) or FLAG-tagged full-length (FL) (P)RR or the extracellular domain (ECD) of (P)RR. Cell lysates (Cell) and culture supernatants (Sup) were analyzed by immunoblotting using an antibody against the FLAG epitope. (B) Immunoblot of cells transfected with (from left to right) GST-fused FL (P)RR or (P)RR ECD; GST with a KDEL ER retention sequence; GST alone; or the empty vector. Cell lysates and culture supernatants were analyzed 48 h after transfection using an antibody against GST. Asterisks indicate non-specific protein bands. (C) Representative images of cells 48 h after transfection with each GST construct. A high signal intensity was observed in the cytoplasm surrounding the nucleus in cells transfected with the FL and ECD (P)RR constructs, similar to the ER localization of the KDEL-fused GST. Scale bar = 20 μm.
To confirm these observations, COS-7 cells were transfected with (P)RR-FL and -ECD fused to glutathione S-transferase (GST) by using the polyethyleneimine transfection method.Citation4) The fusion constructs (Fig. (B)) were generated in the pcDNA3 backbone vector and contained a 24-amino acid signal peptide from the sheep angiotensinogen sequence targeting the protein for secretion into the extracellular spaceCitation5) fused to the N-terminus of Schistosoma japonicum GST. GST-FL and -ECD contained residues 17–350 and 17–304 of human (P)RR, respectively, at the C-terminus, while GST-KDEL contained a C-terminal human calreticulin KDEL endoplasmic reticulum (ER) retention sequence. GST-KDEL was used as a control soluble protein that is retained in the ER through dynamic retrieval.Citation6) KDEL-containing proteins that have escaped from the ER are bound by the KDEL receptor at the Golgi apparatus and are returned to the ER, where they are released from the receptor. After a 48-h transfection, cell lysates and culture supernatants were analyzed by SDS-PAGE followed by immunoblotting using an anti-GST antibody (Santa Cruz Biotechnology Inc., Dallas, TX, USA).
GST alone was predominantly found in the supernatant, while GST-KDEL was mostly present in the cell lysate along with GST-FL and GST-ECD (Fig. (B)). To examine the distribution of the (P)RR ECD in cells, COS-7 cells grown on a cover slip were transfected with these constructs by using the polyethyleneimine method and immunofluorescence labeling was performed using the same anti-GST antibody. After 48 h, cells were fixed in 3.7% formaldehyde in phosphate-buffered saline (PBS; pH 7.4) and permeabilized in PBS containing 0.1% Tween 20 and 0.1% Triton X-100. After blocking with PBS containing 2% FBS and 0.1% Tween 20, cells were incubated at 4 °C overnight with anti-GST antibody, washed with PBS, and then incubated at room temperature for 1 h with Cy2-conjugated anti-rabbit IgG antibody (Jackson ImmunoResearch Laboratories, West Grove, PA, USA). After PBS washes, the cover slips were mounted on slides with mounting medium and fluorescence images were obtained using a fluorescence microscope (BX51; Olympus, Tokyo, Japan). GST-FL, -ECD, and -KDEL localized to intracellular, perinuclear membranous structures, while GST alone was scarcely observed in the cells (Fig. (C)). These results indicate that the (P)RR ECD is retained inside COS-7 cells.
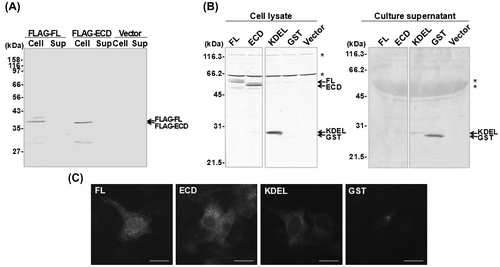
To determine whether the intracellular retention of the (P)RR ECD occurs in other cell types, human hepatoma HepG2 cells (JCRB 1054; Health Science Research Resources Bank, Osaka, Japan) were transiently transfected with the GST constructs by using the polyethyleneimine method. Cells were cultured under the same conditions as COS-7 cells, except that the culture medium contained 5% FBS. After a 48-h transfection, cell lysates and culture supernatants were analyzed by SDS-PAGE followed by immunoblotting using an anti-GST antibody. As in COS-7 cells, GST-FL, -ECD, and -KDEL were detected only in the cell lysates, while the supernatant contained GST alone (Fig. (A)). These results indicate that the (P)RR ECD is retained intracellularly in heterologous cell types.
Fig. 3. Localization of the (P)RR ECD in HepG2 Cells.
Notes: (A) Immunoblot of HepG2 cells transiently transfected with (from left to right) GST-fused full length (FL) (P)RR or the extracellular domain (ECD) of (P)RR; GST with a KDEL ER retention sequence; GST alone; or the empty plasmid pcDNA3 (Vector). Cell lysates and culture supernatants were analyzed 48 h after transfection using an antibody against GST. Asterisks indicate non-specific protein bands. (B) Stable HepG2 cell lines expressing GST-FL, -ECD, or -KDEL were cultured in fresh medium and cell lysates were analyzed by immunoblotting 24 h later. GST fusion proteins were detected in the lysates of the transfected, but not wild-type untransfected cells (WT). Asterisk indicates non-specific protein bands. (C) Phase separation analysis. HepG2 cells stably expressing GST-FL, -ECD, or -KDEL were solubilized in lysis buffer and analyzed by phase separation. Equal portions of cleared total cell lysate (T), aqueous phase (A), wash fraction (W), and detergent phase (D) were analyzed by immunoblotting using antibodies against GST. As a control, the blot was probed with antibodies against the integral membrane protein calnexin (which was detected in the detergent phase), as well as the cytosolic protein actin (which was confined to the aqueous phase). Arrows in (C) indicate bands corresponding to GST fusion proteins.
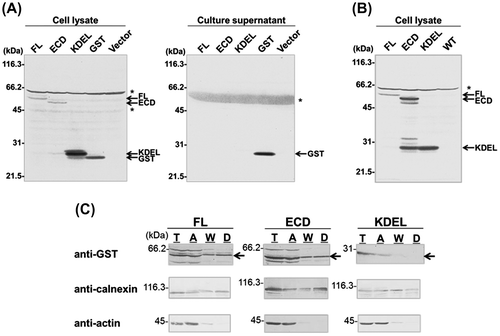
Stable HepG2 cell lines expressing the GST fusion proteins were established to eliminate the possibility that the above observations were due to the nonspecific effects of transient protein expression. To generate stable cell lines, HepG2 cells were transfected using FuGENE 6 transfection reagent (Roche Diagnostics GmbH, Mannheim, Germany) according to the manufacturer’s instructions, and cultured in a selection medium containing 0.2–0.8 mg/mL G-418 (Nacalai Tesque, Kyoto, Japan). Isolated clones expressing each construct were grown in the selection medium and harvested for analysis, as described above. Similar to the transient transfections, GST-FL, -ECD, and -KDEL were found in the cell lysates (Fig. (B)), but not in the culture supernatants (data not shown).
In general, retention of soluble proteins can occur by one of the two mechanisms: (1) static retention achieved by stable association (direct or indirect) with cellular membranes, or (2) dynamic retrieval through transient association with intracellular machinery.Citation7) In the latter case, a KDEL sequence is usually required for the proteins to remain in the ER;Citation8,9) however, the (P)RR ECD lacks this and any other known retention sequence. To identify the mechanism responsible for the intracellular retention of the (P)RR ECD, a detergent-based two-phase separation (i.e. cloud point extraction) method was used.Citation10) This technique is based on the temperature-dependent phase transition of some detergents (e.g. Triton X-114) and provides a convenient way of separating integral membrane and soluble proteins. It has been successfully applied to the separation of KDEL-dependent and -independent soluble ER-resident proteins.Citation11)
The phase separation was performed using the CelLytic MEM Protein Extraction Kit (Sigma-Aldrich, St. Louis, MO, USA) according to the manufacturer’s instructions. Stably transfected HepG2 cells were solubilized in the lysis and separation buffers, centrifuged to remove debris and nuclei, and separated into detergent and aqueous phases containing hydrophobic and hydrophilic proteins, respectively. The detergent (hydrophobic) phase was washed with wash buffer to remove residual hydrophilic proteins. The cleared total cell lysate, wash fractions, and final detergent and aqueous phases were analyzed by SDS-PAGE followed by immunoblotting using anti-GST, anti-calnexin (Santa Cruz Biotechnology Inc.), or anti-actin (Sigma-Aldrich) antibodies.
As expected, the control integral membrane protein calnexin was detected in the detergent phase (Fig. (C)), whereas the control soluble cytosolic protein actin was recovered almost exclusively from the aqueous phase, confirming the correct separation of proteins under these experimental conditions. It has been reported that protein disulfide isomerase, an ER-resident protein containing the KDEL sequence, was recovered in the aqueous and not the detergent phase using Triton X-114.Citation11) Consistent with these findings, in the present experiment, GST-KDEL was recovered only in the aqueous phase (Fig. (C)), which would be predicted for a soluble protein whose intracellular retention is achieved by dynamic retrieval. In addition to the FL fusion protein, GST-ECD was detected in the detergent phase despite the absence of a transmembrane domain, similar to the results obtained from transiently transfected COS-7 cells (data not shown). These results suggest that the intracellular retention of GST-ECD occurs in a different manner than that of GST-KDEL.
Lysyl hydroxylase, an ER luminal protein lacking the KDEL sequence, remains in the ER by static retention.Citation11,12) Co-immunoprecipitation experiments have shown that (P)RR binds to the Wnt receptors Frizzled8 and LRP6, as well as the V-ATPase complex, which is reduced or abolished by the deletion of the (P)RR transmembrane and cytoplasmic domains.Citation13) Thus, it is unlikely that the intracellular retention of the (P)RR ECD occurs through Wnt receptors or the V-ATPase complex. It could, instead, depend on the interactions with an unidentified membrane protein or by post-translational modification of the (P)RR ECD that would allow association with intracellular membranes. Alternatively, our previous observations that the (P)RR ECD required detergent for proper solubilization in a cell-free systemCitation3) could be evidence for its hydrophobicity, in which case a direct interaction with the membrane is possible.
FL (P)RR is cleaved on the luminal side of the juxtamembrane domain by the proteases furinCitation14) and ADAM19.Citation15) The N-terminal fragment that includes most of the ECD, i.e. the soluble (P)RR, is released into the extracellular space, and has been detected in a variety of cell typesCitation14,15) as well as in plasmaCitation14) and urine,Citation16) giving rise to its potential as a biomarker for certain diseases. Considering our findings, the secretion of soluble (P)RR may be regulated. Further studies are necessary to understand how the stable association of (P)RR with intracellular membrane contributes to the soluble (P)RR secretion.
In conclusion, we propose that the ECD of (P)RR is retained inside the cell through stable, direct or indirect, membrane association. These findings provide novel insight into the regulatory mechanism of soluble (P)RR secretion, which may play a role in the biology and physiology of (P)RR.
Acknowledgments
We thank Prof. Yukio Nakamura and Aya Shibata for their participation during the early stages of this work. We also thank Prof. Mohammad N. Uddin, Prof. A.H.M. Nurun Nabi, and Dr Kazal B. Biswas for reviewing the manuscript.
Funding
This work was supported in part by the Japan Society for the Promotion of Science KAKENHI [grant number 22580102] and by a grant from Gifu University (KASSEIKA KEIHI) to T.N.
Notes
Abbreviations: ECD, extracellular domain; ER, endoplasmic reticulum; FBS, fetal bovine serum; FL, full-length; GST, glutathione S-transferase; PAGE, polyacrylamide gel electrophoresis; PBS, phosphate-buffered saline; (P)RR, (pro)renin receptor.
References
- Nguyen G, Delarue F, Burcklé C, Bouzhir L, Giller T, Sraer JD. Pivotal role of the renin/prorenin receptor in angiotensin II production and cellular responses to renin. J. Clin. Invest. 2002;109:1417–1427.10.1172/JCI0214276
- Burckle C, Bader M. Prorenin and its ancient receptor. Hypertension. 2006;48:549–551.10.1161/01.HYP.0000241132.48495.df
- Nabi AH, Biswas KB, Nakagawa T, Ichihara A, Inagami T, Suzuki F. Prorenin has high affinity multiple binding sites for (pro)renin receptor. Biochim. Biophys. Acta. 2009;1794:1838–1847.10.1016/j.bbapap.2009.08.024
- Boussif O, Lezoualc’h F, Zanta MA, Mergny MD, Scherman D, Demeneix B, Behr JP. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc. Nat. Acad. Sci. USA. 1995;92:7297–7301.10.1073/pnas.92.16.7297
- Nakagawa T, Nishiuchi K, Akaki J, Iwata H, Satou R, Suzuki F, Nakamura Y. Efficient production of recombinant human (pro)renin utilizing a decahistidine tag attached at the C-terminus. Biosci. Biotechnol. Biochem. 2007;71:256–260.10.1271/bbb.60455
- Pelham HR. The dynamic organisation of the secretory pathway. Cell Struct. Funct. 1996;21:413–419.10.1247/csf.21.413
- Sato K, Nishikawa S, Nakano A. Membrane protein retrieval from the Golgi apparatus to the endoplasmic reticulum (ER): characterization of the RER1 gene product as a component involved in ER localization of Sec12p. Mol. Biol. Cell. 1995;6:1459–1477.10.1091/mbc.6.11.1459
- Munro S, Pelham HR. A C-terminal signal prevents secretion of luminal ER proteins. Cell. 1987;48:899–907.10.1016/0092-8674(87)90086-9
- Lewis MJ, Pelham HR. Ligand-induced redistribution of a human KDEL receptor from the Golgi complex to the endoplasmic reticulum. Cell. 1992;68:353–364.10.1016/0092-8674(92)90476-S
- Bordier C. Phase separation of integral membrane proteins in Triton X-114 solution. J. Biol. Chem. 1981;256:1604–1607.
- Kellokumpu S, Sormunen R, Heikkinen J, Myllylä R. Lysyl hydroxylase, a collagen processing enzyme, exemplifies a novel class of luminally-oriented peripheral membrane proteins in the endoplasmic reticulum. J. Biol. Chem. 1994;269:30524–30529.
- Suokas M, Lampela O, Juffer AH, Myllylä R, Kellokumpu S. Retrieval-independent localization of lysyl hydroxylase in the endoplasmic reticulum via a peptide fold in its iron-binding domain. Biochem. J. 2003;370:913–920.10.1042/BJ20021533
- Cruciat CM, Ohkawara B, Acebron SP, Karaulanov E, Reinhard C, Ingelfinger D, Boutros M, Niehrs C. Requirement of prorenin receptor and vacuolar H+-ATPase-mediated acidification for Wnt signaling. Science. 2010;327:459–463.10.1126/science.1179802
- Cousin C, Bracquart D, Contrepas A, Corvol P, Muller L, Nguyen G. Soluble form of the (pro)renin receptor generated by intracellular cleavage by furin is secreted in plasma. Hypertension. 2009;53:1077–1082.10.1161/HYPERTENSIONAHA.108.127258
- Yoshikawa A, Aizaki Y, Kusano K, Kishi F, Susumu T, Iida S, Ishiura S, Nishimura S, Shichiri M, Senbonmatsu T. The (pro)renin receptor is cleaved by ADAM19 in the Golgi leading to its secretion into extracellular space. Hypertens. Res. 2011;34:599–605.10.1038/hr.2010.284
- Gonzalez AA, Lara LS, Luffman C, Seth DM, Prieto MC. Soluble form of the (pro)renin receptor is augmented in the collecting duct and urine of chronic angiotensin II-dependent hypertensive rats. Hypertension. 2011;57:859–864.10.1161/HYPERTENSIONAHA.110.167957
